ABSTRACT
We investigated the content of phenolic compounds and antioxidant capacity of two batches of non-heated and heated leaves of the yacon cultivar “Andes no yuki”, grown in Japan. Lyophilized yacon leaves heated at 160°C for 20 min and 100°C for 60 min had a 1.96 to 9.69-times higher total phenolic content than that of the non-heated leaves. Heated leaves exhibited a 1.98 to 4.07-times higher antioxidant capacity than that of the non-heated leaves in three different free radical scavenging assays. Heated leaves were more efficient at attenuating the superoxide anion radical production in human granulocytic cells than the non-heated leaves. High-performance liquid chromatography analysis revealed that, in the heated leaves, the caffeic acid content was 2.13 to 3.64-times higher and the chlorogenic acid content was slightly lower than those in the non-heated leaves. Hence, heat processing may affect the active constituent contents in yacon leaves, potentiating its antioxidant capacity.
Abbreviations: ABTS+: 2,2′-azinobis(2-ethylbenzothiazoline-6-sulfonic acid) cation; DPPH: 1,1-diphenyl-2-picrylhydrazyl, HPLC: high-performance liquid chromatography; NBT: nitroblue tetrazolium; O2−: superoxide anion; PMA: phorbol 12-myristate 13-acetate; PMS: phenazine methosulfate; TEAC: Trolox equivalent antioxidant capacity
Graphical abstract
Outline of increased phenolic content and antioxidant capacity of the heated leaves of yacon (Smallanthus sonchifolius).
Yacon (Smallanthus sonchifolius) is an Andean crop with medicinal properties. This plant was introduced to other countries in Asia, Oceania, and Europe from the Andes [Citation1]. The shape of the yacon tuberous root is similar to that of sweet potato. The yacon root is consumed as a sweet vegetable and is a part of processed foods sold in the local markets of Japan. Most of the aerial part of this plant is the residual obtained upon harvesting. The leaves of this plant are occasionally used for the preparation of herbal tea. Yacon leaves possess antifungal activity [Citation2] and antioxidant activity and even exhibited a hypoglycemic effect in rat models [Citation3]. The major phytochemical constituents of yacon leaves are phenolic compounds, such as chlorogenic acid, caffeic acid, and ferulic acid [Citation4,Citation5]. Additionally, this plant also contains gallic acid, p-coumaric acid [Citation6], rutin, myricetin, quercetin, apigenin, luteolin [Citation6,Citation7], and characteristic caffeoyl derivatives [Citation8]. Therefore, we were interested in establishing yacon (especially the leaves), which is locally cultivated in Japan, as a raw material for manufacturing health-food products.
Our recent studies demonstrated that commercially available yacon herbal tea exhibits antioxidant [Citation9], and anti-inflammatory activities [Citation10] and inhibitory effect against two digestive enzymes, α-glucosidase and α-amylase [Citation11]. We have optimized the extraction parameters for dried yacon herbal tea leaves to enhance their antioxidant activity and total phenolic content using response surface methodology [Citation12]. The polyphenol content varies depending on the variety, harvest time, and processing of plant materials [Citation13]. Generally, a heating process is used for food manufacturing. As the heating process can degrade the thermolabile components, several studies have focused on the effect of heat processing on the antioxidant capacity and polyphenol content of various vegetables [Citation14,Citation15]. An earlier study has demonstrated that phytochemical changes can occur during the commercial production of tea, and that the tea-making process results in 14% loss in the total catechin content [Citation16]. However, the effect of food processing on the stability of the active ingredients, such as polyphenols in the yacon leaves and their function, is not known.
Elevated levels of reactive oxygen species and oxidative stress exert a deleterious effect on normal tissue and cellular functions [Citation17]. Superoxide anion (O2−) radical has been implicated in a variety of pathological processes, such as diabetes, ischemia-reperfusion injury, and chronic heart failure [Citation18]. As superoxide dismutase (SOD) can decrease the level of O2− in our body, the ability to attenuate O2− production can be used for screening compounds that exhibit SOD-like activity. Attenuation of excess O2− production may reduce the risk for developing various diseases. Different free radical scavenging assays using synthetic 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical [Citation19] and 2,2ʹ-azinobis (3-ethylbenzhothiasoline-6-sulfonic acid) cation (ABTS+) radical [Citation20] have been developed for quantitatively evaluating the antioxidative effect of foods and phenolic compounds. Trolox is a known antioxidant, which is used to determine the Trolox equivalent (TE) value or Trolox equivalent antioxidant capacity (TEAC) value in a variety of antioxidant assays [Citation9,Citation12,Citation21].
In this study, we demonstrated that upon heating yacon leaves at 160°C for 20 min followed by heating at 100°C for 60 min, the phenolic compound content and antioxidant capacity of the heated leaf extract increases when compared to those in the non-heated leaf extract. We collected two different batches of yacon leaves from the cultivar “Andes no yuki (SY206)” in two different seasons. The leaves were lyophilized and used for heat processing. The phenolic content of the leaf extract was evaluated by measuring the contents of total polyphenol, total flavonoid, tannin, and proanthocyanidin. To determine the antioxidant activity of the leaf extract, we performed ABTS+, DPPH, and O2− radical scavenging assays. Additionally, we investigated the effect of heated yacon leaves on the attenuation of excessive cellular O2− radical generation in activated human granulocytic neutrophil cells. The phenolic content in the heated leaf extract was also analyzed by high-performance liquid chromatography (HPLC). The total polyphenol contents of heated and non-heated extracts of yacon leaves that were collected in different years was also evaluated.
