ABSTRACT
Myostatin (Mstn) is an important growth/differentiation factor, and knockdown of Mstn reduces fat content. Here, we knocked down Mstn expression in C2C12 myoblasts and then induced adipogenic trans-differentiation in the cells. The effects of Mstn knockdown on lipid droplet contents and H3K27me3 marker expression on adipocyte-specific genes were detected. The results showed that Mstn knockdown reduced the formation of lipid droplets, downregulated the expression of adipocyte-specific genes, and increased H3K27me3 marker expression on adipocyte-specific genes. Chromatin immunoprecipitation analysis showed that the SMAD2/SMAD3 complex could combine with the Jumonji D3 (Jmjd3) promoter and that Mstn regulated Jmjd3 expression through this process. Jmjd3 overexpression removed the H3K27me3 marker and increased the expression of adipocyte-specific genes. Overall, our results showed that Mstn regulated Jmjd3 expression through SMAD2/SMAD3, thus affecting the H3K27me3 marker on adipocyte-specific genes and the trans-differentiation from myocytes to adipocytes.
Graphic abstract-1
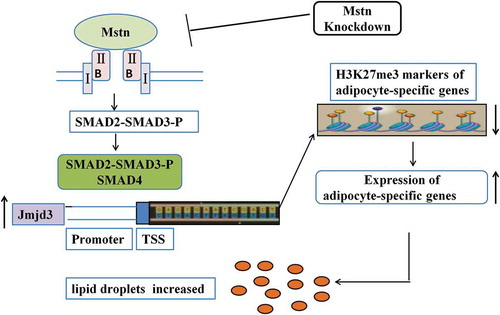
Mstn knockdown decreases the trans-differentiation from myocytes to adipocytes by reducing Jmjd3 expression via the SMAD2/SMAD3 complex.
Intramuscular fat (IMF) is an important factor that affects meat quality and is closely correlated with traits such as flavor, juiciness, and tenderness [Citation1]. To improve the quality of meat, it is necessary to increase the content of IMF. Myostatin (Mstn), also known as growth/differentiation factor-8, is a member of the transforming growth factor β (TGF-β) family and is primarily expressed in skeletal muscle. In previous reports, knockdown or knockout of Mstn significantly increased skeletal muscle development and decreased intramuscular fat contents compared with the results in wild-type animals [Citation2–Citation6]. Zhang et al. [Citation7] found that the absence of Mstn in fat tissues resulted in enhanced peripheral tissue fatty acid oxidation, increased mitochondrial activity, and reduced adipose tissue mass. Similarly, visceral fat was decreased in adult mice upon knockdown of Mstn by siRNA [Citation8]. Studies have demonstrated that Smad2 and Smad3 are downstream of Mstn type Ⅱ receptors; when Mstn combined with its receptors, Smad2 and Smad3 are phosphorylated [Citation9,Citation10] and activated. Accordingly, Smad2 and Smad3 play critical roles in Mstn signaling [Citation11].
Histone H3 lysine 27 trimethylation (H3K27me3) is a prominent histone modification marker. Levels of H3K27me3 are catalyzed by specific histone methyltransferases and demethylases [Citation12,Citation13]. H3K27me3 functions to silence promoters [Citation14]. Many studies have demonstrated that histone methylation and acetylation play important roles in myogenic and adipogenic differentiation. Pan et al. demonstrated that Jumonji D3 (Jmjd3)-mediated H3K27me3 dynamics play important roles in the regulation of fat development [Citation15]. Li et al. demonstrated that the transcription of brown adipocyte-specific genes is regulated by the acetylation and methylation status of H3K27 on the promoter and enhancer regions of Ucp1 and peroxisome proliferator-activated receptor (Ppar) coactivator 1 [Citation16,Citation17].
