ABSTRACT
The present study focused on the effect of paeonol, one of the main components of Guizhi Fuling Pill, on blood pressure, cerebral blood flow, and vascular endothelium injury in spontaneously hypertensive rats to provide theoretical basis for the treatment of hypertension. After treatment with paeonol, the mean arterial pressure (MAP) of LSHRT and HSHRT rats decreased gradually with the prolongation of treatment time. The systolic blood flow velocity (Vs), diastolic blood flow velocity (Vd) and mean blood flow velocity (Vm) were significantly increased after paeonol treatment (p < 0.05). Paeonol effectively improved the blood pressure and increased the cerebral blood flow velocity in spontaneously hypertensive rats. This may be related to the fact that paeonol reduced the blood viscosity and the oxidative stress and improved the antioxidant capacity. Moreover, paeonol protected vascular endothelial cells and reduced vascular endothelial injury in spontaneously hypertensive rats.
Graphical abstract
Paeonol protects against hypertension in spontaneously hypertensive rats by restoring vascular endothelium
Hypertension still remains a major risk factor of heart failure, renal failure, stroke, and myocardial infarction despite dramatic progress in clinical hypertension diagnosis and management [Citation1,Citation2]. Current anti-hypertensive medications in clinic include calcium channel blockers, phosphodiesterase inhibitors, and nitric oxide (NO), prostaglandin and endothelial receptor antagonists [Citation3,Citation4]. However, current therapeutic strategies are often not sufficient to prevent cardiovascular events and side-effects like affecting kidney and liver function and the occurrence of drug-resistance after long-term medication has hindered these treatments to achieve satisfactory therapeutic effects [Citation5], so it is an urgent requirement to identify new potential effective treatments for the treatment of hypertension.
Paeonol has been found to be the main phenolic compound of a Chinese herbal medicine which is prepared from the roots of the plant Paeonia suffruticosa Andrews [Citation6]. Paeonol is traditionally used in clinic in China to improve blood circulation, amenorrhea, dysmenorrhea, and fever as an anti-inflammatory, anti-bacterial, anti-oxidant, antipyretic and analgesic agent [Citation7,Citation8]. Paeonol has been previously suggested to protect against acetaminophen-induced hepatotoxicity in mice and improve Parkinson’s disease in mouse model and diabetic encephalopathy in streptozotocin-induced diabetic rats by attenuating oxidative stress [Citation9–Citation13]. It has been demonstrated that paeonol could reduce lipid peroxidation on mitochondrial membrane by scavenging free radicals, and inhibit permeability and transmission of mitochondrial membrane by reducing thiol oxidation [Citation12–Citation14]. Previous studies have shown that paeonol antagonized acute myocardial ischemia and infarction in rat [Citation15,Citation16]. The vascular endothelium plays an important role in the regulation of vascular tone, tissue blood flow, inflammatory responses, and maintenance of blood fluidity [Citation17]. However, it is rare to investigate the effects of paeonol on the vascular endothelium and whether paeonol could be a potential therapeutic strategy of hypertension is still unknown.
Spontaneously hypertensive rats (SHRs) have been widely used as a primary hypertension animal model [Citation18–Citation20], this study examined the effects of paeonol on blood pressure and blood flow in the artery of spontaneously hypertensive rats and the underlying mechanism via vasomotion was explored. The present study further investigated the endothelial protective effects of paeonol against oxidative stress in rats. We concluded that treatment of paeonol for 12 weeks normalized blood pressure increased the cerebral blood flow through protecting vascular endothelium against oxidative stress and endothelium injury. These results may provide new insights into the role of paeonol to improve cardiovascular diseases such as hypertension, heart failure, ischemic heart diseases, and atherosclerosis.
Materials and methods
Reagent
Paeonol was purchased from the China Food and Drug Testing Institute (catalog number: 110708–201407). The corresponding concentration was prepared in 0.5% sodium carboxymethyl cellulose (CMC-Na) before use.
