ABSTRACT
ChlR is a MarR-type transcriptional regulator that activates the transcription of the chlAII-ho2-hemN operon in response to low oxygen conditions in the cyanobacterium Synechocystis sp. PCC 6803. Upon exposure to low oxygen conditions, ChlR activates transcription of the operon that encodes enzymes critical to tetrapyrrole biosynthesis under low oxygen conditions. We previously identified a super-activator variant, D35H, of ChlR that constitutively activates transcription of the operon. To gain insight into the low-oxygen induced activation of ChlR, we obtained eight additional super-activator variants of ChlR including D35H from pseudorevertants of a chlAI-disrupted mutant. Most substitutions were located in the N-terminal region of ChlR. Mapping of the substituted amino acid residues provided valuable structural insights that uncovered the activation mechanism of ChlR.
GRAPHICAL ABSTRACT
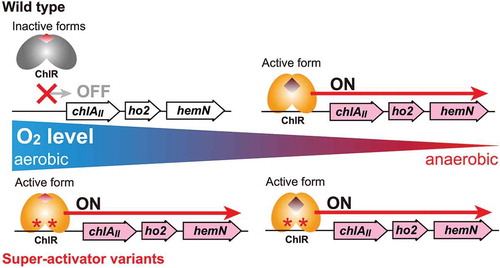
Super-activator variants of ChlR isolated in this work constitutively activate the transcription of the chlAII operon even under aerobic conditions where the wild type ChlR is inactive.
MarR-type transcriptional regulators comprise more than 19,000 members and are distributed among prokaryotes, playing pivotal roles in the regulation of genes involved in important physiological processes such as virulence, multiple antibiotics resistance, oxidative stress, and the degradation of aromatic compounds [Citation1–Citation4]. Transcription of target genes is regulated by MarR-type proteins converted into active or inactive forms upon binding of effector molecules such as organic compounds and antibiotics. Biochemical and molecular genetic analyses including X-ray crystal structure determination have been performed to elucidate the mechanisms of MarR transcriptional regulators, particularly the transcriptional repression/activation of target genes in response to various chemical effectors [Citation5–Citation16].
The MarR-type transcriptional regulators are relatively small proteins, with 130–180 amino acid residues, and they typically function as homodimers. The N-terminal region is known to be critical for the dimerization, and the conserved, centrally located winged helix-turn-helix serves as the DNA binding domain. Well-studied MarR proteins, MarR from Escherichia coli [Citation6] and a MarR homologue of Methanobacterium thermoautotrophicum, MTH313 [Citation9], bind to the operator regions of target genes (mar genes) without effectors, repressing the transcription. The ligand binding of phenolic compounds such as salicylate triggers conformational changes, leading to inactivation and dissociation of the MarR protein from operator regions, induction of the target genes, and eventually conferring multiple antibiotic resistance in the bacterial cells. OhrR, a MarR protein from Bacillus subtilis, is involved in the transcriptional regulation of the ohrA gene for organic peroxide resistance. OhrR is a repressor that binds to the operator regions of ohrA [Citation7]. Upon binding of cumene hydroperoxide, OhrR is inactivated by the oxidation at Cys residue 15 (Cys15) to sulfenic acid followed by S-bacillithiolation with S-bacillithiol [Citation17], resulting in dissociation from the operator region and derepressing the transcription of ohrA.
Thus, MarR-type transcriptional regulators sense environmental or intercellular factors to alter DNA binding capability through conformational changes. To understand the structural basis for sensing effectors and the conversion to inactive/active forms, X-ray crystal structures of many MarR family proteins, including MarR, MTH313, and OhrR, have been studied, revealing effector binding sites and their conformational changes. Structural comparisons between ligand-free and ligand-bound forms of Acinetobacter MarR family repressor HcaR involved in 4-hydroxycinnamic acid catabolism suggested that relatively subtle conformational changes affect the binding ability to DNA [Citation15].
