ABSTRACT
4-(2-Hydroxyphenethyl)-2,6-dimethoxyphenol, a bibenzyl, was isolated from the leaves of Empetrum nigrum var. japonicum, collected from Mount Tateyama. Japanese rock ptarmigans frequently eat the leaves and fruits of this plant. The structure of the bibenzyl was confirmed by NMR spectroscopic analysis and fully characterized. A synthesis of this compound was accomplished by coupling 2-hydroxyphenylacetic acid with syringaldehyde, decarboxylation of the resultant isoaurones, and hydrogenation of the double bond in the corresponding stilbene. This compound displayed cytotoxic activity against human cancer cells (HCT116 and Hela cells) and leukemia cells (HL-60 cells). The present study suggests that this plant serves as a source of biologically active natural products. Also, our findings provide information on the secondary metabolites in the diet of Japanese rock ptarmigans.
Graphical abstract
Isolation, synthesis, and biological activities of a bibenzyl from Empetrum nigrum var. japonicum.
Empetrum nigrum var. japonicum is an evergreen plant that retains its leaves all year round [Citation1]. This plant mainly grows at high altitudes and in cold climates, such as hilly and mountainous areas. In Japan, it is distributed mainly in the Japan Alps and Hokkaido. Japanese rock ptarmigans (Lagopus muta japonica) in the Northern Japan Alps eat the leaves and fruits of Empetrum nigrum var. japonicum [Citation2]. The chemical constituents of related plant species such as Empetrum nigrum L. var. nigrum and Empetrum nigrum subsp. asiaticum have been reported [Citation3–Citation12]. Chalcones, dihydrochalcones, phenanthrenes, dihydrophenanthrenes, bibenzyls, and terpenoids were isolated from the leaves of Empetrum nigrum L. var. nigrum [Citation3−Citation7]. Dihydrochalcones, flavonols, and anthocyanins were isolated from the black crowberry (fruits) of this plant [Citation8,Citation9]. However, little is known about the chemical constituents of Empetrum nigrum var. japonicum [Citation13,Citation14]. In this study, 4-(2-hydroxyphenethyl)-2,6-dimethoxyphenol, a bibenzyl, was isolated from the leaves of Empetrum nigrum var. japonicum. The structure of the bibenzyl was determined by 1D and 2D NMR spectroscopic analysis. Synthesis of this compound was achieved and allowed its complete structural confirmation and biological evaluation. Evaluation of the biological activities revealed that the bibenzyl exhibited cytotoxic activity against human cancer cells such as human colon carcinoma HCT116 cells, human cervix epitheloid carcinoma Hela cells, and human promyelocytic leukemia HL-60 cells.
Materials and methods
General information
All solvents and reagents were used without further purification unless otherwise noted. Analytical TLC was performed using Silica gel 60 F254 plates (0.25 mm) (Merck, Darmstadt, Germany). Flash column chromatography was performed using silica gel (SiliaFlash F60, particle size 40–63 μm, 230–400 mesh ASTM) (SiliCycle, Québec, Canada). Melting point (Mp) data were determined using a MM-2 instrument (Shimadzu corp., Kyoto, Japan) and were uncorrected. IR spectra were recorded on a FT-720 spectrometer (Horiba, Kyoto, Japan), using NaCl (neat) or KBr pellets (solid). 1H and proton-decoupled 13C (13C{1H}) NMR spectra were recorded on a Bruker Avance 400 spectrometer (400 and 100 MHz, respectively) (Bruker, Billerica, MA), using chloroform-d (CDCl3), acetone-d6 or methanol-d4 (CD3OD) as a solvent. Chemical shift values are expressed in δ (ppm) relative to tetramethylsilane (TMS, δ 0.00 ppm) or the residual solvent resonance (CDCl3, δ 77.0 ppm for 13C NMR; acetone-d6, δ 2.04 ppm for 1H NMR and δ 29.4 ppm for 13C NMR; CD3OD, δ 3.30 ppm for 1H NMR and δ 49.0 ppm for 13C NMR). Data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, m = multiplet), coupling constants (J; Hz), and integration. Mass spectra were obtained by a JEOL JMS-700 double-focusing mass spectrometer (JEOL, Tokyo, Japan) using fast atom bombardment (FAB). FAB mass spectra of compounds 1 and 5 were measured using a 2:3 (v/v) mixture of glycerol and 3-nitrobenzyl alcohol as a matrix in positive ion mode. The pH was adjusted with a glass electrode type hydrogen ion concentration indicator HM-30R (DKK-TOA corporation). The optical density at 550 nm (OD550) of each bacterial cell suspension was measured by Ultrospec 2100 pro (GE healthcare). Colorimetric changes were measured using a Spectra Max 190 microplate reader (Molecular Devices).
