ABSTRACT
This study was aimed to investigate the presence of Bacillus coagulans vegetative cells in the intestine and fecal samples in rats fed B. coagulans spores as well as to estimate the ratios of spores and vegetative cells in these samples. A two-step process has been developed to enumerate B. coagulans in different mixed bacterial samples, specifically (1) observation of yellow ring formation on modified GYEA medium upon incubation at 55°C, (2) microscopic examination of spore formation after 7 d of incubation. Our results have demonstrated the presence of vegetative cells in the intestinal and fecal samples in rats fed B. coagulans spores. The ratios of B. coagulans spores and vegetative cells in cecal fluid, colonic content, and feces were approximately 2:8, 2:8, and 4:6, respectively. The existence of B. coagulans vegetative cells improved the intestinal milieu through an elevated short-chain fatty acid concentrations, higher fecal moisture, and lower fecal pH.
Graphical abstract
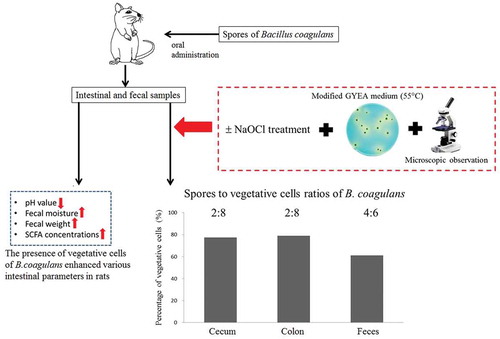
Presence of Bacillus coagulans vegetative cells in rat intestine and its physiological effects.
Bacillus coagulans, one of the promising endospore-forming bacteria used as a probiotic agent, was usually misunderstood as Lactobacillus sporogenes because it exhibits characteristics of both genera Lactobacillus and Bacillus [Citation1,Citation2]. It is capable of developing spores and thus resisting the harsh environmental conditions (e.g. high temperature and acidity) during food processing. These bacteria, which are orally delivered as spores [Citation3], have been commercially available in many food products [Citation2]. Several researches have substantiated different health-promoting effects including modulation of gastrointestinal disorders, immune system stimulation, and lowering of cholesterol through supplementation of B. coagulans spores [Citation4–Citation7].
In general, the probiotic properties of Bacillus spp. were likely to associate with their ratio between spores and vegetative cells in gut [Citation8]. It meant that the germination of spores would take place in the intestine. Intestinal germination of Bacillus spores into vegetative cells has been observed in different animals such as mice, broiler chicken, and piglets [Citation9,Citation10]. Another study has, however, reported that intragastrically administered spores might not germinate and remained metabolically quiescent during passage through the intestinal tract [Citation3]. It has also been postulated that Bacillus spores might germinate, outgrow, and multiply in the upper part of intestinal tract and eventually resporulate after passing into the distal part of the intestine [Citation11,Citation12].
Many researchers have discussed the growth behaviors of Bacillus spp. in the intestine [Citation9,Citation11], but little has been mentioned about the development of B. coagulans spores during the intestinal transit. It was not entirely clear whether these dormant spores in fact reached and germinated in variable proportions at different intestinal locations. There is a literature gap on the comprehension of the development of B. coagulans spores within the intestinal milieu.
There was as yet no specific and accurate culture methods to distinguish B. coagulans in feces from the other fecal bacteria. Without appropriate selective medium available, a heating step was conventionally used to eliminate other bacteria and estimate the number of B. coagulans spores in many studies [Citation1,Citation12]. However, this approach failed to detect the concentration of vegetative cells in fecal or other mixed bacterial samples.
This present study was aimed to investigate the presence of B. coagulans vegetative cells in the intestine and fecal samples in rats after the consumption of B. coagulans spores. A protocol to briefly discern the colonies of B. coagulans from other isolates would be developed. The ratios of spore and vegetative cells in different intestinal and fecal samples were estimated. Different fecal parameters including moisture, pH, short-chain fatty acids (SCFAs) were also evaluated to assess the physiological changes due to the presence of germinated B. coagulans.