Although both Mstn and H3K27me3 are related to fat formation, the relationships between H3K27me3 and Mstn in adipogenic processes remain unclear. Accordingly, in this study, we evaluated the roles of Mstn in the differentiation of C2C12 myoblasts via H3K27me3 marker expression on adipocyte-specific genes. Our findings provide important insights into the roles of Mstn in epigenetic modification.
Materials and methods
Cell culture
C2C12 myoblasts were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20% fetal bovine serum (FBS) and 10% horse serum (HS) at 37°C with 5% CO2. Cells were passaged after reaching 100% confluence.
Plasmid construction and transfection with Mstn short hairpin RNA (shRNA) and the Jmjd3 overexpression vector
We used two shRNAs for Mstn knockdown and a control shRNA. The sequences are shown in Supplemental Table S1, and the vector is shown in Fig S1(a). These fragments were synthesized by TaKaRa and cloned into a pSilencer 2.1-U6 hygro vector. Finally, we selected the shRNA having the highest interference efficiency in C2C12 myoblasts for further analyses. The Jmjd3 overexpression vector pCS2-Jmjd3 (#17,440) was purchased from Addgene, and the vector is shown in Fig S1(b). The shRNA and the Jmjd3 overexpression vector were transfected using Lipofectamine LTX Reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. Transfection was conducted on day 2 after passaging C2C12 cells. RNA was extracted 48 h after transfection.
Adipogenic trans-differentiation and Oil Red O staining
Transfected C2C12 myoblasts were seeded in six-well plates at a cell density of 2 × 104 cells/cm2, and 2 mL of complete medium was added to each well. Cells were transferred to adipogenic differentiation medium (MUBMX-90031, Cyagen, Guangzhou, China) until cell fusion reached 100% or until overfusion was observed, with medium changes every 3 days for 12–20 days.
After adipogenic trans-differentiation was completed, the culture medium was discarded and washed with phosphate-buffered saline (PBS) 1–2 times. An additional 2 mL of 4% neutral formaldehyde was added to each well, and wells were fixed for 30 min. The wells were then washed twice with PBS. An additional 1 mL of a working solution of Oil Red O dye was added to each well for 30 min. The Oil Red O dye solution was aspirated, and the wells were rinsed 2–3 times with PBS. The culture plate was placed under a microscope to observe the effects of lipid staining. Oil Red O was then eluted with isopropanol, and the triglyceride content was determined using a microplate reader at 520 nm absorbance.
Quantitative reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from each sample using TRIzol reagent (Invitrogen), and reverse transcription was performed to generate cDNA using a PrimeScript RT reagent kit (TaKaRa, Shiga, Japan). The samples were then analyzed with SYBR Premix Ex TaqTM II (TaKaRa). For each sample, the target and reference genes were independently amplified in triplicate on the same plate and in the same experimental run. The PCR steps were as follows: 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 34 s. After amplification, melting curve analysis was performed to verify the specificity of the reactions and to analyze gene expression variation. Quantitative RT-PCR was conducted three times and normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh) gene expression. The real-time PCR primers are shown in Table S2.
Western blotting
Cell samples were rinsed with PBS and lysed in 150 μL ice-cold radioimmunoprecipitation assay buffer. The tissue lysates were then centrifuged at 4°C for 30 min at 8000 × g. Proteins in the supernatant were separated by electrophoresis on 10% sodium dodecyl sulfate-polyacrylamide gels and transferred onto polyvinylidene difluoride membranes by performing electroblotting. The membranes were blocked in 5% nonfat milk in Tris-buffered saline with 0.1% Tween-20 (TBST) blocking solution at room temperature for 1 h and subsequently incubated with anti-MSTN (Santa Cruz Biotechnology, Santa Cruz, CA, USA; cat. no. sc-134,345) and anti-JMJD3 (Millipore, USA; cat. no. 09–812) monoclonal antibodies diluted 1:1000 in TBST. The membranes were then incubated for 1 h with horseradish peroxidase-conjugated goat anti-mouse secondary antibodies at 1:10,000 in TBST, followed by detection using the chemiluminescence labeling detection reagent ECL Plus (Thermo Scientific).