Animal group
Thirty male spontaneously hypertensive rats (SHR, 8-week-old, 200–300 g) and 10 Wistar-Kyoto rats (WKY, 8-week-old, 200–300 g) were purchased from Pengyue (license number: SCXK (Lu) 2014-0007, Jinan, China). Rats were maintained in a quiet room with 12 h light and dark cycle light, 24 ± 2°C, 60% humidity and free food and water. All animal work was approved by Animal Experimental Ethics Committee of the Yantai Hospital of Traditional Chinese Medicine. Four animal groups including normal blood pressure rat group (WKY), hypertensive control group (SHR), low dose paeonol group (LSHRT, 2 mg/kg) and high dose paeonol group (HSHRT, 5 mg/kg) with 10 rats per group were studied in our paper. Each group of rats was orally administered once a day for 12 consecutive weeks [Citation21–Citation23].
Determination of rat tail artery blood pressure
The blood pressure of the tail artery was measured before and after the experiment using the animal noninvasive blood pressure analysis system (BP-2000, Visitech, US). The mean arterial pressure (MAP) was calculated after the measurement of diastolic blood pressure (DBP), systolic blood pressure (SBP), 3 times for each rat. The measurement was done at 0, 2, 4, 6, 8, 10, and 12 weeks (at 8, 10, 12, 14, 16, 18, and 20 weeks of age) after paeonol treatment.
Transcranial doppler measurement of cerebral blood flow index
Cerebral blood flow parameters were measured after 12 weeks of administration. Transcranial Doppler system (Multi-Dop x, DWL, Germany) was used to measure the middle cerebral artery blood velocity in model rats. The systolic blood flow velocity (Vs), diastolic blood flow velocity (Vd), mean blood flow velocity (Vm), pulsatility index (PI = (Vs-Vd)/Vm), and resistance index (RI) were measured in each group.
Hemorheological examination
Abdominal aorta blood samples were harvested. A fully automated blood flow rheometer (FASCO-3010, Chongqing University, Weiduo Institute of Biological Engineering, China) was used following the manufacturer’s instruction and low-shear, high-shear whole blood viscosity, plasma viscosity, and fibrinogen determination were examined.
ELISA
1.5 mL of venous blood was drawn from the rat and serum was separated and then stored at −80°C until use. The levels of vWF (CSB-E08438r), ET-1 (CSB-E06979r), AT1 (CSB-E13746r), and SOD (CSB-EL022397RA) in rat serum were determined by ELISA following the manufacturer’s instruction. All the above kits were purchased from CUSABIO (Wuhan, China).
Colorimetric determination of NO
Serum was separated as mentioned above and NO levels in the serum were measured using the NO kit (A012-1, Jiancheng, Nanjing, China) following the manufacturer’s instruction.
Determination of MDA
Serum was separated from venous blood and MDA content in the serum was measured using MDA kit (A003-1, Jiancheng, Nanjing, China) and the operation was strictly performed according to the instructions.
HE staining
Rats were anesthetized with 2% sodium pentobarbital (50 mg/kg, New Asiatic Pharmaceutical, China). The rat’s sternum was cut and the aorta was isolated. The blood vessels were cut and rinsed in cold saline to drain the blood from the blood vessels. Then, the aorta with a length of about 1 cm was fixed in formaldehyde solution and HE staining was performed to observe the morphology of rat aortic tissue cells.