Materials and methods
Materials
The yacon cultivar, “Andes no yuki (SY206)” is cultivated in Japan [Citation22]. This plant is maintained at a field owned by the School of Agriculture, Tokai University (Minamiaso, Kumamoto, Japan). The fresh seed tubers weighing 15–20 g were planted between mid-March and April at 50 cm intervals in plastic film mulched rows 100 cm apart. The fertilizer used for the planted tubers comprised 10 kg of N and 20 kg of P2O5. The leaves were collected in two batches: September (No. 1) and November (No. 2) in 2010. The leaves positioned second from the top of yacon plant were sampled. The leaves were also collected in the November month of 2013, 2015, 2016, and 2017 for comparing the total phenolic content. These leaves were stored at −20°C and then lyophilized. Dimethyl sulfoxide (DMSO), ABTS, caffeic acid, chlorogenic acid, DPPH, Folin-Ciocalteu phenol reagent, HPLC-grade acetic acid, nitroblue tetrazolium (NBT), β-nicotinamide adenine dinucleotide disodium salt (reduced form) (NADH), phenazine methosulfate (PMS), potassium peroxodisulfate were purchased from Nacalai Tesque Inc. (Kyoto, Japan). RPMI-1640 medium, Hank’s balanced salt solution (HBSS), phorbol 12-myristate 13-acetate (PMA), and HPLC-grade methanol were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). We purchased 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt (WST-1) from Dojindo Labs (Kumamoto, Japan). The HL-60 human promyelocytic leukemia cells (JCRB00085) were obtained from the Japanese Collection of Research Bioresources (Tokyo, Japan). Fetal bovine serum (FBS) was purchased from Biowest (Nuaille, France). All other chemicals were of the highest grade and were commercially available.
Preparation of heated leaves and extracts
The yacon leaves were subjected to heat treatment following the procedure used for general green tea production with minor modifications [Citation23]. Briefly, we heated 2.50 g of lyophilized and crushed yacon leaves in an oven at 160°C for 20 min followed by heating at 100°C for 60 min. To eliminate the effect of weight loss due to the heating process, we considered 2.50 g of initial weight for extraction.
Extracts of non-heated leaves and heated leaves were prepared using 50% methanol. Briefly, 2.50 g of lyophilized and crushed leaves were soaked in 32 mL of methanol at 4°C overnight. To prepare the extract of heated yacon leaves, we heated 2.50 g of lyophilized and crushed leaves as mentioned above and then soaked in methanol. The heated and non-heated leaves soaked in methanol were kept at 4°C for another day with 32 mL of MilliQ water. The mixture was filtered and subjected to lyophilization to obtain the extract. The extract was reconstituted in 50% ethanol at 20 mg/mL for further analysis.
Determination of total polyphenol content
The total polyphenol content was determined as reported previously [Citation24] with minor modifications [Citation9]. Briefly, the mixture containing the extract (25 μL) and 10-fold diluted Folin-Ciocalteu phenol reagent solution (125 μL) was incubated with 10% sodium carbonate solution (125 μL) for 10 min at room temperature (20–25°C). The absorbance of the mixture was measured at 600 nm using a grating microplate reader (SH-1000Lab, Corona Electric, Ibaraki, Japan). Chlorogenic acid was used as the standard to generate the calibration curve. The total polyphenol content was expressed in terms of milligram chlorogenic acid equivalent (CAE) per gram of sample (dry weight). Additionally, we also set up assay mixtures with only samples or reagents to determine the background absorbance.
Determination of total flavonoid content
The total flavonoid content was determined as described previously [Citation25]. Briefly, the mixture containing the extract (25 μL) and MilliQ water (125 μL) was incubated with 5% sodium nitrite solution (7.5 μL) for 6 min at room temperature (20–25°C). Then, 10% aluminum chloride solution (7.5 μL) was added to the mixture and incubated for 5 min. Further, 1 M NaOH (50 µL) and MilliQ water (27.5 µL) were added to the mixture and incubated for 5–10 min with continuous shaking. The absorbance of the assay mixture was measured at 510 nm using a microplate reader (SH-1000Lab, Corona Electric, Ibaraki, Japan). (+)-Catechin was used as the standard for generating the calibration curve. The total flavonoid content was expressed in terms of milligram (+)-catechin equivalent (CE) per gram of sample (dry weight).
Determination of tannin content
The tannin content was determined as described previously [Citation26]. Briefly, the mixture containing the extract (12.5 μL) and 66.7 mM potassium-sodium phosphate buffer (pH 7.5) (187.5 μL) was incubated with ferrous-tartrate solution (50 μL), containing 0.1% ferrous sulfate heptahydrate and 0.5% sodium potassium tartrate. The absorbance of the assay mixture was measured at 540 nm using a microplate reader (SH-1000Lab, Corona Electric, Ibaraki, Japan). Ethyl gallate was used as the standard for generating the calibration curve. The tannin content was expressed in terms of milligram ethyl gallate equivalent (EGE) per gram of sample (dry weight).
Determination of proanthocyanidin content
The proanthocyanidin content was determined as described previously [Citation27]. Briefly, the mixture containing the extract (10 µL), methanol (30 µL), 25% sulfuric acid in methanol(100 µL), and 1% vanillin in methanol (100 µL) was incubated at 30°C for 15 min. The absorbance of the assay mixture was measured at 500 nm using a microplate reader (SH-1000Lab, Corona Electric, Ibaraki, Japan). (+)-Catechin was used as the standard for generating the calibration curve. The proanthocyanidin content was expressed in terms of milligram CE per gram of sample (dry weight).
ABTS+ radical scavenging assay
ABTS cation (ABTS+) radical scavenging activity was measured as described previously [Citation20]. Briefly, the ABTS+ mixture solution was prepared by mixing an equal amount of 7.4 mM ABTS and 2.6 mM potassium peroxodisulfate solution for 15 h. The mixture was incubated on a rotator in the dark at room temperature (20–25°C). The ABTS-working solution was prepared by diluting the ABTS+ mixture solution (150 μL) in methanol (2.9 mL) before use. The reaction was initiated by adding the ABTS+ working solution (190 μL) to the extract (10 μL). This mixture was incubated at room temperature (20–25°C) for 2 h in the dark. The absorbance of the solution was measured at 734 nm. Trolox was used as the standard. The radical scavenging activity was expressed in terms of micromole TE per gram of sample (dry weight).