Chromatin immunoprecipitation (ChiP) assay
ChIP assays were performed using a kit (Thermo Scientific; cat. no. 26157), according to the manufacturer’s instructions. Briefly, approximately 1 × 107 cells were fixed with 1% formaldehyde and quenched by glycine. The cells were washed three times with PBS and then harvested in ice-cold PBS containing a 1% Halt Cocktail. DNA was lysed and digested with MNase. The cells were sonicated on ice with pulses to break the nuclear membrane. The resulting lysate was then incubated with anti-SMAD2/SMAD3 antibodies (Abcam, USA; cat. no. ab202445), anti-H3K27me3 antibodies (Abclonal; cat. no. A2363), and protein G beads at 4°C overnight. Incubation with rabbit IgG was used as the negative control. The beads were washed four times, and DNA was eluted using ChIP elution buffer. The elution was incubated at 65°C for 1.5 h. DNA was purified using a DNA purification kit. The purified DNA was assayed by quantitative PCR (qPCR). Assays were repeated at least three times. Data are shown as means ± standard deviations of representative experiments. The sequences of primers are listed in Table S3.
Statistical analysis
The data were statistically compared between Mstn-knockdown and wild-type C2C12 myoblasts using t-tests and expressed as means ± standard errors of the means. Differences with P values of less than 0.05 were considered statistically significant.
Results
Mstn knockdown reduced the formation of lipid droplets and affected the expression of adipocyte-specific genes
Of the two shRNAs for Mstn, we chose to use shRNA2, which exhibited the higher interference efficiency (Fig. S2(a)), and we excluded off-target effects by detection of the expression of adipocyte-specific genes after Mstn-knockdown by shRNA1 (Fig. S2(b)). RNA and proteins from transfected and control cells were extracted, and MSTN expression was evaluated using qRT-PCR and western blotting. Transfection with Mstn shRNA reduced the mRNA and protein expression levels of MSTN, whereas the shRNA control had no effect on the expression of MSTN in C2C12 myoblasts ().
Figure 1. Mstn knockdown reduced the formation of lipid droplets and affected the expression of adipocyte-specific genes. (a, b) MSTN mRNA and protein levels after Mstn knockdown in C2C12 myoblasts. (c) Oil Red O staining of lipid droplets after induction of adipogenic differentiation in C2C12 myoblasts. Left panel: lipid droplets formed from Mstn RNAi C2C12 myoblasts; right panel: control. Lipid droplets were marked with white arrows. (d) Absorbance of lipid droplets at 520 nm. (e) Effects of Mstn knockdown on the expression of the anti-adipogenic factor Gata2 and adipocyte-specific genes. *p < 0.05, **p < 0.01, ***p < 0.001; t-tests were used to generate p values. shRNA C: control shRNA.
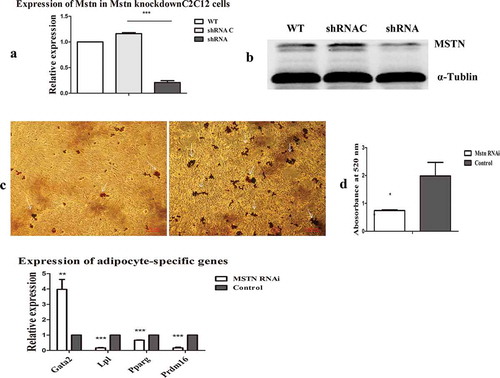
As shown in ), after transfecting the control shRNA and Mstn shRNA into C2C12 myoblasts, the culture was maintained until the cells were overfused to induce adipogenic trans-differentiation. After 12 days, the cells were stained with Oil Red O. The results showed that Mstn knockdown significantly reduced lipid droplet formation. After extracting Oil Red O using propanol, the absorbance was measured at 520 nm using a microplate reader. Consistent with the above findings, we found decrease in lipid droplet formation after Mstn knockdown ()).