Western blot
Tissue samples from the blood vessels were ground in liquid nitrogen and the protein samples were extracted with RIPA lysis buffer (R0020, Solarbio, Beijing, China). The protein concentration was determined with a BCA protein quantification kit (23225, Thermo Fisher Scientific, Waltham, USA). Total protein of 40 μg was loaded and separated by SDS-PAGE (Mini-Protean-3, Bio-Rad, Hercules, CA, USA), transferred to PVDF membrane (Millipore, Massachusetts, USA) and blocked with 5% skimmed milk for 1 h. The primary antibody against each protein (1:1000) was diluted with 5% BSA (rabbit anti-eNOS antibody, ab76198; rabbit anti-NOX4 antibody, ab133303; rabbit anti-VCAM1 antibody, ab134047; Abcam, UK) and incubated overnight at 4°C. The membranes were then washed 3 times with 1 × TBST (TBS, 1 mL/L Tween-20) for 10 min each time, and goat anti-rabbit IgG HRP (1:2000, ab6721, Abcam) was incubated at room temperature for 2 h. The membranes were washed 3 times for 10 min each time and ECL chemiluminescence was used for detection. Quantification was performed using Image J software (NIH). Protein expression levels were normalized to β-actin.
Statistical analysis
SPSS19.0 statistical software was used to analyze the data. The results were expressed as mean ± SD. One-way analysis of variance analysis (ANOVA) followed by Tukey’s test was used for the analysis of data among groups. A value of p < 0.05 was considered to be statistically significant.
Results
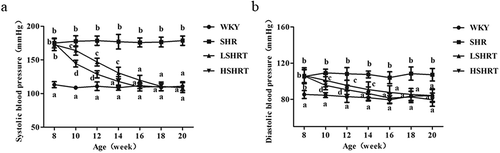
Paeonol reduces blood pressure of the tail artery in rats
To study the effect of paeonol on the blood pressure of rats, blood pressure of the tail artery of the rats was measured at 0, 2, 4, 6, 8, 10, and 12 weeks (at 8, 10, 12, 14, 16, 18, and 20 weeks of age) after paeonol administration. The MAP results are shown in . Before treatment, the MAP of rats in SHR, LSHRT and HSHRT groups was significantly higher than that in WKY group (p < 0.05). The MAP in the SHR group was significantly higher than the other three groups during the entire treatment period. Interestingly, the MAP of LSHRT and HSHRT rats gradually decreased after treatment. No significant difference was observed between HSHRT and WKY groups at 14 weeks of age and between LSHRT group and WKY group at 16 weeks of age, respectively. Both SBP and DBP were significantly reduced after paeonol treatment (,)). These data suggested that paeonol reduced the blood pressure of the tail artery in rats.
Table 1. Mean arterial pressure (mmHg) in various groups after 12 weeks.
Paeonol increases cerebral blood flow index in rats
The cerebral blood flow was measured in rats at 12 weeks after paeonol administration (at 20 weeks of age). The results are shown in . Our results showed that the Vs, Vd, and Vm in SHR group were significantly lower than those in WKY group (p < 0.05) and significantly increased after treatment with high and low doses of paeonol (p < 0.05). PI and RI in both LSHRT and HSHRT group were significantly lower than those in SHR group (p < 0.05), but not significantly different from WKY group. These results suggested that paeonol increased cerebral blood flow velocity in hypertensive rats by increasing vascular compliance and diastolic status while reducing vascular resistance.
Table 2. Cerebral blood flow in various groups after 12 weeks.
Paeonol improves hemorheological parameters in rats
The low-shear whole blood viscosity, high-shear whole blood viscosity and plasma viscosity in SHR group were significantly higher than those in WKY group and significantly decreased after a high dose or low dose of paeonol treatment () (p < 0.05). However, no significant difference was observed between high dose treatment and low dose treatment. This result suggested that paeonol could effectively reduce the blood viscosity of hypertensive rats. Moreover, fibrinogen is a protein that is synthesized by the liver and could cause blood coagulation [Citation24]. The blood fibrinogen levels in the hypertensive group treated with paeonol (2.88 ± 0.52 g/L in LSHRT group, 2.76 ± 0.51 g/L in HSHRT group) were significantly lower than those in SHR group (3.96 ± 0.73 g/L, p < 0.05), whereas no significant difference was observed compared with WKY rats (2.53 ± 0.35 g/L).
Table 3. Hemorheology indexes in various groups after 12 weeks.