Materials and methods
Bacterial culture
A pure strain of B. coagulans in spore form (approximately 5 × 109 spores per gram) was obtained from Syngen Biotech Co., Ltd. (Taiwan) and stored at 4°C until used.
Protocol for the determination of B. coagulans
A two-step process was applied to discern B. coagulans. The bacterial samples were first inoculated on a culture medium which was modified from GYEA medium [Citation13]. The modifications were as follows: pH value was adjusted to 5.5 and bromocresol green was added as a pH indicator. After aerobically incubating at 55°C for 2 d, the colonies, at which a color change occurred in the surrounding area from blue to yellow, were marked as a target. Second, sporulation was induced by continuously placing them at 55°C incubator for a few days. A small fraction from each target colony was individually picked onto a microscope slide and stained with malachite green and safranin for smear preparation. Spore formation in the colonies of B. coagulans would be further discerned by a microscopic examination. The target colonies in which spore formation could be observed were counted.
Diets and experimental design
The protocol used was approved by the Animal Care and Use Committee of National Chung Hsing University. All laboratory animals were cared in accord with the institutional ethical guideline. Sixteen 8-week-old male Sprague Dawley (SD) rats were purchased from BioLASCO Company, Taiwan. These rats weighing 336.6 ± 16.7 g were individually housed in a stainless steel cage under controlled environment (22 ± 1°C, 60 ± 5% humidity), and with a 12-h light/dark cycle.
After an acclimation for 1 week, SD rats were divided into eight weight classes of two each. The animals in each weight class were then randomly assigned to two groups, such as control and BC groups. No significant difference between the mean body weights of these two groups was observed at the beginning of the experiment. Spore biomass of B. coagulans (approximately 5 × 107 CFU/g), which was suspended in 5 mL of sterilized milk, was given daily to the BC group [Citation14]. The animals would finish drinking the sample within 3–4 h. After that, animals were provided with water. B. coagulans was not given to the control group. A chow diet (Laboratory Rodent Diet 5001, PMI Nutrition International/Purina Mills LLC, St. Louis, MO) was fed to all groups during the whole experimental period. Throughout the experiment, feed and water were provided ad libitum. Food intakes and body weights were recorded daily. After daily taking the bacterial sample for 28 d, fresh fecal samples were collected for the analysis of B. coagulans or were stored at – 20°C for further use. At the end of the experiment, animals were sacrificed by carbon dioxide asphyxiation. After laparotomy, samples of cecal fluid and colonic content were collected into sterile containers for immediate analysis.
Estimation of B. coagulans spores and its vegetative cells in intestinal and fecal samples
Different samples collected from cecal fluid, colonic content, and feces were suspended in a sterile phosphate-buffered saline at a ratio of 1:10 (w/v). Serial ten-fold dilutions were made with the phosphate-buffered saline to acquire desired concentration for bacterial enumeration. To enumerate the spore counts (SCs) of B. coagulans, the serial dilution would be treated by a sodium hypochlorite (NaOCl) solution at a concentration of 500 ppm for 30 sec to eliminate the vegetative cells prior to spreading them onto the modified GYEA medium. The total viable counts (TCs) of B. coagulans, which included spores and vegetative cells, were measured on the modified GYEA medium without NaOCl treatment. All agar plates were incubated at 55°C for 2 d. The number of vegetative cells was then calculated by subtracting the SCs from the TCs. All colonies would be further examined by the two-step process as described above to determine as B. coagulans. The colony of B. coagulans would be confirmed genetically by a molecular probing technique using 16S ribosomal RNA gene primers (F: 5ʹ-AGAGTTTGATCCTGGCTCAG-3ʹ; R: 5ʹ- ACGGTTACCTTGTTACGACTT-3ʹ).
Determination of fecal moisture and pH
Fecal samples without feed and urine contamination were collected to determine the moisture content and pH value using the methods as described by Chau et al. [Citation15]. Fecal moisture contents were determined by oven drying at 105°C to constant weight. Fecal pH values were measured by homogenizing the fresh feces with deionized H2O in a 1:4 (w/v) ratio, followed by a centrifugation at 1,006g for 10 min.
