?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The inhibitory effect of WQ-3810 on DNA gyrase was assayed to evaluate the potential of WQ-3810 as a candidate drug for the treatment of quinolone resistant Salmonella Typhymurium infection. The inhibitory effect of WQ-3810, ciprofloxacin and nalidixic acid was compared by accessing the drug concentration that halves the enzyme activity (IC50) of purified S. Typhimurium wildtype and mutant DNA gyrase with amino acid substitution at position 83 or/and 87 in subunit A (GyrA) causing quinolone resistance. As a result, WQ-3810 reduced the enzyme activity of both wildtype and mutant DNA gyrase at a lower concentration than ciprofloxacin and nalidixic acid. Remarkably, WQ-3810 showed a higher inhibitory effect on DNA gyrase with amino acid substitutions at position 87 than with that at position 83 in GyrA. This study revealed that WQ-3810 could be an effective therapeutic agent, especially against quinolone resistant Salmonella enterica having amino acid substitution at position 87.
Graphical abstract
WQ-3810 shows high inhibitory activity against quinolone resistant DNA gyrase.
Nontyphoidal Salmonella, a serotype of rod-shaped, Gram-negative bacteria, is an important causative pathogen of foodborne diseases. The World Health Organization (WHO) estimated that diseases caused by 31 foodborne hazards and nontyphoidal Salmonella infection were among the greatest threats to human health [Citation1]. Salmonella enterica is the main gastroenteritis- and invasive nontyphoidal Salmonella infection-causing bacterial species. In addition, this bacterial species has more than 2,500 serovars and Salmonella enterica serovars Typhimurium and Enteritidis are two of the major foodborne infection-causing serovars [Citation2]. Chloramphenicol, ampicillin and trimethoprim-sulfamethoxazole were formerly used in the treatment of severe gastroenteric cases, particularly in treating susceptible patients such as the elderly and infants [Citation3]. However, an increasing rate of resistance to these antibiotics in nontyphoidal Salmonella has been recently reported in many countries [Citation4,Citation5]. Thus, antibiotic resistant nontyphoidal Salmonella infection has become a serious public health threat worldwide.
Quinolones are synthetic antibacterial drugs used to treat bacterial infections, with nalidixic acid considered the first-generation synthetic quinolone. Initially, nalidixic acid was only effective against Gram-negative bacteria and thus its antibacterial spectrum was narrow. However, the antibacterial activity of nalidixic acid was drastically improved by the addition of a fluorine atom at the position C6 of the quinolone ring [Citation6,Citation7]. Quinolones fluorinated at the position C6 with an improved spectrum are also known as fluoroquinolones. Ciprofloxacin is a representative fluoroquinolone categorized as second-generation quinolone. In general, ciprofloxacin is safe, with high treatment efficacy and a wide antibacterial spectrum. Therefore, ciprofloxacin is a clinically useful antimicrobial drug that has been selected by the WHO as an essential medicament [Citation8]. Currently available fourth-generation fluoroquinolones (e.g. moxifloxacin and gemifloxacin) originated from further development of fluoroquinolones [Citation9]. Quinolones are known to be effective against nontyphoidal Salmonella which are resistant to multiple drugs that have been used in treatment currently, consequently, they are regarded as one of the most clinically important antibiotics. However, clinical isolates of nontyphoidal Salmonella resistant to quinolones have emerged and spread rapidly [Citation10–Citation13]. Hence, previously treatable infectious diseases caused by nontyphoidal Salmonella nowadays are becoming fatal to people with low immunity due to the unavailability of effective antibacterial drugs. Therefore, development of alternative antibacterial agents is currently the most pressing concern for the international medical community.