DPPH radical scavenging assay
DPPH radical scavenging activity was measured as described previously [Citation19]. The reaction was initiated by adding 0.5 mM DPPH dissolved in ethanol (50 μL) into the assay mixture (200 μL) containing the extract (10 μL), 70% ethanol (90 μL), and 0.1 M sodium acetate buffer (pH 5.5, 100 μL). The mixture was incubated for 30 min at room temperature (20–25°C). The absorbance of the solution was measured at 517 nm. Trolox was used as the standard. The radical scavenging activity was expressed in terms of micromole TE per gram of sample (dry weight).
O2− radical scavenging assay
Superoxide anion (O2−) radical scavenging activity was measured on a PMS-NADH-NBT system [Citation28,Citation29]. The reaction was initiated by adding 2 mM NADH (20 μL) into the assay mixture (180 µL) containing the extract (10 μL), 1 mM NBT (20 μL), 0.1 mM PMS (20 μL), 250 mM potassium phosphate buffer (pH 7.4, 40 μL), and MilliQ water (90 μL). The mixture was incubated for 10 min at room temperature (20–25°C). The absorbance of the solution was measured at 570 nm. The radical scavenging activity was expressed in terms of micromole TE per gram of sample (dry weight).
Cellular O2− radical generation assay
HL-60 human promyelocytic leukemia cells were cultured in the RPMI-1640 medium supplemented with 5% FBS, 100 U/mL penicillin G, and 100 μg/mL streptomycin sulfate at 37°C and 5% CO2. For the cellular O2− radical generation assay, DMSO-differentiated HL-60 human granulocyte-like neutrophil cells were prepared by incubating the cells with 1.25% DMSO for 6 days as described previously [Citation30,Citation31]. The cell suspension at 1 × 106 cells/mL in HBSS (250 μL) was preincubated with the extract (1.25 μL) in a 1.5 mL test tube at 37°C for 15 min. A mixture (13.75 μL) containing 20 μM PMA (1.25 μL) in DMSO to induce cellular O2− radical production and 20 mg/mL cytochrome c solution (12.5 μL) in phosphate-buffered saline (PBS, pH 7.4) was added to the cell suspension and incubated at 37°C for 15 min. The cells were immediately placed in ice-cold water for 5 min to terminate O2− radical production. The activated cells were centrifuged and the supernatant was collected. The change in absorbance of cytochrome c in the supernatant was determined at 550 nm to indirectly measure the level of O2− radical.
HPLC analysis of chlorogenic acid and caffeic acid
HPLC analysis of the yacon leaf extract was performed based on earlier studies [Citation21,Citation32]. Briefly, HPLC-UV analysis was performed using an LC-2000 PLUS Series HPLC with a gradient pump, column oven, manual injector, UV-VIS detector (JASCO Co., Tokyo, Japan) and a 3-µm YMC-Pack ODS-A column (4.6 mm × 150 mm) (YMC Co., Ltd., Kyoto, Japan). The flow rate was set to 1.0 mL/min and the column was maintained at 30°C. The detection wavelength was set to 350 nm. A gradient elution was performed with two mobile phases: solution A (0.25% aqueous acetic acid, v/v) and B (methanol). Initially, the analysis was started with 85% A and 15% B, then gradually changed to 65% A and 35% B for 15 min and maintained for 5 min. Thereafter, the 35% B was allowed to reach 100% B at 50 min and maintained for 10 min. The gradient system was recovered to initial conditions at 60.01 min for 10 min. Chromatogram data were recorded and analyzed using Chromato-REC recorder and Chromato-Pro software (Run Time Co., Tokyo, Japan), respectively. The yacon leaf extracts (40 µg) and different concentrations of standard compounds (chlorogenic acid and caffeic acid) were reconstituted in an initial mobile phase solution (85% A and 15% B) for analysis. The peaks were identified in the test samples by co-chromatography with their respective standards. The concentration of chlorogenic acid and caffeic acid used for generating a calibration curve ranged from 1.56 to 12.5 µg/mL and from 1.56 to 25.0 µg/mL, respectively.
Statistical analysis
The data were expressed as the mean ± standard deviation of four independent experiments. The data were analyzed using a statistical add-on software program (Statcel 4, OMS Co., Saitama, Japan). In the Dunnett’s test, the difference was considered statistically significant when the P value was less 0.01. One-way analysis of variance (ANOVA), followed by the post-hoc Tukey-Kramer test was used for multiple comparisons and the difference was considered statistically significant when the P value was less than 0.05.
Results and discussion
We previously studied the multifunctional role of yacon (Smallanthus sonchifolius), especially the leaves, to evaluate its potential health benefits. The aim of this study was to comparatively evaluate the phenolic content and the antioxidant capacity of the heated and non-heated leaves of the yacon cultivar “Andes no yuki” grown in Japan.