Collection of Empetrum nigrumvar. japonicum
The leaves and stems of Empetrum nigrum var. japonicum were collected in Mt. Tateyama with permissions from Ministry of the Environment of Japan (No. 1,605,111), Forestry Agency of Japan (No.172 and No. 320 in 2016), and Toyama prefecture (No. 117) in accordance with the Environment Research and Technology Development Fund (4–1604).
Extraction and purification of compounds
The leaves (3 g) of Empetrum nigrum var. japonicum were pulverized and suspended with methanol (100 mL). The suspension was stirred at room temperature for 1 day. The mixture was filtered. The filtrate was concentrated to afford a crude extract (803 mg). This crude extract was separated by silica gel column chromatography with hexane/EtOAc (4/1 to 1/1) to give fractions 1–6. Fraction 6 (116.2 mg) was separated by silica gel column chromatography with hexane/EtOAc (2/1) to give benzoic acid (51.6 mg) and compound 1 (23.7 mg).
Physical properties of 4-(2-hydroxyphenethyl)-2,6-dimethoxyphenol (1)
Mp = 111–114°C; IR (KBr) νmax = 3395, 2957, 2934, 2852, 2835, 1615, 1592, 1521, 1507, 1464, 1459 cm−1; 1H and 13C{1H} NMR data in acetone-d6, see ; 1H NMR (400 MHz, CD3OD) δ 6.99 (dd, J = 8.0, 1.7 Hz, 1H), 6.94 (ddd, J = 8.0, 7.4, 1.7 Hz, 1H), 6.74 (dd, J = 8.0, 1.2 Hz, 1H), 6.68 (ddd, J = 8.0, 7.4, 1.2 Hz, 1H), 6.40 (s, 2H), 3.76 (s, 6H), 2.85–2.81 (m, 2H), 2.80–2.75 (m, 2H); 13C{1H} NMR (100 MHz, CD3OD) δ 156.3, 148.9 (2C), 134.6, 134.3, 131.4, 129.4, 127.9, 120.4, 115.8, 106.7 (2C), 56.6 (2C), 37.2, 33.9; HRMS (FAB/double-focusing MS) m/z: [M]+ Calcd for C16H18O4 274.1205; Found 274.1208.