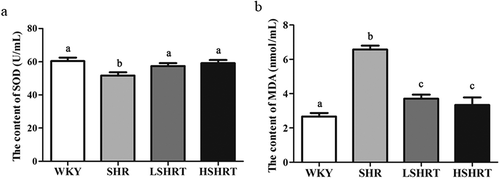
Paeonol protects against oxidative stress in rats
To study the effect of paeonol on the oxidative stress, the oxidative stress factors SOD and MDA in the serum of each group were detected. The serum SOD content in SHR group (51.61 ± 2.47 U/mL) was significantly lower than that in WKY group (60.34 ± 1.41 U/mL, p < 0.05). The SOD content was significantly increased after a high dose or low dose of paeonol treatment (), p < 0.05). The MDA content in SHR group is significantly higher than that in WKY group (p < 0.05), and it is obviously decreased after the paeonol treatment ()). These data suggested that paeonol effectively increased the antioxidant capacity of rats.
Paeonol restores the function and structure of vascular endothelium in rats
It is well known that vWF is involved in coagulation, reflecting the degree of endothelial dysfunction [Citation25]; AT1 could induce vasoconstriction and result in hypertension, which can cause endothelial injury, extracellular matrix deposition and atherosclerosis [Citation26]; ET-1 has a strong vasoconstriction activity which can cause vascular damage in hypertensive rats [Citation27]. ELISA results showed the serum levels of vWF ()), AT1 ()), and ET-1 ()) in SHR group were significantly higher than those in WKY group (p < 0.05). After treatment with high dose or low dose of paeonol, the levels of vWF ()), AT1 ()), and ET-1 ()) were significantly decreased although still higher than WKY group, indicating that paeonol significantly reduced blood levels of vWF, AT1, and ET-1 in hypertensive rats.
Figure 3. Serum levels of vWF (a), NO (b), AT1 (c), and ET-1 (d) in different groups of rats. N = 10, Different alphabets indicated significant differences.
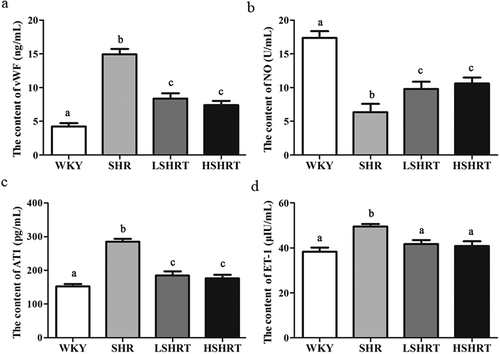
NO is an important vasodilating factor synthesized by endothelial cells, and its content indirectly reflects the functional status of endothelial cells [Citation19]. The NO content in SHR group (6.34 ± 0.47 U/mL) was significantly lower than that of WKY group (17.38 ± 0.41 U/mL, p < 0.05, )), and significantly increased after paeonol treatment (p < 0.05). These results showed that paeonol significantly reduced serum levels of vWF, AT1, and ET-1 and improved serum NO level. Moreover, paeonol protected the vascular endothelium and lower blood pressure.
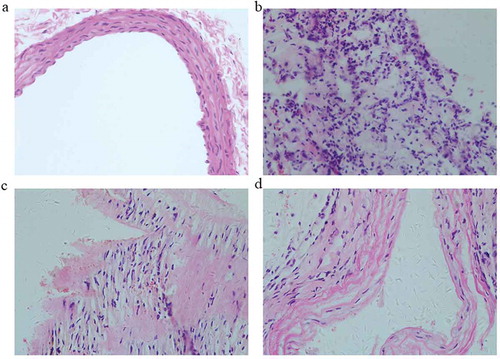
Then the structure of aorta was examined. Results of HE staining showed that the aortic intima was smooth and the endothelial cells were intact in WKY group (). However, the aortic intima was destroyed and the endothelial cells were defective in SHR group. Compared with SHR group, the rat aortic intima was significantly improved and no obvious defect was observed in the endothelial cells after paeonol treatment.