Determination of fecal short-chain fatty acids
The SCFA concentrations in the fecal samples were determined according to the methods as described by Huang et al. [Citation16] with slight modifications. A fresh fecal sample was first homogenized with 0.9% (w/v) cold saline at a ratio of 1:10 (w/v); then, centrifuged at 1,006g for 10 min. Two mL of the supernatant was mixed with 10 μL of isocaporic acid (internal standard) and 20 μL of 50% (w/v) sulfuric acid. After extracting with diethyl ether, 1 μL of the ether layer was analyzed by a column (Agilent J & W HP-INNO Wax GC Column, 30 m, 0.25 mm. 0.25 µm) using gas chromatograph (Agilent Technologies 7890A, California, USA) fitted with flame ionization detector. Helium was supplied as the carrier gas at a flow rate of 7 mL/min. The conditions were as follows: initial oven temperature held at 80°C for 1 min and raised to 140°C at a rate of 20°C/min, then held at 140°C for another 1 min and raised again to 220°C at a rate of 20°C/min, and lastly held at 220°C for 2 min; the temperatures of injector and detector were 140°C and 250°C, respectively.
Statistical analysis
All values expressed as mean ± standard deviation (SD) were analyzed by one-way ANOVA using the software of Statistical Product and Service Solutions (SPSS) (IBM Corp, version 20.0, Armonk, NY, USA). Differences with p < 0.05 were considered statistically significant.
Results and discussion
Protocol for the determination of B. coagulans
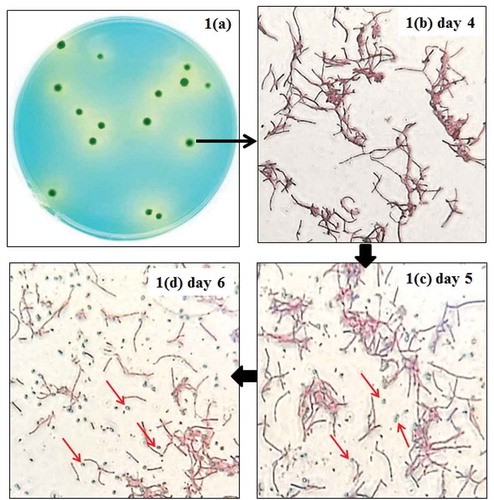
In this study, a protocol was designed to determine the presence of B. coagulans. A glucose yeast extract agar (GYEA) medium was modified by adjusting to pH 5.5 and bromocresol green indicator was added. It was aimed to highlight the lactic acid-producing bacteria (e.g. B. coagulans) on the agar plate due to the accumulation of lactic acid. This medium was incubated at 55°C to restrict the growth of many microorganisms which were unable to grow at a temperature above 45°C [Citation17] and select B. coagulans colonies. ) displays the colony morphology of pure strain of B. coagulans on the modified GYEA medium. The growth of B. coagulans resulted in a drop of pH below 3.8 and accompanied a color change from blue to yellow. A yellow circular ring surrounding each blue colony of B. coagulans could be observed.
Figure 1. Colony morphology of pure Bacillus coagulans cultured on the modified GYEA medium and their micrographs after 4–6 d of incubation.
*Spores of B. coagulans were pointed by red arrows.
A further examination was, however, necessary to distinguish B. coagulans from other possible acid-producing bacteria on the agar plate. Based on the spore-forming characteristics of B. coagulans, sporulation was induced on the agar plate by continuously incubating them at 55°C for a period of time [Citation18]. A tiny fraction of bacteria on each individual colony featuring a yellow ring would be picked, stained, and observed under an optical microscope to examine if any signs of sporulation appeared on a daily basis. The agar plates with approximately 30 to 80 colonies were selected to allow the examination to be feasible. It should be noted that perhaps a relatively large number of colonies on the agar plate needed to be examined individually. In the present study, the colony which was surrounded by a circular ring on the agar plate and composed of the spore-forming cells was counted as B. coagulans.