Table 1. 1H and 13C NMR spectroscopic data for compound 1.a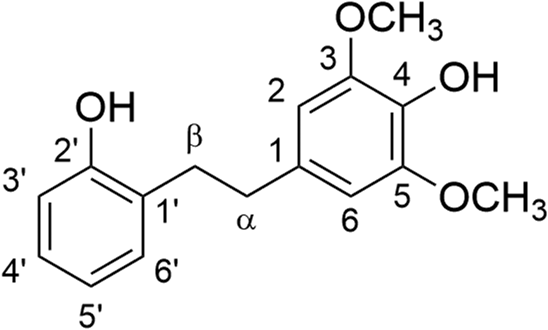
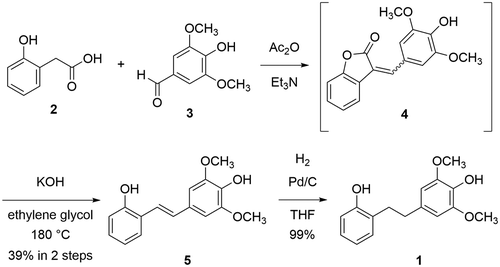
Synthesis of (E)-4-(2-hydroxystyryl)-2,6-dimethoxyphenol (5)
A solution of 2-hydroxyphenylacetic acid (2) (2.28 g, 15.0 mmol), syringaldehyde (3) (2.73 g, 15.0 mmol) in a mixture of triethylamine (3.8 mL) and acetic anhydride (4.2 mL) was stirred at 110°C for 5 h. The mixture was concentrated. The resulting residue was passed through the short pad of silica gel to yield a crude isoaurone 4 (3.10 g). A solution of the crude isoaurone 4 (3.10 g) and potassium hydroxide (85%, 5.80 g, 87.9 mmol) in ethylene glycol (58 mL) was stirred at 180°C for 1.5 h. The reaction was quenched by the addition of 1 M aqueous HCl solution. The mixture was diluted with EtOAc. The layers were separated, the organic layer was washed with water, brine, dried over Na2SO4, and concentrated. The residue was purified by silica gel chromatography (hexane/EtOAc = 3/1) to yield 5 (1.58 g, 39% over two steps). Mp = 89–90°C; IR (KBr) νmax = 3481, 3415, 3008, 2964, 2840, 1612, 1599, 1585, 1456 cm−1; 1H NMR (400 MHz, CDCl3, TMS) δ 7.51 (d, J = 7.1 Hz, 1H), 7.21 (d, J = 16.3 Hz, 1H), 7.14 (dd, J = 7.8, 7.1 Hz, 1H), 7.04 (d, J= 16.3 Hz, 1H), 6.95 (dd, J = 7.8, 7.1 Hz, 1H), 6.82 (d, J = 7.8 Hz, 1H), 6.77 (s, 2H), 5.57 (s, 1H), 5.03 (s, 1H), 3.95 (s, 6H); 13C{1H} NMR (100 MHz, CDCl3) δ 152.8, 147.2 (2C), 134.7, 130.3, 129.2, 128.4, 127.1, 124.7, 121.2, 121.1, 115.9, 103.3 (2C), 56.3 (2C); HRMS (FAB/double-focusing MS) m/z: [M]+ Calcd for C16H16O4 272.1049; Found 272.1048.
Synthesis of 4-(2-hydroxyphenethyl)-2,6-dimethoxyphenol (1)
A suspension of 5 (706 mg, 2.59 mmol) and 10% palladium on carbon (90.1 mg) in THF (10 mL) was stirred under an H2 atmosphere at room temperature for 18 h. The mixture was filtered through a short pad of Celite and washed with EtOAc. The filtrate was concentrated to yield pure 1 (681 mg, 96%). The 1H NMR spectrum of synthetic 1 in CD3OD was identical with that of natural 1.
Cell culture
HCT116, Hela, HL-60, and Jurkat cells were purchased from the Riken Cell Bank (Tsukuba, Japan). BALL-1 cells were obtained from the Cell Resource Center for Biomedical Research, Tohoku University (Sendai, Japan). HCT116 and Hela cells were cultured at 37°C with 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) (Nacalai tesque, Kyoto, Japan) supplemented with 10% fetal bovine serum (FBS) (BioWest, Nuaillé, France). HL-60, Jurkat, and BALL-1 cells were cultured at 37°C with 5% CO2 in Roswell Park Memorial Institute (RPMI) 1640 medium (Nacalai tesque, Kyoto, Japan) supplemented with 10% FBS.