Next, control shRNA and Mstn shRNA were transfected into C2C12 myoblasts for 48 h, and RT-PCR was performed to detect the expression levels of the adipogenic factors GATA binding protein 2 (Gata2), lipoprotein lipase (Lpl), Ppar gamma (Pparg), and PR/SET domain 16 (Prdm16). As shown in ), the expression of the anti-adipogenic factor Gata2 was upregulated after Mstn knockdown, and the expression levels of the adipocyte-specific genes Lpl, Pparg, and Prdm16 were reduced. These findings indicated that Mstn knockdown reduced the trans-differentiation from myocytes to adipocytes.
Mstn knockdown increased the H3K27me3 modification on adipocyte-specific genes by reducing the expression of the histone demethylase Jmjd3
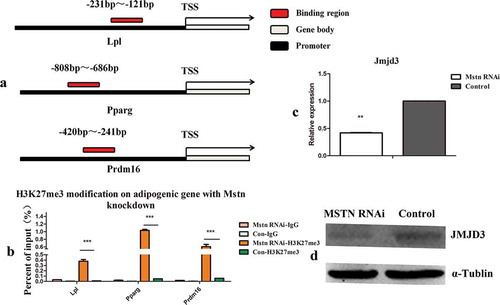
Histone modification is an important factor that regulates gene expression. To detect the effects of Mstn knockdown on histone modification in the promoter of adipocyte genes, control shRNA and Mstn shRNA were transfected into C2C12 myoblasts for 48 h, and histone modifications in the promoter of adipocyte-specific genes were detected by ChIP-qPCR. The combination regions detected are shown in ). The histone modification H3K27me3 was detected. Moreover, as shown in ), H3K27me3 modification on adipocyte-specific genes significantly increased when Mstn expression was knocked down. qPCR and western blotting showed that JMJD3 mRNA and protein levels were significantly decreased (). Thus, these results showed that Mstn increased the H3K27 modification on adipocyte-specific genes, mainly by decreasing Jmjd3 expression.
Figure 2. Mstn knockdown increased the H3K27me3 modification on adipocyte-specific genes by reducing the expression of the histone demethylase Jmjd3. (a) Schematic diagram of the detected binding region of H3K27me3 modification. The detected binding region was located on the promoter of adipocyte-specific genes. (b) The H3K27me3 modification on adipocyte-specific genes was detected by ChIP-qPCR. (c, d) JMJD3 mRNA and protein levels after Mstn knockdown. *p < 0.05, **p < 0.01, ***p < 0.001; t-tests were used to generate p values. Con: control.
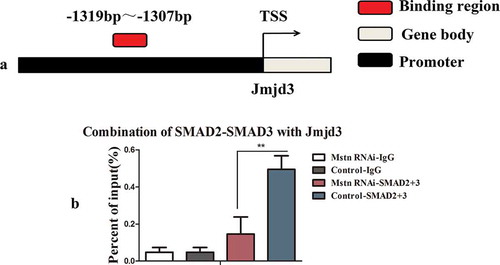
Mstn regulated Jmjd3 expression via SMAD2/SMAD3
Because Jmjd3 expression was decreased following downregulation of Mstn, and Smad2 and Smad3 are downstream of Mstn type Ⅱ receptors, we predicted the binding of SMAD2/SMAD3 transcription factors to the Jmjd3 (NC_000077.6) promoter region using the JASPAR database (http://jaspar.binf.ku.dk/cgi-bin/jaspar_db.pl). The results of the predication are shown in Fig. S3(a,b) and suggested that SMAD2/SMAD3 could combine with the promoter of Jmjd3. The combination region was from −1319 to −1307 on the promoter of Jmjd3 ()). Accordingly, we then performed ChIP assays with an anti-SMAD2/SMAD3 monoclonal antibody and amplified Jmjd3 in the binding product, as shown in Fig. S3(c). The region we detected by ChIP-qPCR was from −1374 to −1236 on the promoter of Jmjd3. The ChIP-qPCR results showed that the SMAD2/SMAD3 complex combined with the promoter of Jmjd3 ()). These results indicated that Mstn could regulate the expression of Jmjd3 via SMAD2/SMAD3.