Resistance to quinolones is typically caused by bacterial mutation due to amino acid substitutions in DNA gyrase, the target enzyme [Citation14]. DNA gyrase consists of two GyrA and two GyrB subunits. The function of DNA gyrase is to introduce negative supercoils into the DNA to relieve the accumulation of twisted bacterial DNA prior to enzymic translocation [Citation15]. Therefore, this enzyme is essential for DNA replication and transcription. Quinolones bind to DNA gyrases and inhibit enzymic activity, resulting in a bactericidal effect. However, quinolones cannot bind to DNA gyrases when a specific mutation has occurred on the binding site of DNA gyrases because this mutation first alters the structure of the target enzyme and subsequently the binding affinity of fluoroquinolones to this enzyme, resulting in quinolone resistance. Mutations have been frequently reported at codons 83 and 87 of the amino acid sequence of GyrA in quinolone-resistant Salmonella [Citation16–Citation19]. Amino acids at these positions are considered to be strongly involved in the binding to quinolones [Citation20–Citation22]. Therefore, it is critical to find quinolones showing high affinity to DNA gyrases with amino acid substitutions at positions 83 and 87.
WQ-3810 is a relatively new fluoroquinolone. Although only a few studies have assayed the potency of WQ-3810, its strong antibacterial activity has already been tested against certain pathogens [Citation23,Citation24]. For example, in a previous study, WQ-3810 showed a stronger antibacterial activity against every single clinical isolate of Escherichia coli and Acinetobacter baumannii with different amino acid substitutions in GyrA than did other fluoroquinolones, including ciprofloxacin [Citation24]. We performed similar experiments using a distinct novel quinolone, DC-159a, to reveal that it significantly inhibit the activity of mutant DNA gyrase comparing to nalidixic acid and ciprofloxacin. WQ-3810 is structurally very different from DC-159a and ciprofloxacin [Citation25]. WQ-3810 possesses unique substituents at positions N1 and C7, in addition to fluorination at the position C6 in the quinolone ring. Substituents at N1 and C7 are thought to be involved in the binding affinity to DNA gyrase [Citation26,Citation27]. Thus, it was expected that WQ-3810 would exert higher inhibitory effect on DNA gyrases carrying amino acid substitutions at 83 and 87 in GyrA than DC-159a and other quinolones. Therefore, the objective of the present study was to assess the inhibitory effect of WQ-3810 on S. Typhimurium DNA gyrase carrying amino acid substitutions that putatively cause quinolone resistance. In addition, we examined the antibacterial activity of WQ-3810 against nontyphoidal Salmonellae.
Materials and methods
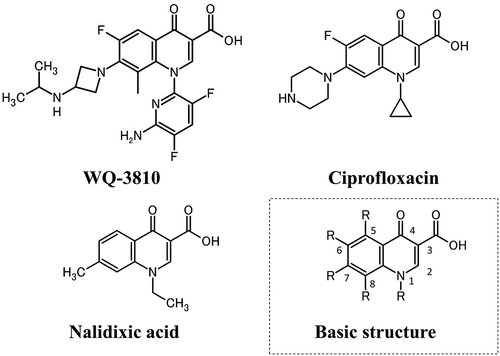
Quinolones and reagents
WQ-3810 was kindly provided by Wakunaga Pharmaceutical Co., Ltd. The inhibitory effect of WQ-3810 was assayed by comparing it with that of ciprofloxacin and nalidixic acid, which were tested in a previous study [Citation25]. Ciprofloxacin is clinically crucial for treating infectious human diseases as explained in the introduction [Citation8]. On the other hand, nalidixic acid is less important as a therapeutic agent because other more effective quinolones are available, but it is important as a standard for screening quinolone-resistant bacteria [Citation28]. The chemical structures of these quinolones are shown in . Ciprofloxacin and nalidixic acid were purchased from LKT Laboratories, Inc. (St Paul, MN, USA) and Wako Pure Chemical Industries, Ltd (Tokyo, Japan), respectively. Relaxed pBR322 DNA was purchased from John Innes Enterprises Ltd (Norwich, UK) and Ethidium bromide was obtained from Wako Pure Chemical Industries, Ltd.
Recombinant DNA gyrases
The inhibitory effect of quinolones was tested against recombinant wild-type (WT) and mutant DNA gyrases. Mutant DNA gyrases with four single amino acid substitutions, Ser83Phe, Asp87Asn, Asp87Gly, and Asp87Tyr, and one double amino acid substitution, Ser83Phe-Asp87Asn, were selected because these amino acid substitutions have been frequently detected in quinolone-resistant nontyphoidal Salmonella [Citation29–Citation31]. Every recombinant DNA gyrases used in the present work were produced in our previous study [Citation25].