Phenolic contents in yacon non-heated and heated leaves
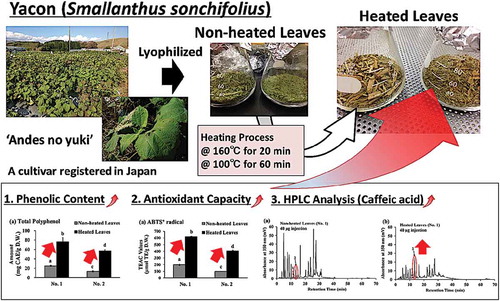
We determined the phenolic contents of heated and non-heated yacon leaves using extracts of two different batches of leaves that were collected in different seasons (see Materials, leaves collected in September (No. 1) and November (No. 2) in 2010). The contents of total polyphenol, total flavonoid, tannin, and proanthocyanidin were expressed as the chlorogenic acid, (+)-catechin, ethyl gallate, and (+)-catechin equivalent values, respectively. As shown in ), the total polyphenol content in 1 g of heated leaves was 3.06 to 4.25-times higher than that in 1 g of non-heated leaves (76.1 mg vs. 24.9 mg in batch No. 1, and 56.9 mg vs. 13.4 mg in batch No. 2, respectively). As shown in ), the total flavonoid content in 1 g of heated leaves was 4.32 to 4.51-times higher than that in 1 g of non-heated leaves (51.4 mg vs. 11.4 mg in No. 1, and 30.5 mg vs. 7.06 mg in No. 2, respectively). As shown in ), the tannin content in 1 g of heated leaves was 1.96 to 2.01-times higher than that in 1 g of non-heated leaves (27.9 mg vs. 14.2 mg in No. 1, and 13.8 mg vs. 6.86 mg in No. 2, respectively). The methodology used for measuring tannin content in this study is used to detect catechins and gallic acid, especially in green tea [Citation33]. Next, we determined the content of a condensed tannin, proanthocyanidin, in the yacon leaves. As shown in ), the proanthocyanidin content in 1 g of heated leaves was 2.57 to 9.69-times higher than that in 1 g of non-heated leaves (5.61 mg vs. 0.579 mg in No. 1, and 1.51 mg vs. 0.587 mg in No. 2, respectively). The phenolic content in 1 mg of the heated leaf extract was higher than that in 1 mg of the non-heated leaf extract (data not shown). All four phenolic contents in the heated leaves were significantly higher than those in the non-heated leaves (P < 0.05). We observed a similar trend in both the batches of yacon leaves. The extract yield of heated leaves was 1.55 to 1.64-times higher than that of non-heated leaves (25.2% vs. 16.3% in No. 1, and 23.5% vs. 14.3% in No. 2, respectively). The difference in the extract yield may be one of the reasons for explaining these differential phenolic contents between non-heated and heated yacon leaves. A previous study demonstrated that various heat treatments (frying in oil and griddled) tended to increase the concentration of phenolic compounds in vegetables [Citation15]. The cooking process may result in the thermal destruction of the cell wall and sub-cellular compartments of plant material. Upon roasting, a time-dependent increase in the contents of chlorogenic acid and several flavonoids was observed in mulberry leaves (200°C; 1, 3, 5 min) [Citation13]. However, another study demonstrated that chlorogenic acid is sensitive to thermal degradation [Citation34]. Chlorogenic acid, caffeic acid, ferulic acid, and caffeoyl derivatives, such as caffeoylaltraric acid and caffeoylquinic acid are the characteristic phenolics present in the yacon leaves [Citation4,Citation5,Citation8]. Therefore, it is important to investigate the effect of heat processing on the contents and biological functions of caffeoylquinic acid derivatives, such as chlorogenic acid and caffeic acid, in yacon leaves.
Figure 1. Total polyphenol (a), total flavonoid (b), tannin (c) and proanthocyanidin contents (d) in yacon non-heated and heated leaves. Data shown represent mean ± standard deviation (S.D.) from four independent experiments. Two batches of extracts prepared from yacon leaves collected in September 2010 (No. 1) or November 2010 (No. 2) were used in this study. Values not sharing a common superscript letter are considered significantly different (P < 0.05, one-way analysis of variance (ANOVA) followed by Tukey-Kramer test). CAE; chlorogenic acid equivalent, CE; (+)-catechin equivalent, EGE; ethyl gallate equivalent, D.W.; dry weight of sample.
Radical scavenging activity of non-heated and heated yacon leaves
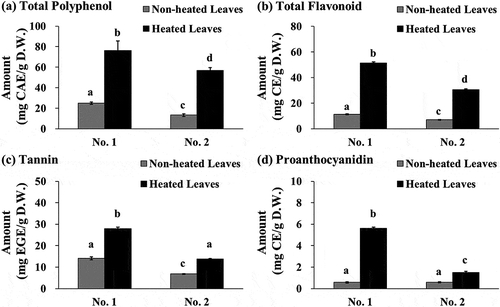
We determined the antioxidant capacity of heated and non-heated yacon leaves using three different radical scavenging assays. In the ABTS+ radical scavenging assay, the TEAC value of 1 g of heated leaves was 3.12 to 4.07-times higher than that of 1 g of non-heated leaves (620 µmol TE vs. 199 µmol TE in No. 1, and 407 µmol TE vs. 100 µmol TE in No. 2, respectively) ()). In DPPH radical scavenging assay, the TEAC value of 1 g of heated leaves was 3.02 to 3.13-times higher than that in 1 g of non-heated leaves (497 µmol TE vs. 159 µmol TE in No. 1, and 221 µmol TE vs. 73.1 µmol TE in No. 2, respectively) ()). In O2− radical scavenging assay, the TEAC value of 1 g of heated leaves was 1.98 to 2.12-times higher than that of 1 g of non-heated leaves (5,393 µmol TE vs. 2,718 µmol TE in No. 1, and 3,713 µmol TE vs. 1,749 µmol TE in No. 2, respectively) ()). Notably, the antioxidant capacity of heated leaves was significantly higher than that of the non-heated leaves in all three radical scavenging assays (P < 0.05). Additionally, the antioxidant capacity evaluated based on EC50 values (determined as µg/mL of the extract) of the heated leaves was higher than that of the non-heated leaves. The EC50 values of heated and non-heated leaves determined in ABTS+ radical scavenging assay were 12.4 µg/mL vs. 25.0 µg/mL in No. 1, and 17.6 µg/mL vs. 43.5 µg/mL in No. 2, respectively. Those in DPPH radical scavenging assay were 13.9 µg/mL vs. 28.3 µg/mL in No. 1, and 29.2 µg/mL vs. 53.8 µg/mL in No. 2, while those in O2− radical scavenging assay were 174 µg/mL vs. 223 µg/mL in No. 1, and 235 µg/mL vs. 304 µg/mL in No. 2, respectively. The high TEAC values of yacon leaves obtained in the O2− radical scavenging assay concur with the results of our previous studies [Citation9,Citation12]. Several studies have focused on the effect of heat processing on the antioxidant capacity and the polyphenol contents of various vegetables [Citation14]. Additionally, a previous study demonstrated that the roasting process enhances the antioxidant capacity of bitter melon by increasing the phenolic constituents using DPPH and ABTS+ radical scavenging assays and the ferric reducing antioxidant power (FRAP) assay [Citation35].