Overexpression of Jmjd3 erased the H3K27me3 markers and promoted the expression of adipocyte-specific genes
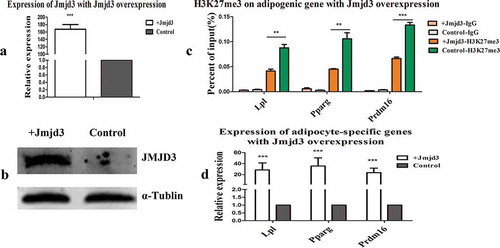
We transfected C2C12 myoblasts with the Jmjd3 overexpression vector (Fig. S1(b)), which increased the mRNA and protein expression levels of JMJD3, as shown in . We then detected the expression of the H3K27me3 marker and adipocyte-specific genes. The results showed that overexpression of Jmjd3 eliminated the H3K27me3 marker ()) and promoted the expression of adipocyte-specific genes ()).
Figure 4. Overexpression of Jmjd3 erased the H3K27me3 markers and promoted the expression of adipocyte-specific genes. (a, b) JMJD3 mRNA and protein levels after Jmjd3 overexpression in C2C12 myoblasts. (c) H3K27me3 modification on adipocyte-specific genes after overexpression of Jmjd3. (d) Expression of adipocyte-specific genes after overexpression of Jmjd3. *p < 0.05, **p < 0.01, ***p < 0.001; t-tests were used to generate p values. + Jmjd3: overexpression of Jmjd3.
Discussion
Carcass fat can be subdivided into at least three separate compartments, including IMF. IMF is an important factor that affects meat quality and is closely correlated with traits such as flavor, juiciness, and tenderness [Citation18]. In Mstn-null animals, skeletal muscles were significantly increased, and the fat content decreased compared with that in wild-type animals [Citation19,Citation20]. Similar phenomena were observed in natural Mstn mutated cattle, sheep, dogs, humans, and adult rats in which MSTN protein expression was blocked [Citation1,Citation21–Citation24]. Therefore, it is important to explore how Mstn regulates IMF formation to produce high-quality meat.
In the current study, Mstn was knocked down in C2C12 myoblasts using RNA interference (RNAi), and Mstn knockdown was found to reduce the formation of lipid droplets when adipogenic trans-differentiation was induced, consistent with previous studies indicating that Mstn knockdown inhibited trans-differentiation from myocytes to adipocytes [Citation4,Citation5]. Further detection of the expression of the adipogenic-specific genes revealed that the expression of the anti-adipogenic gene Gata2 was upregulated and that the expression of the adipocyte-specific genes Lpl, Pparg, and Prdm16 were decreased during Mstn knockdown. Thus, these findings suggested that Mstn knockdown inhibited the activation of adipogenic genes and thus blocked trans-differentiation from myocytes to adipocytes, consistent with previous studies [Citation4,Citation5].
Many studies have demonstrated that histone methylation and acetylation play important roles in the processes of myogenic and adipogenic differentiation. Naren et al. found that overexpression of Dnmt3a increased cell proliferation via methylation of the p21 promoter and suppressed the adipogenesis of porcine intramuscular pre-adipocytes through methylation of the Pparg promoter [Citation25]. A recent study demonstrated that the levels of both H3K9 and H3K27 acetylation are markedly reduced at the Gata2 locus during differentiation of 3T3-L1 cells [Citation26]. Pan et al. demonstrated that Jmjd3-mediated H3K27me3 dynamics played important roles in the regulation of fat development. In this study, we detected H3k27me3 markers on the promoters of adipocyte-specific genes and demonstrated that Mstn knockdown reduced the H3K27me3 markers on adipocyte-specific genes by downregulating the histone demethylase Jmjd3 [Citation15].