DNA gyrase supercoiling assay
DNA supercoiling activity assays of the purified DNA gyrases were carried out in triplicate in a reaction mixture. Relaxed pBR322 (302.4 ng) and DNA gyrases (GyrA: 30.6 ng, GyrB: 28.3 ng) were incubated at 35ºC in a gyrase assay solution [35 mM Tris-HCl, 6 mM MgCl2, 1.8 mM spermidine, 24 mM KCl, 5 mM DTT, 0.36 mg/mL of BSA and 6.5% glycerol (w/v)]. This reaction mixture contained serially diluted concentrations of WQ-3810. After 40-min incubation, the reaction was stopped by adding 8 μL of a stop solution (5% SDS, 25% glycerol and 0.25mg/mL of bromophenol blue) [Citation32]. 10 μL of the reaction mixture was loaded onto 1% agarose gel in 0.5xTris-borate-EDTA (TBE) buffer for electrophoresis for two hours at 40 mA. The supercoiling activity of DNA gyrases can be easily distinguished and separated by gel electrophoresis because gyrases introduce negative supercoils into relaxed DNA, and supercoiled DNA is more compact than relaxed DNA [Citation33–Citation35]. After electrophoresis, the agarose gel was stained with 0.5 μg/mL of ethidium bromide. The presence of supercoiled DNA was confirmed under UV light. The amount of supercoiled DNA was measured by the intensity of its band using digital image processing software ImageJ (http://rsbweb. nih.gov/ij).
The concentration of each quinolone required to reduce by 50% DNA gyrase activity (IC50) was used as the baseline to assess their inhibitory effect. Intensity of the supercoiled DNA band of a quinolone-free sample was defined as 100% control. Data of the decreasing DNA band intensity caused by incremental quinolone concentration was fitted to a four-parameter log-logistic model. IC50 was estimated using a model for dose-response [Citation36,Citation37]. In addition to the calculation of the parameters of the model and the estimation of IC50, the 95% confidence interval (95%CI) of IC50 was also estimated. All analyses were conducted using statistical software R version 3.2.5 and the add-on package “drc” version 3.0 [Citation38,Citation39].
Antimicrobial susceptibility testing for nontyphoidal salmonella
Nontyphoidal Salmonella strains tested in the present study were S. Typhimurium NBRC 13,245 and S. Enteritidis NBRC 3313. An antimicrobial susceptibility test ran in triplicate for each strain was used to evaluate the permeability of WQ-3810 as well as the antibacterial activity of WQ-3810. The minimum inhibitory concentration (MIC) was measured by micro-broth dilution methods as per the recommended protocol of the Clinical and Laboratory Standards Institute(CLSI) [Citation40]. A suspension of each strain was transferred to wells in a 96-well tray containing serially diluted concentrations of WQ-3810 and incubated overnight at 37°C. The MIC was defined as the lowest concentration of the quinolone required to completely inhibit all visible bacterial growth in a well.