Evaluation of cytotoxic activity
Cell growth was evaluated using cell counting kit SF (Nacalai tesque, Kyoto, Japan) according to the manufacturer’s instructions based on the WST-8 [4-(3-(2-methoxy-4-nitrophenyl)-2-(4-nitrophenyl)-2H-tetrazol-3-ium-5-yl)-3-sulfobenzenesulfonate, sodium salt] assay [Citation15]. For the assay, the cells were cultured in a 96-well plate, with each well containing 5000 cells in a total volume of 100 μL. The concentration of dimethyl sulfoxide (DMSO) in the cell cultures was 0.1% (v/v). The plates also included blank wells (0 cells/100 μL) and control wells (5000 cells/100 μL). Adherent HCT116 and Hela cells were preincubated for 24 h before exposure to the test compounds. The plates were incubated with various concentrations of each compound for 48 h. At the end of incubation, 10 μL of the WST-8 solution was then added, and the resulting mixture was incubated for 2 h at 37°C. The absorbance values were then measured at 450 nm with a 96-well plate reader. Cell growth inhibition was evaluated as the ratio of the absorbance of the sample to that of the control.
Evaluation of antimicrobial activity
The broth microdilution method [Citation16] was used to determine the minimum inhibitory concentration (MIC) of the compounds against Escherichia coli (NBRC 3301) and Bacillus subtilis (NBRC 3134). Triplicate twofold serial dilutions of the test samples (90 µL/well) were prepared in sterile flat-bottom 96-well plates by dilution with Cation-adjusted Mueller-Hinton broth (CMHB) (Kyokuto Pharmaceutical Industrial Co. Ltd, Tokyo, Japan). After the bacterial cell suspension was adjusted to 1.5 × 108 CFU/mL, which is comparable to 0.5 McFarland turbidity standard [optical density at 550 nm (OD550) = 0.125], and diluted 30 times with phosphate-buffered saline (PBS) (Nacalai tesque, Kyoto, Japan) (5.0 × 106 CFU/mL), the adjusted cell suspension (10 µL) was added into the wells, except for the blank. The final concentration of bacteria in the assay was 5 × 105 CFU/mL, and that of DMSO was 1.28% v/v. The plates were incubated at 37°C for 18 h. After incubation, the MIC was determined as the lowest concentration at which no growth was observed in triplicate wells, visually and by measuring OD550. Ampicillin (Nacalai tesque, Kyoto, Japan) was used as a positive control.
Results
Isolation and structural characterization of 4-(2-hydroxyphenethyl)-2,6-dimethoxyphenol (1)
Separation of the extract from the leaves of Empetrum nigrum var. japonicum using silica gel yielded compounds 1 and benzoic acid. The molecular formula of C16H18O4 for compound 1 was determined by HRMS (FAB). The peak that corresponds to the molecular ion [M]+ was observed at m/z 274 in the FAB mass spectrum of 1 (Figure S10 in Supplementary Material) [Citation17]. The 1H and 13C NMR data for 1 are summarized in . The HMBC spectrum suggested that 1 is a bibenzyl, which has a 4-hydroxy-2,6-dimethoxylbenzyl moiety and a 2-hydroxybenzyl moiety. Taken together, the structure of 1 was determined to be 4-(2-hydroxyphenethyl)-2,6-dimethoxyphenol. Although the isolation of 1 from diseased Discorea mangenotiana [Citation18] and Dioscorea opposite [Citation19] was reported previously, only 1H NMR data for 1 was reported. A structural assignment and characterization for 1 was not made. In order to determine the structure of 1 unambiguously and to obtain larger quantities for biological experiments, a synthetic route toward 1 was examined.
Synthesis of 4-(2-hydroxyphenethyl)-2,6-dimethoxyphenol (1)
The synthesis of 4-(2-hydroxyphenethyl)-2,6-dimethoxyphenol (1) starts from 2-hydroxyphenylacetic acid (2) and syringaldehyde (3) (). A coupling of 2 with 3 using triethylamine and acetic anhydride gave a mixture of E and Z-aurone 4 [Citation20]. Without purification, crude 4 was treated with potassium hydroxide in ethylene glycol at 180°C. Hydrolysis of 4 and decarboxylation of the resultant carboxylate occurred to give E-stilbene 5 in 39% yield over two steps. Finally, hydrogenation of 5 gave 1 in 99% yield. The 1H NMR spectrum of synthetic 1 was identical with that of natural 1, indicating that the structure of 1 was unambiguously determined.