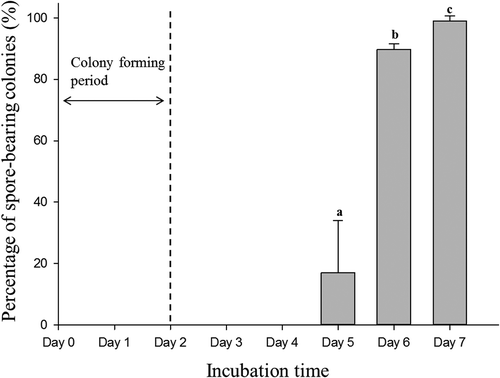
demonstrates the percentage of spore-bearing colonies over the total colonies featuring a yellow ring at different incubation times. Obvious colonies were developed in 2 d after the inoculation of bacterial culture. Our results showed that spores could be observed in some of the target colonies on the fifth day, indicating that sporulation was initiated within 5 d after inoculation. An apparent increase (p < 0.05) in the percentage of spore-bearing colonies was observed on the sixth day. Approximately 100% of the colonies had spores on the seventh day.
Figure 2. Percentages of spore-bearing colonies over the total colonies of Bacillus coagulans with yellow ring on the modified medium at different incubation times.
Bars (mean ± SD, n = 3) with different letters are significantly different (p < 0.05).
*Day 0 refers to the time point of inoculation prior to incubation.
–d) illustrates the micrographs of the existence of spores in the target colony being surrounded by a yellow ring. After 4 d of incubation, only vegetative form of B. coagulans was observed in all of the colonies on the agar plate ()). However, spores seen as green ellipses were found and increased in number gradually on the following days (,d)). It was inferred that sporulation was induced by nutrient depletion during the continuous incubation. Besides, the trait of this modified GYEA medium (e.g. the presence of manganese) might also stimulate the sporulation of B. coagulans at 55°C [Citation18].
Taken together, the determination of B. coagulans could be achieved by a two-step process: 1) observation of yellow ring formation on modified GYEA medium upon incubation at 55°C, 2) microscopic examination of spore formation after 7 d of incubation. This approach could be useful for facilitating the distinguishment of B. coagulans spores and vegetative cells in mixed bacterial samples, particularly fecal solution.
Estimation of B. coagulans spores and vegetative cells in fecal samples
As there was no available selective medium to isolate B. coagulans specifically in a fecal sample in which a complex microbial community existed, it was difficult to distinguish B. coagulans from the other enteric bacteria. In the face of this technical barrier in estimating the number of B. coagulans in vivo, the two-step process as above mentioned has been applied.
The vegetative cell numbers of B. coagulans were roughly estimated from the difference between TCs and SCs. In the past, the determination of the numbers of B. coagulans spores was usually done by a short period of heat treatment (e.g. 75°C for 30 min) to eliminate the other unwanted bacteria. No heat treatment, on the other hand, was needed for the estimation of TCs. However, the heat treatment would trigger a phenomenon of “heat activation”, further promote spore germination, and generate a higher value of SCs [Citation12]. A relatively higher value of SCs than TCs was commonly seen. The same problem (e.g. SCs > TCs) has also been encountered by means of heat treatment in our preliminary studies. In order to tackle this problem, a treatment step using NaOCl has been developed to eliminate most of the unwanted bacteria in the fecal sample effectively. It enabled the enumeration of the B. coagulans spores and vegetative cells without heat activation. As shown in , the SCs and TCs of B. coagulans in fecal samples were estimated by treating their serial dilutions with or without NaOCl, respectively.
Table 1. The numbers of Bacillus coagulans spores and vegetative cells in feces after the consumption of B. coagulans spores.