Results
Inhibitory effect on recombinant DNA gyrase
IC50s were estimated using the purified DNA gyrases. Electrophoretic patterns of enzymic activity attenuated by IC50s of quinolones are shown in and IC50s are summarized in . IC50s of nalidixic acid against all WT and mutant DNA gyrases were found to be the highest. Although no significant differences were found between the IC50s of WQ-3810 [0.31 μg/mL; 95%CI: 0.24–0.37 μg/mL] and ciprofloxacin (0.25 μg/mL; 95%CI: 0.19–0.32 μg/mL) against WT DNA gyrases, they were markedly lower than that of nalidixic acid (28 μg/mL; 95%CI: 14–42 μg/mL). Moreover, IC50s of WQ-3810 against every single mutant DNA gyrase were significantly lower than those of ciprofloxacin and nalidixic acid. IC50s of every quinolone against double mutant DNA gyrases were significantly greater than those against WT and single mutant DNA gyrases. For example, IC50s of ciprofloxacin against double mutant DNA gyrases was 1,920-fold greater than those against WT DNA gyrases and 123-fold to 184-fold greater than those against single mutant DNA gyrases (). Nonetheless, compared with those of ciprofloxacin and nalidixic acid, IC50s of WQ-3810 against double mutant DNA gyrases were considerably lower. The IC50 of WQ-3810 against double mutant DNA gyrase was 45-fold greater that that against WT DNA gyrases, it was less than 1/45 that of ciprofloxacin. In the present study, when taking into consideration the position of the amino acid substitution, IC50s of WQ-3810 against mutant DNA gyrases with an amino acid substitution at 87 were lower than those against DNA gyrases with an amino acid substitution at 83. Moreover, while the IC50 of WQ-3810 against DNA gyrase with Ser83Phe was 1.6 μg/mL (95%CI: 1.4–1.9 μg/mL), those against DNA gyrases with amino acid substitutions at position 87 were no greater than 0.92 μg/mL
Table 1. IC50s of quinolones against WT and mutant DNA gyrases.
Figure 2. Inhibitory activity of WQ-3810 compared with that of ciprofloxacin and nalidixic acid. Supercoiled DNA bands were easily distinguished and separated from the bands of relaxed DNA. Asterisks (*) indicates the band of supercoiled DNA. It was observed that as the concentration of quinolone increased, the intensity of the supercoiled DNA band decreased. The data of ciprofloxacin and nalidixic acid shown in this figure were reported in a previous study [Citation25].
![Figure 2. Inhibitory activity of WQ-3810 compared with that of ciprofloxacin and nalidixic acid. Supercoiled DNA bands were easily distinguished and separated from the bands of relaxed DNA. Asterisks (*) indicates the band of supercoiled DNA. It was observed that as the concentration of quinolone increased, the intensity of the supercoiled DNA band decreased. The data of ciprofloxacin and nalidixic acid shown in this figure were reported in a previous study [Citation25].](/cms/asset/0c524d93-1422-4253-81b8-2f3552b23f5c/tbbb_a_1650634_f0002_b.gif)
MICs against nontyphoidal Salmonella
MICs estimated during the antimicrobial susceptibility testing are summarized in . WQ-3810 had different MICs against S. Typhimurium and S. Enteritidis. While the antibacterial activity of WQ-3810 was higher than that of nalidixic acid, it was lower than that of ciprofloxacin.
Table 2. MICs for S. Typhimurium and S. Enteritidis.
Discussion
In the supercoiling activity assay, WQ-3810 exerted a higher inhibitory effect on mutant DNA gyrases with quinolone resistance-conferring amino acid substitutions than did ciprofloxacin and nalidixic acid. It is worth noting that the inhibitory effect of WQ-3810 differed depending on the position of the amino acid substitution in DNA gyrases. Indeed, WQ-3810 showed a high affinity to DNA gyrases with the amino acid at position 87 regardless of the occurrence of an amino acid substitution. This finding seems to indicate that binding of WQ-3810 to DNA gyrases is more dependent on the amino acid at position 83, that is serine, than at position 87. WQ-3810 possesses unique substituents in its chemical structure at N1 and C7, and these substituents have been proposed to contribute to the affinity to DNA gyrases [Citation26,Citation41]. Previous work proposed a model of a quinolone-binding pocket in DNA gyrases [Citation42]. In that model, ciprofloxacin is positioned in the binding pocket between GyrA and GyrB. The substituent at N1 interacts with residues in GyrA and the substituent at C7 interacts with residues in GyrB, including serine at position 83 and aspartic acid at position 87. In the model, a change of substituent at N1 of the quinolone ring causes different binding affinity to amino acids at positions 83 and 87 in GyrA. In the case of WQ-3810, it possesses a 2-amino-6-tert-butylamino-3,5-difluoropyridin moiety at position N1, whereas ciprofloxacin has a cyclopropyl moiety at the same position. Hence, the substituent of WQ-3810 at N1 is structurally larger than that of ciprofloxacin. In addition, it is likely that the substituent at N1 in WQ-3810 is closer to the amino acid at position 83. These unique characteristics likely help WQ-3810 develop stronger interaction and affinity to DNA gyrases.