Biological activities of 4-(2-hydroxyphenethyl)-2,6-dimethoxyphenol (1)
The cytotoxic activity of 1 against five cancer cells (HCT116, Hela, HL-60, Jurkat and BALL-1 cells) was evaluated via the WST-8 method [Citation15] after treatment with test compounds for 48 h ( and Figure S10 in the Supplementary Information). SN-38, the active metabolite of irinotecan, was used as a positive control in this study. The 50% inhibitory concentrations (IC50s) of cell viability of 1 are summarized in . Compound 1 showed cytotoxic activity against HCT116, Hela, and HL-60 cells with IC50 values below 100 μM. On the other hand, this compound did not affect the proliferation of Jurkat and BALL-1 cells. The antibacterial activity of 1 against Gram-positive B. subtilis and Gram-negative E. coli was evaluated by broth microdilution assay [Citation16]. However, compound 1 did not show antibacterial activity against B. subtilis and E. coli at concentrations below 128 μg/mL (Figure S11 in the Supplementary Information).
Table 2. Cytotoxic activity of 1 against human cancer cells.a
Conclusion
In this study, the isolation, structural determination, synthesis, and biological evaluation of 4-(2-hydroxyphenethyl)-2,6-dimethoxyphenol (1) are reported. The structure of this compound was fully characterized by 1D and 2D NMR spectroscopy. The synthesis of 1 was accomplished in only three steps from commercially available 2-hydroxyphenylacetic acid and syringaldehyde. The synthesis enabled unambiguous structural confirmation and biological studies. The biological evaluation revealed that 1 exhibits cytotoxic activity against HCT116, Hela and HL-60 cells at concentrations below 100 μM. However, this compound is not toxic against Jurkat and BALL-1 cells at concentrations below 100 μM, nor is it toxic against B. subtilis and E. coli at concentrations below 128 μg/mL. Jurkat and BALL-1 cells are derived from T-cell and B-cell acute lymphoblastic leukemia, respectively. These results suggest that this compound might exert the growth inhibitory activity against cancer cells without affecting the growth of lymphocytes and enteric bacteria. Interesting biological activities and structure–activity relationships of structurally related natural products such as resveratrol [Citation21] and combretastatins [Citation22] have been reported. Thus, further biological studies including elucidation of the structure-activity relationships and the mechanism of action are currently underway. In addition, the present study gives useful information on the diet of Japanese rock ptarmigans because they frequently eat the leaves of Empetrum nigrum var. japonicum in the Northern Japan Alps. The exploration of other secondary metabolites in this plant is ongoing in our laboratories.
Author contributions
S.Tsuchida, K.U. and K.K. designed the research; S.O., R.K., N.T., S.Tsuchida, and K.U. performed the research. S.Tomoshige and K.K. analyzed the data; N.T., S.Tsuchida, K.U., S.Tomoshige and K.K. wrote the article.
Supplementary_material190823.doc
Download MS Word (1.4 MB)Acknowledgments
This research was supported by the Environment Research and Technology Development Fund [ERTDF/No. 4-1604 and 4-1903] of the Environmental Restoration and Conservation Agency of Japan.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental material
The supplemental data for this article can be accessed here.
Additional information
Funding
References
- Kim KC, Kim D, Kim SC, et al. Empetrum nigrum var. japonicum extract suppresses ultraviolet B-induced cell damage via absorption of radiation and inhibition of oxidative stress. Evid Based Complement Alternat Med. 2013. Article ID 983609.
- Kobayashi A, Nakamura H. Seasonal change of food items of the Japanese rock ptarmigan. Jpn J Ornith. 2011;60:200–215.