In the animal feeding experiment, the average food intake (28.4–28.9 g/day) and final body weight (434.3–439.0 g) were found to be comparable between control and BC groups. After a 28-d oral administration of pure strain of B. coagulans spores (approximately 5 × 107 spores), the SCs and vegetative cell counts of B. coagulans in the fecal samples collected from the BC group were found to be 3.64 × 105 and 6.31 × 105 CFU/g, respectively (). The target colony discerned by the two-step process was analyzed by 16S rDNA testing. The results of 16S rDNA sequence blasting showed that the colony was identified as a Bacillus species with the highest similarity (99.8%) to multiple B. coagulans strains in the NCBI BLAST database. As shown in , no spore-bearing colony was detected in the fecal sample from animals in the control group after treatment with the two-step process, implying that spore formers (e.g. Bacillus spp.) were not initially detected in this study. It should be noted the colony numbers less than 1 × 102 CFU/g were the limit of quantification and were regarded as “not detected”. It is believed that Bacillus spp., such as B. coagulans, are not native inhabitants of intestine, but transiently colonized in the intestine after ingesting the materials contaminated with soil microflora [Citation19]. Our results from dosing of pure strain of B. coagulans spores showed that both spores and vegetative cells of B. coagulans existed simultaneously in the fecal samples (). It was inferred that the germination of spores might occur along the gastrointestinal tract.
Ratios of B. coagulans spore and its vegetative cells
Regarding the spore germination of Bacillus species, it was unlikely to reach an agreement with the germination in gastrointestinal tract because the anaerobic mammalian intestinal tract made difficult to the germination of Bacillus spore [Citation19]. Nevertheless, some researches have demonstrated the germination of Bacillus spores in different animals model [Citation9,Citation10]. The occurrence of germination in the animal intestine might be associated with the microorganism itself, gastrointestinal section, intestinal milieu, intestinal maturity, and animal age [Citation8].
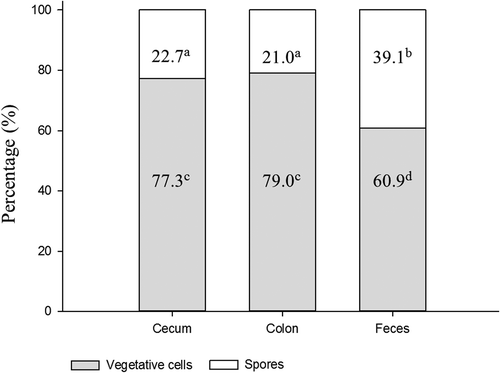
This study was the first description of exploring the ratios of B. coagulans spore and its vegetative cells at the different intestinal sections. To date, there was no relevant information available. The ratios between spore and vegetative cells of B. coagulans in cecal fluid, colonic content, and feces were 2.3:7.7, 2.1:7.9, and 3.9:6.1, respectively (). This result showed that vegetative cells of B. coagulans were found in all intestinal and fecal samples, the percentages of vegetative cells in cecum and colon (77% and 79%, respectively) were apparently (p < 0.05) higher than that in feces (61%). This difference implied that the resporulation of B. coagulans might possibly occur in the distal part of colon prior to defecation.
Figure 3. The ratios of Bacillus coagulans spores and vegetative cells at different intestinal sections.
a-bPercentages of spore numbers (mean ± SD, n = 8) among different bars with different superscripts are significantly different (p < 0.05).
c-dPercentages of vegetative cell numbers (mean ± SD, n = 8) among different bars with different superscripts are significantly different (p < 0.05).
Analysis of intestinal parameters after consumption of B. coagulans spores
shows the differences in fecal moisture, weight, and pH between the control and BC groups. As compared with the control group, fecal moisture content was significantly (p < 0.05) increased in the BC group with a daily supplementation of B. coagulans (104%). A similar trend was also observed in fecal weight between control and BC groups (14.1 and 15.7 g/day, respectively). The difference in fecal moisture could be ascribed to the profuse lactic acid production by viable B. coagulans [Citation20]. A significant (p < 0.05) reduction in fecal pH from 6.22 to 5.95 was noted in the BC group over the control group. In agreement with the findings as described by Ara et al. [Citation21], the consumption of B. coagulans spores could lead to a reduction in the fecal pH within a short time.