The substituent at C7 of the quinolone ring is thought to be critical for potent antimicrobial activity [Citation26,Citation27,Citation41]. In the present work, when compared with ciprofloxacin and nalidixic acid, WQ-3810 showed lower IC50s. In the aforementioned model, C7 is bound to GyrB and thus, it can be inferred that IC50s are likely affected by C7. Based on this, IC50s of quinolones against double mutant DNA gyrases estimated in the present study were likely influenced by the binding force to substituents at C7 in GyrB. Quinolone resistant nontyphoidal Salmonella with amino acid substitutions in GyrB is much rarer than those with amino acid substitutions in GyrA [Citation43–Citation45]. This might be because of the fatal effect of amino acid substitutions in GyrB on bacterial survival. Assuming that the proportion of GyrB in binding of WQ-3810 to DNA gyrase is large, it can be expected that WQ-3810 is less likely to produce quinolone resistant Salmonella than other quinolones. Although no universally accepted explanation for this phenomenon is available to date, the results of the present study will likely contribute to the understanding of the relationship between the structure and the antibacterial activity of quinolones.
Comparing to the IC50 of DC-159a in our previous study [Citation25], it is apparent that WQ-3810 exhibit a stronger inhibitory effect on DNA gyrase with amino acid substitutions at position 87 than with that at position 83 in GyrA. Though there was no significant difference between the IC50 of WQ-3810 and DC-159a against wildtype DNA gyrase, the IC50 for mutant DNA gyrase was different between WQ-3810 and DC-159a. While DC-159a showed significantly high inhibitory effect on regardless of amino acid substitution on DNA gyrase, WQ-3810 showed higher inhibitory effect on DNA gyrase having amino acid substitution at position 87 than DC-159a. The substituent at N1 in WQ-3810 might be playing an important role in this phenomenon. Detailed analysis of structure-activity relationship in our future study may lead to a practical idea of designing novel fluoroquinolones effective for quinolone-resistant Salmonella with various kind of mutations in gyrA.
While its inhibiting concentration depended on the amino acid substitution, it was demonstrated that WQ-3810 could inhibit the activity of DNA gyrases at a lower concentration than the other quinolones. However, to exert a bactericidal effect, a quinolone has to be taken up by the bacterial cell and accumulate intracellularly until reaching the concentration required to inhibit DNA gyrase activity. This is observed when uptake is suppressed and bacteria readily show quinolone resistance afterward [Citation46]. To investigate the antibacterial activity against nontyphoidal Salmonella bacteria, apart from S. Typhimurium, MICs were also measured against S. Enteritidis. According to National Center for Biotechnology Information (NCBI) databases [Citation47], these two strains have identical amino acid sequences in GyrA and are defined as wild type; therefore, the binding affinity of WQ-3810 to DNA gyrases in both strains is thought to be the same. Considering that there was no difference between the IC50s of WQ-3810 and ciprofloxacin against WT DNA gyrases, the likely reason for the MIC of WQ-3810 being higher than that of ciprofloxacin is that WQ-3810 is less permeable and easier to be excreted than ciprofloxacin. Thus, it can be argued that in terms of permeability and accumulation, WQ-3810 is an inferior drug compared with ciprofloxacin. Nonetheless, since its IC50s were very low, it would be expected that MICs of WQ-3810 against strains with mutant DNA gyrases would be lower than those of ciprofloxacin. In particular, a strong antimicrobial effect would be expected against strains with amino acid substitutions at position 87. Therefore, permeability and accumulation rate should be also important factors to consider when evaluating the antibacterial activity of WQ-3810.