Jmjd3 (also known as Kdm6b) is a JmjC domain-containing histone lysine demethylase that, together with Utx, belongs to the KDM6 family [Citation27,Citation28]. Jmjd3 mediates the epigenetic activation of genes by catalyzing the demethylation of the gene repression histone mark H3K27me3. Jmjd3 has known epigenetic functions in development, differentiation, immunity, and tumorigenesis [Citation29,Citation30] and was recently shown to play roles in the epigenetic activation of brown fat development and white fat plasticity [Citation15]. In this study, Mstn knockdown promoted H3K27me3 marker expression on adipocyte-specific genes and reduced the expression of these genes. We found that the expression of one of the demethylases, Jmjd3, significantly decreased. This indicated that Mstn knockdown reduced the expression of Jmjd3 to inhibit the expression of adipocyte-specific genes, leading to reduced formation of lipid droplets.
Because SMAD2 and SMAD3 are transcription factors that act downstream of the MSTN/TGF-β pathway [Citation11], we predicted the binding of SMAD2/SMAD3 to the Jmjd3 promoter region using the JASPAR database and performed ChIP assays with an anti-SMAD2/SMAD3 monoclonal antibody. By ChIP-qPCR, we found that SMAD2/SMAD3 could combine with the promoter of Jmjd3. Moreover, more Jmjd3 was amplified with the same amount of DNA, potentially because phosphorylated/activated SMAD2/SMAD3 was decreased when Mstn was knocked down [Citation9,Citation10], leading to decreased combination with Jmjd3.
Overall, we demonstrated that Mstn regulated the expression of Jmjd3 via SMAD2/SMAD3 and further increased the H3K27me3 marker on adipocyte-specific genes to decrease the expression of these genes. Moreover, overexpression of Jmjd3 in C2C12 myoblasts erased the H3K27me3 markers on adipocyte-specific genes and promoted the expression of these genes.
Conclusion
In conclusion, we found that Mstn knockdown inhibited the formation of lipid droplets in myoblasts, upregulated the expression of an anti-adipogenic gene, and downregulated the expression of adipocyte-specific genes. We also demonstrated that SMAD2/SMAD3 combined with the promoter of Jmjd3 and that Mstn regulated the expression of the histone demethylase Jmjd3 by SMAD2/SMAD3, thus affecting H3K27me3 markers on the adipogenic-specific gene promoter region. These changes decreased the expression of these genes, further blocking trans-differentiation from myocytes to adipocytes.
Author Contribution
LG and GL conceived and designed the experiments; LG and CB performed the experiments; MY and XW analyzed the data; LY provide technical support. LG and GL drafted the manuscript. All authors read and approved the final manuscript.
Supplemental_Material.docx
Download MS Word (1.1 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Fernandez X, Monin G, Talmant A, et al. Influence of intramuscular fat content on the quality of pig meat. Meat Sci. 1999;53(1):67–72.
- McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA. 1997;94:12457–12461.
- Singh R, Braga M, Pervin S. Regulation of brown adipocyte metabolism by myostatin/follistatin signaling. Front Cell Dev Biol. 2014;2:60.
- Li N, Yang Q, Walker RG, et al. Myostatin attenuation in vivo reduces adiposity, but activates adipogenesis. Endocrinology. 2016;157:282–291.
- Lin J, Arnold HB, Della-Fera MA, et al. Myostatin knockout in mice increases myogenesis and decreases adipogenesis. Biochem Biophys Res Commun. 2002;291:701–706.
- Zhao B, Wall RJ, Yang J. Transgenic expression of myostatin propeptide prevents diet-induced obesity and insulin resistance. Biochem Biophys Res Commun. 2005;337(1):248–255.
- Zhang C, McFarlane C, Lokireddy S, et al. Inhibition of myostatin protects against diet-induced obesity by enhancing fatty acid oxidation and promoting a brown adipose phenotype in mice. Diabetologia. 2012;55:183–193.