Table 2. Differences of fecal moisture, fecal weight, and fecal pH in rats fed diets with or without Bacillus coagulans spores.
presents the fecal SCFA profiles in rats with or without the administration of B. coagulans spores. The result showed that the consumption of B. coagulans spores (5 × 107 CFU/g) in BC group resulted in a drastic elevation in the concentration of acetic acid (from 96.1 to 147.7 μmol/g feces) and butyric acid (34.9 to 99.0 μmol/g feces). Hence, the total SCFA concentrations were effectively (p < 0.05) elevated by about 1.7 fold (from 169.3 to 279.2 μmol/g feces) after the administration of B. coagulans, even there was no apparent change in the levels of propionic acid in fecal samples. Based on the significantly higher SCFA concentrations in the BC group over the control, it was speculated that germinated B. coagulans might be able to promote the growth and acid-production of other intrinsic acid-producing bacteria in the digestive tract. The apparent elevation in the SCFA concentrations might probably be attributed to the sum of organic acids produced by B. coagulans and other lactic acid bacteria (e.g. Bifidobacterium spp. and Lactobacillus spp.).
Figure 4. Differences in the fecal short-chain fatty acids between the control and BC groups.
Bars (mean ± SD, n = 8) of each fatty acid denoted with * differ from its corresponding control group significantly (p < 0.05).
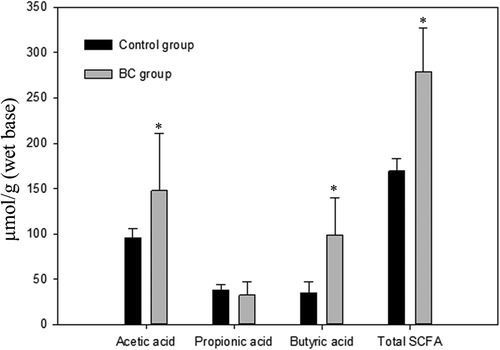
The above findings proved that spores would germinate into vegetative cells in a variable proportion. It was inferred that a relatively higher level of total SCFAs was attributed to the enhanced fermentation due to the increased concentration of germinated B. coagulans in colon. Some studies have reported the beneficial functions of SCFAs (i.e. acetate, propionate, and butyrate) in promoting the intestinal health. According to Grider et al. [Citation22], SCFAs might trigger a peristaltic reflex to accelerate the intestinal transit through a chemical stimulation of mucosa. It was speculated that the shorter intestinal transit time would impede the water reabsorption in the gut and therefore led to significantly higher fecal moisture in the B. coagulans spore fed group (). Moreover, acetate and butyrate could stimulate the intestinal mucosa to secrete the mucin which might act the intestinal barrier from physical or chemical injury [Citation23,Citation24].
Conclusion
In this study, a two-step process has been developed to enumerate the number of B. coagulans. More specifically, the estimation of the viable counts of B. coagulans spores and vegetative cells was successfully performed by using NaOCl as a tool to eliminate other vegetative bacteria. Regarding the presence of B. coagulans in different intestinal and fecal samples, about 61–79% of B. coagulans were found to be vegetative form. It is the first description of the ratio of B. coagulans spore and its vegetative cells at the different intestinal sections. The existence of B. coagulans vegetative cells might improve the intestinal milieu through an elevated SCFA concentration, higher fecal moisture, and lower fecal pH.
Author Contributions
C.Y. Saw participated in the experimental design, experimental work, data analyses, and manuscript preparation. T.J. Chang and Y.Q. Lau contributed to drafting the manuscript and arranging native English speakers to double-check the manuscript. P.Y. Chen, F.J. Dai, and T.Y. Chen contributed to technical supports of different experiments, information collection, and results analyses. C.F. Chau conceived this study and supervised all the experiments and progresses. All authors discussed the results and checked the manuscript.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
2019-07-12--Supplementary_file.docx
Download MS Word (455 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Ripamonti B, Agazzi A, Baldi A, et al. Administration of Bacillus coagulans in calves: recovery from faecal samples and evaluation of functional aspects of spores. Vet Res Commun. 2009;33(8):991–1001.
- Konuray G, Erginkaya Z. Potential use of Bacillus coagulans in the food industry. Foods. 2018;7(6):1–10.
- Spinosa MR, Braccini T, Ricca E, et al. On the fate of ingested Bacillus spores. Res Microbiol. 2000;151(5):361–368.