- Wollenweber E, Dörr M, Stelzer R, et al. Lipophilic phenolics from the leaves of Empetrum nigrum — chemical structures and exudate localization. Bot Acta. 1992;105:300–305.
- Arriaga-Giner FJ, Wollenweber E, Dörr M. Bibenzyl form crowberry leaves. Phytochemistry. 1993;33:725–726.
- Toiron C, Rumbero A, Wollenweber E, et al. A new skeletal triterpenoid isolated from Empetrum nigrum. Tetrahedron Lett. 1995;36:6559–6562.
- Jarevång T, Nilsson MC, Wallstedt A, et al. A bibenzyl from Empetrum nigrum. Phytochemistry. 1998;48:893–896.
- Muravnik LE, Shavarda AL. Leaf glandular trichomes in Empetrum nigrum: morphology, histochemistry, ultrastructure and secondary metabolites. Nord J Bot. 2012;30:470−481.
- Ogawa K, Sakakibara H, Iwata R, et al. Anthocyanin composition and antioxidant activity of the crowberry (Empetrum nigrum) and other berries. J Agric Food Chem. 2008;56:4457–4462.
- Jurikova T, Mlcek J, Skrovankova S, et al. Black crowberry (Empetrum nigrum L.) flavonoids and their health promoting activity. Molecules. 2016;21:1685.
- Krasnov EA, Ermilova EV, Kadyrova TV, et al. Phenolic components of Empetrum nigrum extract and the crystal structure of one of them. Chem Nat Compd. 2000;36:493–496.
- Li H, Jean S, Webster D, et al. Dibenz[b, f]oxepin and antimycobacterial chalcone constituents of Empetrum nigrum. J Nat Prod. 2015;78:2837–2840.
- Bao S, Wang Q, Bao W, et al. Structure elucidation and NMR assignments of a new dihydrochalcone from Empetrum nigrum subsp. asiaticum (Nakai ex H.Ito) Kuvaev. Nat Prod Res. 2018. DOI:10.1080/14786419.2018.1542390
- Zhang YL, Mei RQ, Liu X, et al. Chemical constituents of Empetrum nigrum var. japonicum. Chin Tradit Herbal Drugs. 2014;49:2293–2298.
- Zhao M, Huang SL, Xu YH, et al. Triterpenoids from Empetrum nigrum var. japonicum. Chin Tradit Herbal Drugs. 2018;49:69–74.
- Ishiyama M, Miyazono Y, Sasamoto K, et al. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta. 1997;44:1299–1305.
- Clinical and Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—ninth edition. Wayne, PA: CLSI document M07-A9; 2012.
- Takayama M, Fukai T, Nomura T, et al. Further study of the matrix effect on the extent of fragmentation in molecular ions M+· produced under fast atom bombardment (FAB) conditions. Int J Mass Spectrom Ion Proc. 1990;96:169–179.
- Kaganda NG, Adesanya SA. A new dihydrostilbene from diseased Dioscorea mangenotiana. J Nat Prod. 1990;53:1345–1346.
- Yang MH, Yoon KD, Chin YW, et al. Phenolic compounds with radical scavenging and cyclooxygenase-2 (COX-2) inhibitory activities from Dioscorea opposite. Bioorg Med Chem. 2009;17:2689–2694.
- Huang X, Liu J, Sheng J, et al. Decarboxylation of α,β-unsaturated aromatic lactones: synthesis of E-ortho-hydroxystilbenes from 3-arylcoumarins or isoaurones. Green Chem. 2018;20:804–808.
- Huang XT, Liu X, Xie ML, et al. Resveratrol: review on its discovery, anti-leukemia effects and pharmacokinetics. Chem Biol Interact. 2019;306:29–38.
- Seddigi ZS, Malik MS, Saraswati AP, et al. Recent advances in combretastatin based derivatives and prodrugs as antimitotic agents. MedChemComm. 2017;8:1592–1603.