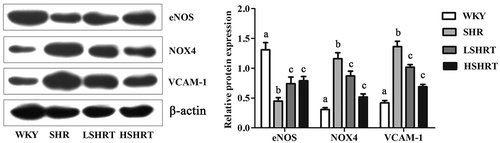
Paeonol decreased eNOS, NOX4, and VCAM-1 proteins in rats
NO is synthesized by eNOS in endothelial cells [Citation28]. The expression of eNOS in SHR group was significantly lower than that in WKY group. However, the expression of eNOS was significantly increased after paeonol treatment (; p < 0.05). NOX4 is also involved in the physiological functions of the vasculature as well as hypertension and may function as the major catalytic component of an endothelial NADPH oxidase [Citation29]. Several studies have indicated that NOX4 activity is increased in cerebral arteries during chronic hypertension, which is associated with greater production of superoxide and vasodilatation in spontaneously hypertensive rats [Citation18,Citation30,Citation31]. In addition, ET-1 stimulates VCAM-1 expression in spontaneously hypertensive rats [Citation32]. Compared with WKY rats, the expressions of NOX4 and VCAM-1 in the aortic tissues of SHR group were significantly increased (; p< 0.05) and the expressions of NOX4 and VCAM-1 were significantly decreased after paeonol treatment (; p < 0.05). These data demonstrated that paeonol protected endothelial cells by down-regulating the expression of NOX4 and VCAM-1 protein [Citation6,Citation33,Citation34].
Discussion
Hypertension still remains a major risk factor of heart failure, renal failure, stroke, and myocardial infarction. However, current therapeutic strategies are often not sufficient to prevent cardiovascular events and side-effects [Citation35–Citation37]. Chinese herbal medicine paeonol is traditionally used in clinic in China to improve blood circulation [Citation16]. Increased blood pressure and decreased cerebral blood flow were observed in spontaneously hypertensive rats with increased ROS generation and increased nitric oxide (NO) bioavailability in aortae, which caused damage to vascular endothelium. The present study demonstrated that chronic treatment with paeonol in vivo confers vascular protection by alleviating oxidative stress and restoring vascular endothelium.
Previous studies have shown that paeonol antagonized acute myocardial ischemia and infarction in rat [Citation15,Citation16]. In this study, long-term administration with paeonol at both low (LSHRT) and high (HSHRT) doses significantly decreased the blood pressure and improved cerebral blood flow compared with WKY and SHR group. Hypertension in human is associated with oxidative stress [Citation38,Citation39]. Paeonol has been previously suggested to attenuate oxidative stress in other disease models such as diabetes, hepatotoxicity [Citation9,Citation11]. In this study, paeonol treatment increased SOD content and decreased MDA content in both low (LSHRT) and high (HSHRT) group.
NO synthesized by eNOS in endothelial cells serves a key role in the cardiovascular system through dilating blood vessels to relieve hypertension [Citation40]. Hypertension induced by NO inhibition promotes oxidative stress [Citation41]. In the present study, the production of NO was remarkably decreased in SHR group compared with WKY group. Interestingly, paeonol treatment significantly improved serum NO level, which may be associated with the increased SOD content and decreased MDA content after paeonol treatment. The intact structure and function of vascular endothelium are critical in the regulation of vascular tone, tissue blood flow, inflammatory responses, and maintenance of blood fluidity [Citation17]. Paeonal treatment reduced vWF, AT1, and ET-1 levels which play important roles in maintaining the intact structure and function. Therefore, paeonol treatment protected the vascular endothelium to lower blood pressure.
To further illuminate the mechanism of the protective effect of paeonol on endothelial cells, our results showed that the expression of eNOS which synthesizes NO was up-regulated and the activity of NOS was increased. However, the expression of NOX was increased in hypertension but decreased after paeonol treatment. The expression of VCAM-1, as the downstream effector of ET-1 signaling, was increased in hypertension, and paeonol treatment significantly decreased the expression of VCAM-1.