Previous studies have shown that log (MIC) and log (IC50) correlate in quinolones [Citation33,Citation48]. Since S. Typhimurium and S. Enteritidis showed different MICs, regression lines were calculated based on the log (IC50) and log (MIC) of each quinolone for WT, and log (MIC) was calculated from the regression coefficient of the calculated regression lines and log (IC50) against mutant DNA gyrases. The MIC was obtained by converting from the log (MIC). Assuming that WQ-3810 would show a similar logarithmic correlation, its MICs were estimated from IC50s. The estimated MIC range of ciprofloxacin for nontyphoidal Salmonella with single mutant DNA gyrases was 0.33–0.60 μg/mL (data not shown). Since this estimate was within the MIC range for clinical isolates reported in previous studies [Citation18,Citation19], it was considered to be acceptable. Furthermore, while the estimated range of MICs of WQ-3810 against strains having DNA gyrases with Ser83Phe was 0.20–0.25 μg/ml, its range of MICs for strains containing amino acid mutations at position 87 in GyrA was 0.08–0.14 μg/ml, which was very low. In a past epidemiological study, the number of nontyphoidal Salmonella strains containing amino acid substitutions at position 87 was greater than that with amino acid substitutions at position 83 [Citation17]. In light of this, WQ-3810, with a higher antibacterial activity than ciprofloxacin and a higher antimicrobial activity against strains with amino acid substitutions at position 87, will likely be an effective quinolone antibacterial drug.
Conclusion
To summarize the present study, it was demonstrated that WQ-3810, which has a unique structure at positions N1 and C7, exerted a higher inhibitory effect on DNA gyrases with amino acid substitutions at 87 in GyrA than did ciprofloxacin and nalidixic acid. It was shown that WQ-3810 was expected to exhibit effective antibacterial activity against nontyphoidal Salmonella even if they are quinolone resistant. Furthermore, although further studies are required for the structure activity relationship of quinolones, the results obtained in this study were important findings for development of quinolones which are particularly effective for quinolone resistant bacteria.
Author contribution
All authors contributed to research concept, design, and planning. KK performed all the experiments done in this study and analysed and interpreted the data obtained. SK greatly contributed to the preparation of the test material. CN and YS made a contribution to the interpretation of the results obtained from the experiment. The manuscript was created by KK. All authors revised the article about important content and approved the final manuscript.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Acknowledgments
This work was supported in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, for the Joint Research Program of the Research Center for Zoonosis Control, Hokkaido University to YS, and in part by the Japan Initiative for Global Research Network on Infectious Diseases, Grant No. JP18fm0108008 from the Japan Agency for Medical Research and Development (AMED) to YS. Finally, we are grateful to Wakunaga Pharmaceutical Co., Ltd. for providing WQ-3810.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- World Health Organization. WHO estimates of the global burden of foodborne diseases [Internet]. World Health Organization, editor. 2016. Available from: http://apps.who.int/iris/bitstream/10665/199350/1/9789241565165_eng.pdf?ua=1
- Hohmann EL. Nontyphoidal salmonellosis. Clin Infect Dis [Internet]. 2001;32:263–269. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11170916
- Bruzzese E, Giannattasio A, Guarino A. Antibiotic treatment of acute gastroenteritis in children. F1000Res [Internet]. 2018;7:193. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29511533
- Eng SK, Pusparajah P, Ab Mutalib NS, et al. Salmonella: a review on pathogenesis, epidemiology and antibiotic resistance. Front Life Sci [Internet]. 2015;8:284–293.
- V. T. Nair D, Venkitanarayanan K, Kollanoor JA. Antibiotic-resistant salmonella in the food supply and the potential role of antibiotic alternatives for control. Foods. 2018;7:167.
- Emmerson AM, Jones AM. The quinolones: decades of development and use. J Antimicrob Chemother [Internet]. 2003;51(Suppl 1):13–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12702699
- Andersson MI, MacGowan AP. Development of the quinolones. J Antimicrob Chemother [Internet]. 2003;51(Suppl 1):1–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12702698
- World Health Organizatio. 19th WHO model list of essential medicines [Internet]. 2015. Available from: http://www.who.int/medicines/publications/essentialmedicines/EML_2015_FINAL_amended_NOV2015.pdf?ua=1.
- Redgrave LS, Sutton SB, Webber MA, et al. Fluoroquinolone resistance: mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol [Internet]. 2014;22:438–445.
- Martins M, McCusker MP, Viveiros M, et al. A simple method for assessment of MDR bacteria for over-expressed efflux pumps. Open Microbiol J. 2013;7:72–82.