- Haldar L, Gandhi DN. Cholesterol-lowering effects of Bacillus coagulans B37 and Bacillus pumilus B9 strains in a rat animal model. Indian J Anim Res. 2019;53(4):469–475.
- Baron M. A patented strain of Bacillus coagulans increased immune response to viral challenge. Postgrad Med. 2009;121(2):114–118.
- Hun L. Bacillus coagulans significantly improved abdominal pain and bloating in patients with IBS. Postgrad Med. 2009;121(2):119–124.
- Dolin BJ. Effects of a proprietary Bacillus coagulans preparation on symptoms of diarrhea-predominant irritable bowel syndrome. Methods Finds Exp Clin Pharmacol. 2009;31(10):655–659.
- Bernardeau M, Lehtinen MJ, Forssten SD, et al. Importance of the gastrointestinal life cycle of Bacillus for probiotic functionality. J Food Sci Technol. 2017;54(8):2570–2584.
- Jadamus A, Vahjen W, Simon O. Growth behaviour of a spore forming probiotic strain in the gastrointestinal tract of broiler chicken and piglets. Arch Anim Nutr. 2001;54(1):1–17.
- Casula G, Cutting SM. Bacillus probiotics: spore germination in the gastrointestinal tract. Appl. Environ Microb. 2002;68(5):2344–2352.
- Leser TD, Knarreborg A, Worm J. Germination and outgrowth of Bacillus subtilis and Bacillus licheniformis spores in the gastrointestinal tract of pigs. J Appl Microbiol. 2008;104(4):1025–1033.
- Hoa TT, Duc LH, Isticato R, et al. Fate and dissemination of Bacillus subtilis spores in a murine model. Appl Environ Microbiol. 2001;67(9):3819–3823.
- United States Pharmacopeia. Monograph for Bacillus coagulans GBI-30, 6086, first supplement, vol. 9. FCC, 2014;1816–1824.
- Haldar L, Gandhi DN. Effect of oral administration of Bacillus coagulans B37 and Bacillus pumilus B9 strains on fecal coliforms, Lactobacillus and Bacillus spp. in rat animal model. Vet World. 2016;9(7):766–772.
- Chau CF, Huang YL, Chang FY. Effects of fibre derived from passion fruit seed on the activities of ileum mucosal enzymes and colonic bacterial enzymes in hamsters. J Sci Food Agric. 2005;85(12):2119–2124.
- Huang YL, Chu HF, Dai FJ, et al. Intestinal health benefits of the water-soluble carbohydrate concentrate of wild grape (Vitis thunbergii) in hamsters. J Agric Food Chem. 2012;60(19):4854–4858.
- Martínez JE. Hyperthermophilic microorganisms and USP hot water systems. Pharm Technol. 2004;28(2):50–65.
- Amaha M, Ordal ZJ, Touba A. Sporulation requirements of Bacillus coagulans var. thermoacidurans in complex media. J Bacteriol. 1956;72(1):34–41.
- Elshaghabee FM, Rokana N, Gulhane RD, et al. Bacillus as potential probiotics: status, concerns, and future perspectives. Front Microbiol. 2017;8:1–15.
- Etheridge RD, Seerley RW, Huber TL. The effect of diet on fecal moisture, osmolarity of fecal extracts, products of bacterial fermentation and loss of minerals in feces of weaned pigs. J Anim Sci. 1984;58(6):1403–1411.
- Ara K, Meguro S, Hase T, et al. Effect of spore-bearing lactic acid-forming bacteria (Bacillus coagulans SANK 70258) administration on the intestinal environment, defecation frequency, fecal characteristics and dermal characteristics in humans and rats. Microb Ecol Health Dis. 2002;14(1):4–13.
- Grider JR, Piland BE. The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):429–437.
- Barcelo A, Claustre J, Moro F, et al. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut. 2000;46(2):218–224.
- Snyder JD, Walker A. Structure and function of intestinal mucin: developmental aspects. Int Arch Allergy Immunol. 1987;82(3–4):351–356.