Figure 2. TEAC values of yacon non-heated and heated leaves in ABTS+ radical (a), DPPH radical (b), and O2− radical scavenging assays (c). Two batches of extracts prepared from yacon leaves collected in September 2010 (No. 1) or November 2010 (No. 2) were used in this study. Data shown represent mean ± standard deviation (S.D.) from four independent experiments. Values not sharing a common superscript letter are considered significantly different (P < 0.05, one-way analysis of variance (ANOVA) followed by Tukey-Kramer test). TEAC; Trolox equivalent antioxidant capacity, TE; Trolox equivalent, D.W.; dry weight of sample, O2−; superoxide anion.
Suppressive effect of yacon non-heated leaf and heated leaf extracts on cellular O2− radical generation
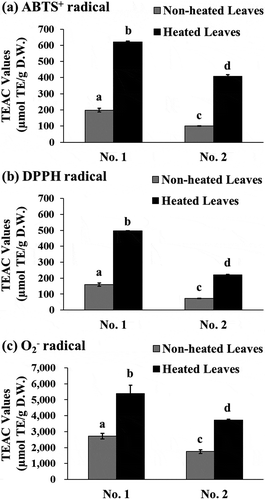
Recently, we had demonstrated the potent antioxidant capacity of several herbal teas using a human-cell-based assay [Citation9,Citation21]. In this study, we investigated the effect of heated and non-heated yacon leaf extracts on the excessive production of O2− in PMA-activated human granulocytic neutrophil cells. We observed a concentration-dependent attenuation of cellular O2− radical generation by the heated and non-heated leaf extracts in both batch No. 1 ()) and No. 2 ()) leaves. The IC50 value for the attenuation of O2− radical generation of the batch No. 1 heated leaf extract was 2.71-times higher than that of the batch No. 1 non-heated leaf extract (27.4 µg/mL and 74.3 µg/mL in batch No. 1, respectively) ()). A similar trend was observed in the leaves of batch No. 2, where the IC50 value of heated leaf extract was 69.9 µg/mL and that of non-heated leaf extract was more than 100 µg/mL ()). Interestingly, the inhibitory effect of the heated leaves on the cellular O2− radical generation was higher than that of Trolox in all cases. It is important to investigate the antioxidant capacity of agricultural products and their processed forms in some cellular systems. Several studies have demonstrated the cellular antioxidant activity of various antioxidants, fruits, and vegetables using dichloro-dihydro-fluorescein diacetate (DCFH-DA), an oxidation indicator [Citation36,Citation37]. Another study demonstrated that steam-processed broccoli exhibit high antioxidant activity in chemical and cellular assays by measuring the level of reactive oxygen species [Citation38].
Figure 3. Effect of the extracts prepared from yacon non-heated and heated leaves and Trolox on cellular O2− radical generation in PMA-stimulated human granulocytic neutrophil cells. Two batches of extracts prepared from yacon leaves collected in September 2010 (a) or November 2010 (b) were used in this study. Data shown represent mean ± standard deviation (S.D.) from four independent experiments. The statistical significance between treated and untreated cells was evaluated using Dunnett test (**P < 0.01). PMA; phorbol 12-myristate 13-acetate.
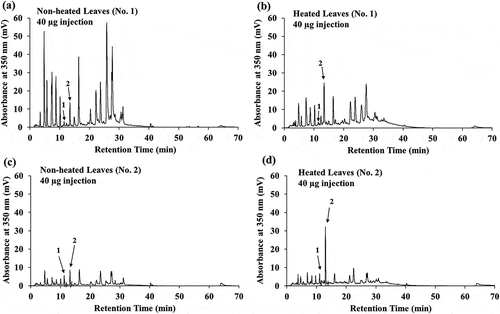
HPLC analysis of yacon non-heated and heated leaf extracts
The effect of heat processing on the antioxidant constituents in the yacon leaves is currently unknown. Hence, we determined the contents of chlorogenic acid and caffeic acid in heated and non-heated yacon leaf extracts by HPLC as described previously [Citation21,Citation32]. The HPLC chromatogram of batch No. 1 non-heated and heated leaf extracts (40 µg) is shown in ,b), respectively. The HPLC chromatogram of batch No. 2 non-heated and heated leaf extracts (40 µg) is shown in ,d), respectively. Several peaks observed in the batch No. 1 non-heated leaf extract at 5–10 min and 15–40 min (see )) were observed as smaller peaks in the batch No. 1 heated leaf extract (see )). However, the intensity of peak corresponding to caffeic acid in the batch No. 1 heated leaf extract (see )) was higher than that in the batch No. 1 non-heated leaf extract (see )). A similar trend was observed between the batch No. 2 non-heated (see )) and heated leaves (see )). We observed a small change in the intensity of the peak corresponding to chlorogenic acid between heated and non-heated leaves. However, the effect of heat treatment on the contents of other phytochemical constituents of yacon leaves is not known. Hence, a systematic analysis is required to evaluate the phytochemical changes.
Figure 4. High-performance liquid chromatography (HPLC) chromatogram of the extracts prepared from yacon non-heated and heated leaves. Extracts of non-heated leaves (a) and heated leaves (b) prepared from yacon collected in September 2010 (No. 1), and those prepared from non-heated leaves (c) and heated leaves (d) collected in November 2010 (No. 2) were analyzed using HPLC. The phytochemicals from 40 µg of the extract were detected at 350 nm. The retention time of chlorogenic acid (peak 1) and caffeic acid (peak 2), indicated by arrows, was approximately 11.4 min and 13.4 min, respectively. Individual figures represent data from three repetitions.