- Bruzzese E, Giannattasio A, Guarino A. High rate of reduced susceptibility to ciprofloxacin and ceftriaxone among nontyphoid Salmonella clinical isolates in Asia. F1000Res [Internet]. 2018;7:193. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29511533
- Herikstad H, Hayes P, Mokhtar M, et al. Emerging quinolone-resistant Salmonella in the United States. Emerg Infect Dis [Internet]. 2018;3:371–372. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29511533.
- Piddock LJV. Fluoroquinolone resistance in Salmonella serovars isolated from humans and food animals. FEMS Microbiol Rev [Internet]. 2002;26:3–16. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29511533
- Ruiz J. Mechanisms of resistance to quinolones: target alterations, decreased accumulation and DNA gyrase protection. J Antimicrob Chemother. 2003;51:1109–1117.
- Bush NG, Evans-Roberts K, Maxwell A. DNA topoisomerases. EcoSal Plus [Internet]. 2015;6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26435256
- Reche MP, García de Los Ríos JE, Jiménez PA, et al. gyrA Mutations associated with nalidixic acid-resistant salmonellae from wild birds. Antimicrob Agents Chemother [Internet]. 2002;46:3108–3109. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12183286.
- Utrarachkij F, Nakajima C, Changkwanyeun R, et al. Quinolone resistance determinants of clinical Salmonella enteritidis in Thailand. Microb Drug Resist [Internet]. 2017;23:885–894. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8437229.
- Piddock LJ, Ricci V, McLaren I, et al. Role of mutation in the gyrA and parC genes of nalidixic-acid-resistant Salmonella serotypes isolated from animals in the United Kingdom. J Antimicrob Chemother [Internet]. 1998;41:635–641. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8849216.
- Griggs DJ, Gensberg K, Piddock LJ. Mutations in gyrA gene of quinolone-resistant Salmonella serotypes isolated from humans and animals. Antimicrob Agents Chemother [Internet]. 1996;40:1009–1013. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8849216
- de Souza RB, Magnani M, Ferrari RG, et al. Detection of quinolone-resistance mutations in Salmonella spp. Strains of epidemic and poultry origin. Braz J Microbiol [Internet]. 2011;42:211–215. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24031623.
- Aldred KJ, McPherson SA, Turnbough CL, et al. Topoisomerase IV-quinolone interactions are mediated through a water-metal ion bridge: mechanistic basis of quinolone resistance. Nucleic Acids Res. 2013;41:4628–4639.
- Palù G, Valisena S, Ciarrocchi G, et al. Quinolone binding to DNA is mediated by magnesium ions. Proc Natl Acad Sci U S A. 1992;89:9671–9675.
- Itoh K, Kuramoto Y, Amano H, et al. Discovery of WQ-3810: design, synthesis, and evaluation of 7-(3-alkylaminoazetidin-1-yl)fluoro-quinolones as orally active antibacterial agents. Eur J Med Chem [Internet]. 2015;103:354–360.
- Kazamori D, Aoi H, Sugimoto K, et al. In vitro activity of WQ-3810, a novel fluoroquinolone, against multidrug-resistant and fluoroquinolone-resistant pathogens. Int J Antimicrob Agents [Internet]. 2014;44:443–449.
- Koide K, Kongsoi S, Ouchi Y, et al. Antibacterial activity of DC-159a against Salmonella Typhimurium. Microb Drug Resist [Internet]. 2018. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30036136
- Renau TE, Sanchez JP, Gage JW, et al. Structure-activity relationships of the quinolone antibacterials against mycobacteria: effect of structural changes at N-1 and C-7. J Med Chem. 1996;39:729–735.
- Chu DTW, Fernandes PB. Structure-activity relationships of the fluoroquinolones. Antimicrob Agents Chemother. 1989;33:131–135.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 27th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute. Perform. Stand. Antimicrob. susceptibility testing. 27th ed. CLSI Suppl. M100. Wayne, PA Clin. Lab. Stand. Inst; 2017.