- Mosler S, Relizani K, Mouisel E, et al. Combinatory effects of siRNA-induced myostatin inhibition and exercise on skeletal muscle homeostasis and body composition. Physiol Rep. 2014;2(3):e00262.
- Langley B, Thomas M, Bishop A, et al. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem. 2002;277(51):49831–49840.
- Rebbapragada A, Benchabane H, Wrana JL, et al. Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol Cell Biol. 2003;23(20):7230–7242.
- Roberta S, Giulia M, Maria P, et al. Smad2 and 3 transcription factors control muscle mass in adulthood. Am J Physiol Cell Physiol. 2009;296:C1248–C1257.
- Black JC, Van Rechem C, Whetstine JR. Histone lysine methylation dynamics: establishment,regulation, and biological impact. Mol Cell. 2012;48(4):491–507.
- Mosammaparast N, Shi Y. Reversal of histone methylation: biochemical and molecular mechanisms of histone demethylases. Annu Rev Biochem. 2010;79:155–179.
- Schuettengruber B, Chourrout D, Vervoort M, et al. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128(4):735–745.
- Pan D, Huang L, Zhu LJ, et al. Jmjd3-mediated H3K27me3 dynamics orchestrate brown fat development and regulate white fat plasticity. Dev Cell. 2015;35(5):568–583.
- Li FF, Wu R, Cui X, et al. Histone Deacetylase 1 (HDAC1) negatively regulates thermogenic program in brown adipocytes via coordinated regulation of histone H3 Lysine 27 (H3K27) deacetylation and methylation. J Biol Chem. 2016;291(9):4523–4536.
- Zha L, Li FF, Wu R, et al. The histone demethylase UTX promotes brown adipocyte thermogenic program via coordinated regulation of H3K27 demethylation and acetylation. J Biol Chem. 2015;290(41):25151–25163.
- Grunert KG, Bredahl L, Brunsø K. Consumer perception of meat quality and implications for product development in the meat sector. Meat Sci. 2004;66(2):259–272.
- McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest. 2002;109(5):595–601.
- Hamrick MW, Pennington C, Webb CN, et al. Resistance to body fat gain in ‘double-muscled’ mice fed a high-fat diet. Int J Obes. 2006;30(5):868–870.
- Clop A, Marcq F, Takeda H, et al. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat Genet. 2006;38(7):813–818.
- Mosher DS, Quignon P, Bustamante CD, et al. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet. 2007;3(5):e79.
- Grobet L, Martin LJ, Poncelet D, et al. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet. 1997;17(1):71–74.
- Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA. 2001;98(16):9306–9311.
- Qimuge N, He Z, Qin J, et al. Overexpression of DNMT3A promotes proliferation and inhibits differentiation of porcine intramuscular preadipocytes by methylating p21and PPARg promoters. Gene. 2019;696:54–62.
- Ishijima Y, Ohmori S, Uneme A, et al. The Gata2 repression during 3T3-L1 preadipocyte differentiation is dependent on a rapid decrease in histone acetylation in response to glucocorticoid receptor activation. Mol Cel End. 2019;483:39–49.
- Shi Y. Histone lysine demethylases: emerging roles in development, physiology and disease. Nat Rev Genet. 2007;8(11):829–833.
- Hong S, Cho YW, Yu LR, et al. Identification of JmjC domain-containing UTX and JMJD3 as histone H3 lysine 27 demethylases. Proc Natl Acad Sci USA. 2007;104(47):18439–18444.
- Chen S, Ma J, Wu F, et al. The histone H3 Lys 27 demethylase JMJD3 regulates gene expression by impacting transcriptional elongation. Genes Dev. 2012;26(12):1364–1375.
- Yang L, Song LS, Liu XF, et al. The maternal effect genes UTX and JMJD3 play contrasting roles in Mus musculus preimplantation embryo development. Sci Rep. 2016;6:26711.