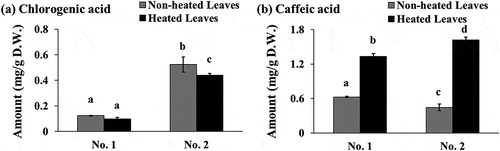
In a previous study, the contents of chlorogenic acid and caffeic acid in 1 g of leaves from four different types of yacon cultivars, historically imported from New Zealand to Czech, was reported to be in the range of 0.037–0.160 mg and 0.044–0.097 mg, respectively [Citation5]. As shown in ), the chlorogenic acid content in 1 g of heated leaves was 0.789 to 0.840-fold higher when compared to that in 1 g of non-heated leaves (0.0970 mg vs. 0.123 mg in No. 1, and 0.440 mg vs. 0.524 mg in No. 2, respectively). As shown in ), the caffeic acid content in 1 g of heated leaves was 2.13 to 3.64-times higher than that in 1 g of non-heated leaves (1.33 mg vs. 0.625 mg in No. 1, and 1.62 mg vs. 0.445 mg in No. 2, respectively). The chlorogenic acid content in the yacon leaves determined in this study was comparable to that reported in an earlier study [Citation5], while that of caffeic acid seemed to be higher than that reported in an earlier study. The caffeic acid content in the heated leaves was significantly higher than that in the non-heated leaves (P < 0.05). Contrastingly, the chlorogenic acid content in the heated leaves was slightly lower than that in the non-heated leaves. A similar trend was obtained in both the batches of yacon leaves collected in different seasons. In our previous report, we had demonstrated the antioxidant capacity of caffeic acid in several antioxidant assays [Citation21]. Therefore, caffeic acid may be one of the active constituents that contributes to the antioxidant activity, which can be potentiated by heat processing of yacon leaves. Chlorogenic acid is formed by the esterification of quinic acid and caffeic acid [Citation34]. Our results partly concur with those of a previous study, which reported that chlorogenic acid is sensitive to thermal degradation [Citation34]. Caffeoyl derivatives, such as caffeoylaltraric acid and caffeoylquinic acid are the characteristic phytochemicals present in yacon [Citation4,Citation5,Citation8]. In this study, we observed an increase in the caffeic acid content of heated leaves, which may have resulted from degradation of the caffeoyl derivatives. Nevertheless, the effect of heat processing on other active constituents of the yacon leaves must be investigated. In this study, we also demonstrated that the phenolic content, including total polyphenol, total flavonoid, tannin, and proanthocyanidin, was high in the heated yacon leaves (see –d)). We preliminary investigated the effect of heat processing on the contents of gallic acid, (+)-catechin, (-)-epicatechin, and rutin, which are reported to be present in yacon leaves [Citation6,Citation7,Citation39]. A qualitative HPLC analysis revealed that there was an increase in the intensity of gallic acid-like peak at 3.5 min and a small increase in the intensity of (-)-epicatechin-like peak at 12.5 min in the heated leaf extracts (data not shown). Additionally, the intensity of the rutin-like peak at 25 min was almost comparable in both heated and non-heated leaves, while (+)-catechin-like peak was undetectable in this experimental setting. The effect of heat processing on the contents of phytochemicals in the yacon leaves is still not completely understood. The total tannin and proanthocyanidin contents in the heated yacon leaves were higher than those in the non-heated leaves (see ,d)). The methodology used to quantify proanthocyanidin in this study is based on the detection of flavan-3-ol compounds, such as catechins [Citation40]. Therefore, catechins comprising a galloyl group may be one of the candidates that can be affected by heat processing. Additionally, other phenolic acids (p-coumaric acid), flavonoids (myricetin, quercetin, apigenin, and luteorin), and tannins (gallic acid and its derivatives, e.g. galloyl catechins) present in yacon leaves [Citation6,Citation7,Citation39] may be the candidates responsible for the variable antioxidant activity.
Figure 5. Chlorogenic acid (a) and caffeic acid (b) contents in the yacon non-heated and heated leaves. Data shown represent the mean ± standard deviation (S.D.) from three independent high-performance liquid chromatography (HPLC) runs (see ; chlorogenic acid (peak 1), caffeic acid (peak 2)). Two batches of extracts prepared from yacon leaves collected in September 2010 (No. 1) or November 2010 (No. 2) were used in this study. Values not sharing a common superscript letter are considered significantly different (P < 0.05, one-way analysis of variance (ANOVA) followed by Tukey-Kramer test). D.W.; dry weight of sample.
Total polyphenol content in non-heated yacon leaves and heated leaves collected in five different years
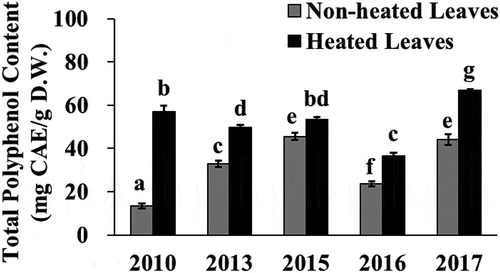
In this study, we observed that the phenolic content and the antioxidant capacity of the heated leaves of the yacon cultivar “Andes no yuki” were significantly higher than those of the non-heated leaves (P < 0.05). The phenolic content and the antioxidant capacity of the batch No. 1 non-heated leaves (collected in September 2010) were mostly higher than those of the batch No. 2 non-heated leaves (collected in November 2010) (see –). Those of the batch No. 1 heated leaves were also higher than those of the batch No. 2 heated leaves. Thus, the content of phenolic antioxidants and antioxidant capacity may be affected by seasonal differences. We also determined the total polyphenol contents in the non-heated and heated yacon leaves collected in the month of November in five different years: 2010, 2013, 2015, 2016, and 2017. As the tuberous root part is usually harvested in November in the test area (Minamiaso, Kumamoto, Japan), we investigated if the residual aerial part of this plant was usable after harvesting. As shown in , the total polyphenol content in 1 g of non-heated leaves varied from 13.4 to 45.5 mg, while that of heated leaves varied from 36.5 to 66.8 mg. The total polyphenol content in 1 g of heated leaves was 4.25-, 1.51-, 1.17-, 1.54-, and 1.52-times significantly higher than that in 1 g of non-heated leaves collected in 2010, 2013, 2015, 2016 and 2017, respectively (P < 0.05). Notably, we observed an enhanced total polyphenol content in the heated leaves collected in five different years. The lower total polyphenol content observed in the yacon leaves collected in 2010, 2013, and 2016 may have resulted from insufficient growth due to intense heat during the respective summer season (especially in August in all cases and until October in 2016). The plants harvested from these three years exhibited short height and decreased yield of tuberous roots (data not shown). These results indicate that the polyphenol content of yacon leaves may vary depending on the season and cultivation year. However, the heated leaves may be a better source of antioxidants compared to the non-heated leaves. The reasons for the drastic difference in the polyphenol contents between heated and non-heated leaves observed only in 2010 are unclear. Further studies are needed to evaluate the biological activity of heated leaves of other yacon cultivars and/or leaves harvested in different months.