- Piddock L. Fluoroquinolone resistance in Salmonella serovars isolated from humans and food animals. FEMS Microbiol Rev [Internet]. 2002;26:3–16. Available from: http://www.sciencedirect.com/science/article/pii/S0168644501000766
- Ling JM, Chan EW, Lam AW, et al. Mutations in topoisomerase genes of fluoroquinolone-resistant Salmonellae in Hong Kong. Antimicrob Agents Chemother. 2003;47:3567–3573.
- Casin I, Breuil J, Darchis JP, et al. Fluoroquinolone resistance linked to GyrA, GyrB, and ParC mutations in Salmonella enterica typhimurium isolates in humans. Emerg Infect Dis. 2003;9:1455–1457.
- Fisher LM, Pan X-S. Methods to assay inhibitors of DNA gyrase and topoisomerase IV activities. Methods Mol Med [Internet]. 2008;142:11–23.
- Kongsoi S, Yokoyama K, Suprasert A, et al. Characterization of Salmonella Typhimurium DNA gyrase as a target of quinolones. Drug Test Anal. 2015;7:714–720.
- Kongsoi S, Changkwanyeun R, Yokoyama K, et al. Amino acid substitutions in GyrA affect quinolone susceptibility in Salmonella Typhimurium. Drug Test Anal [Internet]. 2016;8:1065–1070.
- Kim H, Nakajima C, Yokoyama K, et al. Impact of the E540V amino acid substitution in GyrB of Mycobacterium tuberculosis on quinolone resistance. Antimicrob Agents Chemother. 2011;55:3661–3667.
- Lyles RH, Poindexter C, Evans A, et al. Nonlinear model-based estimates of IC(50) for studies involving continuous therapeutic dose-response data. Contemp Clin Trials [Internet]. 2008;29:878–886. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18582601.
- Sebaugh JL. Guidelines for accurate EC50/IC50 estimation. Pharm Stat. 2011;10:128–134.
- R Core Team. R: A language and environment for statistical computing. [Internet]. R Found. Stat. Comput. Vienna, Austria; 2016. Available from: https://www.r-project.org/
- Ritz C, Baty F, Streibig JC, et al. Dose-response analysis using R. PLoS One. 2015;10:1–13.
- Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. Wayne, PA: Clinical and Laboratory Standards Institute; 2012.
- Lee S, Park T, Lee Y. Structure-activity relationship of fluoroquinolone in Escherichia coli. Arch Pharm Res [Internet]. 1998;21:106–112. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9875416
- Heddle J, Maxwell A. Quinolone-binding pocket of DNA gyrase: role of GyrB. Antimicrob Agents Chemother. 2002;46:1805–1815.
- Eaves DJ, Randall L, Gray DT, et al. Prevalence of mutations within the quinolone resistance-determining region of gyrA, gyrB, parC, and parE and association with antibiotic resistance in quinolone-resistant Salmonella enterica. Antimicrob Agents Chemother. 2004;48:4012–4015.
- Randall LP, Coldham NG, Woodward MJ. Detection of mutations in Salmonella enterica, gyrA, gyrB, parC and parE genes by denaturing high performance liquid chromatography (DHPLC) using standard HPLC instrumentation. J Antimicrob Chemother. 2005;56:619–623.
- PoRen H, LeeJene T, SungPin T, et al. Ciprofloxacin-resistant Salmonella enterica typhimurium and choleraesuis from pigs to humans, Taiwan. Emerg Infect Dis. 2004;10:60–68.
- Fàbrega A, Madurga S, Giralt E, et al. Mechanism of action of and resistance to quinolones. Microbiol Biotechnol [Internet]. 2009;2:40–61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21261881.
- Benson DA, Karsch-Mizrachi I, Lipman DJ, et al. GenBank. Nucleic Acids Res [Internet]. 2005;33:D34–D38. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15608212.
- Guillemin I, Sougakoff W, Cambau E, et al. Purification and inhibition by quinolones of DNA gyrases from Mycobacterium avium, Mycobacterium smegmatis and Mycobacterium fortuitum bv. peregrinum. Microbiology. 1999;145:2527–2532.