In summary, the present results demonstrated that long-term administration of paeonol in spontaneously hypertensive rats confers protection against endothelial dysfunction and normalized blood pressure by alleviating oxidative stress and protecting vascular endothelium. These data provided further evidence which supported the potential use of paeonol as a novel therapeutic agent or health supplement for patients with cardiovascular diseases, particularly in the treatment of hypertension.
Author contribution
Zhonghui Gai, Zhenxing Wang, and Qiao Zhu conducted program design, experimental operation, data analysis, manuscript writing, and manuscript review.
Lei Zhang and Jun Ma conducted experimental operation, data analysis, and manuscript writing.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Liu D, Huang Y, Jia C, et al. Administration of antagomir-223 inhibits apoptosis, promotes angiogenesis and functional recovery in rats with spinal cord injury. Cell Mol Neurobiol. 2015 May;35:483–491. PubMed PMID: 25416533; eng.
- Antonopoulos AS, Margaritis M, Shirodaria C, et al. Translating the effects of statins: from redox regulation to suppression of vascular wall inflammation. Thromb Haemost. 2012 Nov;108:840–848. PubMed PMID: 22872079; eng.
- Wu S, Han J, Li WQ, et al. Hypertension, antihypertensive medication use, and risk of psoriasis. JAMA Dermatol. 2014 Sep;150:957–963. PubMed PMID: 24990147; PubMed Central PMCID: PMCPmc4184206. eng.
- Zhang FL, Guo ZN, Xing YQ, et al. Hypertension prevalence, awareness, treatment, and control in northeast China: a population-based cross-sectional survey. J Hum Hypertens. 2017 Dec;32:54–65. PubMed PMID: 29180804; eng.
- Siu D. A new way of targeting to treat coronary artery disease. J Cardiovasc Med (Hagerstown). 2010 Jan;11:1–6. PubMed PMID: 19829140; eng.
- Choy KW, Lau YS, Murugan D, et al. Chronic treatment with paeonol improves endothelial function in mice through inhibition of endoplasmic reticulum stress-mediated oxidative stress. PloS One. 2017;12(May):e0178365. PubMed PMID: 28562691; PubMed Central PMCID: PMCPmc5451063. eng.
- Fu PK, Wu CL, Tsai TH, et al. Anti-inflammatory and anticoagulative effects of paeonol on LPS-induced acute lung injury in rats. Evid Based Complement Alternat Med. 2012;2012:837513. PubMed PMID: 22454687; PubMed Central PMCID: PMCPmc3291481. eng.
- Zhang LH, Xiao PG, Huang Y. Recent progresses in pharmacological and clinical studies of paeonol. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1996 Mar;16:187–190. PubMed PMID: 9208544; chi.
- Ding Y, Li Q, Xu Y, et al. Attenuating oxidative stress by paeonol protected against acetaminophen-induced hepatotoxicity in mice. PLoS One. 2016;11(May):e0154375. PubMed PMID: 27144271; PubMed Central PMCID: PMCPmc4856301. eng.
- Shi X, Chen YH, Liu H, et al. Therapeutic effects of paeonol on methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid-induced Parkinson’s disease in mice. Mol Med Rep. 2016 Sep;14:2397–2404. PubMed PMID: 27484986; PubMed Central PMCID: PMCPmc4991680. eng.
- Liu J, Wang S, Feng L, et al. Hypoglycemic and antioxidant activities of paeonol and its beneficial effect on diabetic encephalopathy in streptozotocin-induced diabetic rats. J Med Food. 2013 Jul;16:577–586. PubMed PMID: 23875897; eng.
- Liu J, Shen HM, Ong CN. Role of intracellular thiol depletion, mitochondrial dysfunction and reactive oxygen species in salvia miltiorrhiza-induced apoptosis in human hepatoma HepG2 cells. Life Sci. 2001 Sep 7;69:1833–1850. PubMed PMID: 11693264; eng.