Figure 6. Total polyphenol content in the yacon non-heated and heated leaves among five different cultivation years. Yacon leaves collected in November 2010, 2013, 2015, 2016, and 2017 were used in this study. Data shown represent mean ± standard deviation (S.D.) from four independent experiments. Values not sharing a common superscript letter are considered significantly different (P < 0.05, one-way analysis of variance (ANOVA) followed by Tukey-Kramer test). CAE; chlorogenic acid equivalent, D.W.; dry weight of sample.
Conclusion
Using the yacon cultivar “Andes no yuki” grown in Japan, we demonstrated that heated yacon leaves exhibit a higher phenolic content and a higher antioxidant capacity to scavenge free radicals compared to the non-heated leaves. HPLC analysis revealed that the caffeic acid content in the heated leaves was higher than that in the non-heated leaves. Additionally, the enhanced caffeic acid content was observed with a small decrease in the chlorogenic acid content of the heated leaves. The total polyphenol content in the heated yacon leaves collected in five different years was higher than that in the non-heated yacon leaves. These results suggest that heat processing may affect the contents of active constituents in yacon leaves, thereby potentiating its antioxidant capacity. Nevertheless, the effect of different heating processes on the active constituent contents and biological functions of yacon leaves must be evaluated. Further studies are also needed to evaluate the effect of heat processing of leaves from other yacon cultivars and/or leaves harvested at different months on their biological activity.
Author contributions
Y. Ueda designed the study, performed experiments, drafted and prepared the manuscript. Y. Matsuda, T. Murata, Y. Hoshi, and K. Kabata interpreted the data of phenolic compounds. M. Ono provided support for HPLC analysis. H. Kinoshita and K. Igoshi interpreted the antioxidant assay data. S. Yasuda designed the study with Y. Ueda, interpreted the results, and prepared the manuscript.
Acknowledgments
We thank Ms. T. Morita and Ms. A. Sakai-Sakaguchi for their technical assistance. We would like to thank Dr. Michael James Rupp (Tokai University Kyushu Campus Liberal Arts Center) for volunteering his linguistic support in English and editing skills, and Editage (www.editage.com) for English language editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ojansivu I, Ferreira CL, Salminen S. Yacon, a new source of prebiotic oligosaccharides with a history of safe use. Trends Food Sci Technol. 2011;22:40–46.
- Lin F, Hasegawa M, Kodama O. Purification and identification of antimicrobial sesquiterpene lactones from yacon (Smallanthus sonchifolius) leaves. Biosci Biotechnol Biochem. 2003;67:2154–2159.
- Aybar MJ, Sanchez RAN, Grau A, et al. Hypoglycemic effect of the water extract of Smallantus sonchifolius (yacon) leaves in normal and diabetic rats. J Ethnopharmacol. 2001;74:125–132.
- Valentova K, Sersen F, Ulrichova J. Radical scavenging and anti-lipoperoxidative activities of Smallanthus sonchifolius leaf extracts. J Agric Food Chem. 2005;53:5577–5582.
- Valentova K, Lebeda A, Dolezalova I, et al. The biological and chemical variability of yacon. J Agric Food Chem. 2006;54:1347–1352.
- Andrade EF, Souza-Leone R, Ellendersen LN. Phenolic profile and antioxidant activity of extracts of leaves and flowers of yacon (Smallanthus sonchifolius). Ind Crops Prod. 2014;62:499–506.
- Russo D, Malafronte N, Frescura D, et al. Antioxidant activities and quali-quantitative analysis of different Smallanthus sonchifolius [(Poepp. and Endl.) H. Robinson] landrace extracts. Nat Prod Res. 2015;29:1673–1677.
- Chagas-Paula DA, Oliveira TB, Faleiro DPV, et al. Outstanding anti-inflammatory potential of selected Asteraceae species through the potent dual inhibition of cyclooxygenase-1 and 5-lipoxygenase. Planta Med. 2015;81:1296–1307.
- Sugahara S, Ueda Y, Fukuhara K, et al. Antioxidant effects of herbal tea leaves from yacon (Smallanthus sonchifolius) on multiple free radical and reducing power assays, especially on different superoxide anion radical generation systems. J Food Sci. 2015;80:C2420–C2429.
- Ueda Y, Sugahara S, Matsuda Y, et al. Effects of hot-water extract of herbal tea leaves from yacon (Smallanthus sonchifolius): lipoxygenase inhibition and suppression on nitric oxide generation in RAW264.7 mouse macrophage-like cells. Proc Sch Agric Tokai Univ. 2017;36:37–43. (in Japanese).
- Ueda Y, Sugahara S, Matsuda Y, et al. In vitro α-glucosidase and α-amylase inhibitory effects of herbal tea leaves from yacon (Smallanthus sonchifolius). Bull Inst Adv Biosci. 2017;1:33–37. (in Japanese).
- Ueda Y, Apiphuwasukcharoen N, Tsutsumi S, et al. Optimization of hot-water extraction of dried yacon herbal tea leaves: enhanced antioxidant activities and total phenolic content by response surface methodology. Food Sci Technol Res. 2019;25:131–139.
- Lee WJ, Choi SW. Quantitative changes of polyphenolic compounds in mulberry (Morus alba L.) leaves in relation to varieties, harvest period, and heat processing. Prev Nutr Food Sci. 2012;17:280–285.
- Ramírez-Anaya Jdel P, Samaniego-Sánchez C, Castañeda-Saucedo MC, et al. Phenols and the antioxidant capacity of Mediterranean vegetables prepared with extra virgin olive oil using different domestic cooking techniques. Food Chem. 2015;188:430–438.
- Juaniz I, Ludwig IA, Huarte E, et al. Influence of heat treatment on antioxidant capacity and (poly) phenolic compounds of selected vegetables. Food Chem. 2016;197:466–473.
- Friedman M, Levin CE, Choi SH, et al. Changes in the composition of raw tea leaves from the Korean yabukida plant during high-temperature processing to pan-fried kamairi-cha green tea. J Food Sci. 2009;74:C406–C412.
- Gutowski M, Kowalczyk S. A study of free radical chemistry: their role and pathophysiological significance. Acta Biochim Pol. 2013;60:1–16.
- Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58:87–114.
- Blois MS. Antioxidant determinations by the use of the stable free radical. Nature. 1958;26:1199–1200.
- Thaipong K, Boonprakob U, Crosby K, et al. Comparison of ABTS, DPPH, FRAP and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal. 2006;19:669–675.
- Sugahara S, Chiyo A, Fukuoka K, et al. Unique antioxidant effects of herbal leaf tea and stem tea from Moringa oleifera L. especially on superoxide anion radical generation systems. Biosci Biotechnol Biochem. 2018;82:1973–1984.
- Fujino M, Nakanishi T, Ishihara J, et al. A new yacon cultivar, “Andes no yuki” and “Salad Okame”. Bull NARO West Reg Agric Res Cent. 2008;7:131–143.
- Yoshida T. Illustration food processing. Yoshida T, ed. Tokyo, Japan: Kogyo Chosakai; 2003. p. 217–222.
- Singleton VL, Rossi JA Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158.
- Chang CH, Lin HY, Chang CY, et al. Comparisons on the antioxidant properties of fresh, freeze-dried and hot-air-dried tomatoes. J Food Eng. 2006;77:478–485.
- Sriwilaijaroen N, Fukumoto S, Kumagai K, et al. Antiviral effects of Psidium guajava Linn. (guava) tea on the growth of clinical isolated H1N1 viruses: its role in viral hemagglutination and neuraminidase inhibition. Antiviral Res. 2012;94:139–146.
- Suda I, Oki T, Nishiba Y, et al. Polyphenol contents and radical-scavenging activity of extracts from fruits and vegetables in cultivated in Okinawa, Japan. J Jpn Soc Food Sci Technol. 2005;52:462–471 (In Japanese).
- Gulcin I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology. 2006;217:213–220.
- Wang BS, Yu HM, Chang LW, et al. Protective effects of pu-erh tea on LDL oxidation and nitric oxide generation in macrophage cells. LWT-Food Sci Technol. 2008;41:1122–1132.
- Nakamura Y, Murakami A, Ohto Y, et al. Suppression of tumor promoter-induced oxidative stress and inflammatory responses in mouse skin by a superoxide generation inhibitor 1ʹ-acetoxychavicol acetate. Cancer Res. 1998;58:4832–4839.
- Kim HW, Murakami A, Nakamura Y, et al. Screening of edible Japanese plants for suppressive effects on phorbol ester-induced superoxide generation in differentiated HL-60 cells and AS52 cells. Cancer Lett. 2002;176:7–16.
- Liu L, Jiang W, Zhang L, et al. Chemical correlation between Shuanghuanglian injection and its three raw herbs by LC fingerprint. J Sep Sci. 2011;34:1834–1844.
- Iwasa K, Ôta I, Torii H. Improvement of official chemical analysis of tea (Part 3): examination of tannin determination. Tea Res J. 1970;40:69–73. (In Japanese).
- de Maria CAB, Trugo LC, de Mariz E Miranda LS, et al. Stability of 5-caffeoylquinic acid under different conditions of heating. Food Res Int. 1998;31:475–477.
- Choi JS, Kim HY, Seo WT, et al. Roasting enhances antioxidant effect of bitter melon (Momordica charantia L.) increasing in flavan-3-ol and phenolic acid contents. Food Sci Biotechnol. 2012;21:19–26.
- Wolfe KL, Liu RH. Cellular antioxidant activity (CAA) assay for assessing antioxidant, foods, and dietary supplements. J Agric Food Chem. 2007;55:8896–8907.
- Girard-Lalancette K, Pichette A, Legault J. Sensitive cell-based assay using DCFH oxidation for the determination of pro- and antioxidant properties of compounds and mixtures: analysis of fruit and vegetable juices. Food Chem. 2009;115:720–726.
- Roy MK, Juneja LR, Isobe S, et al. Steam processed broccoli (Brassica oleracea) has higher antioxidant activity in chemical and cellular assay systems. Food Chem. 2009;114:263–269.
- Marchyshyn S, Hudz N, Dakhym I, et al. Analysis of phenolic compounds from Polymnia sonchifolia Poepp. & Endl. leaves by HPLC-method. Pharma Innovation. 2017;6:980–983.
- Oki T, Sugawara T, Sato-Furukawa M, et al. 4-Dimethylaminocinnamaldehyde (DMAC) method for determination of total proanthocyanidin content in grain legumes. J Jpn Soc Food Sci Technol. 2013;60:301–309. (In Japanese).