- Tang Y, Wang M, Le X, et al. Antioxidant and cardioprotective effects of Danshensu (3-(3, 4-dihydroxyphenyl)-2-hydroxy-propanoic acid from salvia miltiorrhiza) on isoproterenol-induced myocardial hypertrophy in rats. Phytomedicine. 2011 Sep;18:1024–1030. PubMed PMID: 21665454; eng.
- Li H, Xie YH, Yang Q, et al. Cardioprotective effect of paeonol and danshensu combination on isoproterenol-induced myocardial injury in rats. PloS One. 2012;7:e48872. PubMed PMID: 23139821; PubMed Central PMCID: PMCPmc3490947. eng.
- Ma L, Chuang CC, Weng W, et al. Paeonol protects rat heart by improving regional blood perfusion during no-reflow. Front Physiol. 2016 Jul;7:298. PubMed PMID: 27493631; PubMed Central PMCID: PMCPmc4954854. eng.
- Zhang JY, Zhao L, Li YK, et al. Effect of paeonol on blood pressure and blood flow in artery of spontaneously hypertensive rats and its mechanisms related on vasomotion. Zhongguo Zhong Yao Za Zhi. 2015 Dec;40:4903–4907. PubMed PMID: 27245041; chi.
- Sandoo A, van Zanten JJ, Metsios GS, et al. The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J. 2010 Dec 23;4:302–312. PubMed PMID: 21339899; PubMed Central PMCID: PMCPmc3040999. eng.
- Yao H, Ago T, Kitazono T, et al. NADPH oxidase-related pathophysiology in experimental models of stroke. Int J Mol Sci. 2017 Oct 11;18:2123. PubMed PMID: 29019942; PubMed Central PMCID: PMCPmc5666805. eng.
- Sarkar O, Li Y, Anand-Srivastava MB. Nitric oxide attenuates overexpression of Gialpha proteins in vascular smooth muscle cells from SHR: role of ROS and ROS-mediated signaling. PloS One. 2017;12:e0179301. PubMed PMID: 28692698; PubMed Central PMCID: PMCPmc5503203. eng.
- Hosoo S, Koyama M, Kato M, et al. The restorative effects of eucommia ulmoides oliver leaf extract on vascular function in spontaneously hypertensive rats. Molecules. 2015 Dec 9;20:21971–21981. PubMed PMID: 26690110; PubMed Central PMCID: PMCPmc6331908. eng.
- Hu J, Li YL, Li ZL, et al. Chronic supplementation of paeonol combined with danshensu for the improvement of vascular reactivity in the cerebral basilar artery of diabetic rats. Int J Mol Sci. 2012 Nov;13:14565–14578. PubMed PMID: 23203081; PubMed Central PMCID: PMCPmc3509597. eng.
- Yang Q, Wang S, Xie Y, et al. Effect of salvianolic acid B and paeonol on blood lipid metabolism and hemorrheology in myocardial ischemia rabbits induced by pituitruin. Int J Mol Sci. 2010 Sep;11:3696–3704. PubMed PMID: 21152295; PubMed Central PMCID: PMCPmc2996798. eng.
- Chen Y, Liu Z, Zhou F, et al. Evaluating pharmacological effects of two major components of Shuangdan oral liquid: role of Danshensu and Paeonol in diabetic nephropathy rat. Biomol Ther (Seoul). 2016 Sep;24:536–542. PubMed PMID: 27582557; PubMed Central PMCID: PMCPmc5012880. eng.
- Sechi LA, Zingaro L, Catena C, et al. Relationship of fibrinogen levels and hemostatic abnormalities with organ damage in hypertension. Hypertension. 2000 Dec;36:978–985. PubMed PMID: 11116111; eng.
- Lip GY, Foster W, Blann AD. Plasma von Willebrand factor levels and surrogates of atherosclerosis. J Thromb Haemost. 2005 Apr;3:659–661. PubMed PMID: 15842350; eng.
- Yatabe J, Sanada H, Midorikawa S, et al. Effects of decreased renal cortical expression of G protein-coupled receptor kinase 4 and angiotensin type 1 receptors in rats. Hypertens Res. 2008 Jul;31:1455–1464. PubMed PMID: 18957817; PubMed Central PMCID: PMCPmc3731072. eng.
- Lariviere R, Thibault G, Schiffrin EL. Increased endothelin-1 content in blood vessels of deoxycorticosterone acetate-salt hypertensive but not in spontaneously hypertensive rats. Hypertension. 1993 Mar;21:294–300. PubMed PMID: 8478038; eng.
- Tonelli AR, Haserodt S, Aytekin M, et al. Nitric oxide deficiency in pulmonary hypertension: pathobiology and implications for therapy. Pulm Circ. 2013 Jan;3:20–30. PubMed PMID: 23662172; PubMed Central PMCID: PMCPmc3641730. eng.
- Ago T, Kitazono T, Ooboshi H, et al. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004 Jan;109:227–233. PubMed PMID: 14718399; eng.
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007 Jan;87:245–313. PubMed PMID: 17237347; eng.
- Paravicini TM, Chrissobolis S, Drummond GR, et al. Increased NADPH-oxidase activity and Nox4 expression during chronic hypertension is associated with enhanced cerebral vasodilatation to NADPH in vivo. Stroke. 2004 Feb;35:584–589. PubMed PMID: 14739416; eng.
- Ono H, Ichiki T, Ohtsubo H, et al. CAMP-response element-binding protein mediates tumor necrosis factor-alpha-induced vascular cell adhesion molecule-1 expression in endothelial cells. Hypertens Res. 2006 Jan;29:39–47. PubMed PMID: 16715652; eng.
- Pan LL, Dai M. Paeonol from Paeonia suffruticosa prevents TNF-alpha-induced monocytic cell adhesion to rat aortic endothelial cells by suppression of VCAM-1 expression. Phytomedicine. 2009 Nov;16:1027–1032. PubMed PMID: 19541467; eng.
- Cheng YC, Sheen JM, Hu WL, et al. Polyphenols and oxidative stress in atherosclerosis-related ischemic heart disease and stroke. Oxid Med Cell Longev. 2017;2017:8526438. PubMed PMID: 29317985.
- Armario P, Waeber B. Therapeutic strategies to improve control of hypertension. J Hypertens. 2013 Mar;31(Suppl 1):S9–S12. PubMed PMID: 23389085; eng.
- Rubio-Guerra AF, Duran-Salgado MB. Recommendations for the treatment of hypertension in elderly people. Cardiovasc Hematol Agents Med Chem. 2015;12:146–151. PubMed PMID: 25761106; eng.
- Stewart MH, Lavie CJ, Ventura HO. Emerging therapy in hypertension. Current hypertension reports. Curr Hypertens Rep. 2019 Mar;21:23. PubMed PMID: 30826948; eng.
- Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004 Sep;44:248–252. PubMed PMID: 15262903; eng.
- Rincon J, Correia D, Arcaya JL, et al. Role of Angiotensin II type 1 receptor on renal NAD(P)H oxidase, oxidative stress and inflammation in nitric oxide inhibition induced-hypertension. Life Sci. 2015 Mar;124:81–90. PubMed PMID: 25623850; PubMed Central PMCID: PMCPmc6037991. eng.
- Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006 Apr;113:1708–1714. PubMed PMID: 16585403; eng.
- Khattab M, Ahmad M, Al-Shabanah OA, et al. Effects of losartan on blood pressure, oxidative stress, and nitrate/nitrite levels in the nitric oxide deficient hypertensive rats. Recept Channels. 2004;10:147–157. PubMed PMID: 15989079; eng.