Some MarR family regulators function as activators, such as ChlR [Citation11], BldR [Citation18], and LdtR [Citation14]. In contrast to many studies on repressors, few studies have reported on MarR family activators [Citation18]. It remains largely unknown how MarR family activators trigger the transcription of their target genes in response to effectors and environmental cues. ChlR is a cyanobacterial MarR family activator, identified in the cyanobacterium Synechocystis sp. PCC 6803 (Synechocystis 6803) [Citation11,Citation19]. ChlR is activated in low oxygen conditions, leading to the transcription of a small operon, chlAII-ho2-hemN, that encodes three enzymes in tetrapyrrole biosynthesis to produce chlorophyll, heme, and bilin pigments under low oxygen conditions. These enzymes, ChlAII, HO2, and HemN, bypass oxygen-dependent reactions catalyzed by ChlAI, HO1, and HemF, respectively, to support photosynthetic growth under low oxygen conditions ()) [Citation20–Citation22]. The induction of the chlAII operon by ChlR is critical for photosynthetic growth under low-oxygen conditions since the mutant lacking chlR does not grow photosynthetically under such conditions [Citation11]. In the process of identifying the chlR gene in the Synechocystis 6803 genome, we found that a single mutation in the coding region of chlR, a D35H substitution, resulted in the constitutively active form of ChlR, known as the “super-activator”, which led to the transcription of the chlAII operon even under aerobic conditions.
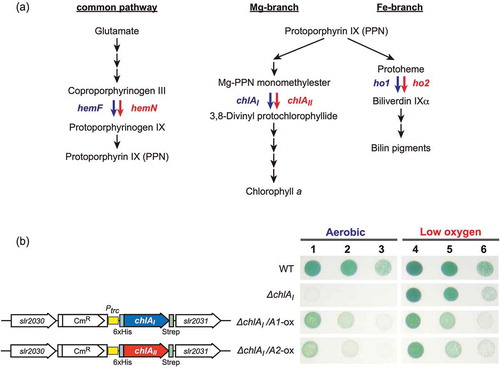
Figure 1. Chlorophyll, heme, and bilin biosynthesis pathways (a) and complementation of ∆chlAI with overexpression of chlAII (b).
(a) Chlorophyll, heme, and bilin biosynthesis pathways. In cyanobacteria, all tetrapyrrole pigments are produced from glutamate and shared with the common pathway from glutamate to protoporphyrin IX (PPN). Two biosynthetic pathways toward chlorophyll (Mg-branch) and heme/bilin (Fe-branch) diverge from PPN. Three reactions catalyzed by two different enzymes, aerobic and low-oxygen/anaerobic enzymes, are shown blue and red arrows, respectively. Coproporphyrinogen III oxidase in the common pathway is catalyzed by HemF (aerobic) and HemN (anaerobic). 3,8-divinyl protochlorophyllide formation from Mg-PPN monomethyester cyclase in the Mg-branch is catalyzed by two ChlA isoforms, ChlAI (aerobic) and ChlAII (low-oxygen). Biliverdin IXα formation from protoheme is catalyzed by two HO isoforms, HO1 (aerobic) and HO2 (low-oxygen), in the Fe-branch. (b) Photoautotrophic growth of WT, ∆chlAI, ΔchlAI mutants overexpressing ChlAI (ΔchlAI/A1-ox), and ChlAII (ΔchlAI/A2-ox). ∆chlAI/A1-ox, ∆chlAI/A2-ox, WT, and ΔchlAI cells were all grown under aerobic and low-oxygen conditions for 4 days. Spots 1, 4; 2, 5; and 3, 6 indicate that the initial cell densities were 1.0, 0.1 and 0.01 at OD730, respectively. Spots 1 to 3 and 4 to 6 were incubated under aerobic and low oxygen conditions, respectively. Gene arrangements of ∆chlAI/A1-ox and ∆chlAI/A2-ox at the genome neutral site (slr2030-slr2031) are shown (left). chlAI and chlAII were ectopically overexpressed under the trc promoter in ∆chlAI/A1-ox and ∆chlAI/A2-ox.
Another study on Synechococcus sp. PCC 7002 ChlR suggested that the formation of an oxygen-labile iron-sulfur cluster, a [4Fe-4S] cluster, on ChlR under low-oxygen conditions leads to its activation. Upon exposure of activated ChlR to oxygen, the [4Fe-4S] cluster is destroyed into a [2Fe-2S] cluster, resulting in the inactivation of ChlR [Citation23]. However, it is still unknown which Cys residues are involved in holding the [4Fe-4S] cluster, and there is no direct evidence that active ChlR carrying the [4Fe-4S] cluster binds to the operator to initiate the transcription of the chlAII operon. In addition, most of the MarR family proteins studied so far are repressors unlike ChlR; thus, the molecular mechanism for ChlR activation warrants further investigation.
In this study, to obtain insight into the activation mechanism of ChlR, we generated chlR-specific mutants via error-prone PCR (epPCR), followed with the positive screening of pseudorevertants from ∆chlAI. We then identified eight single amino acid substitutions including D35H that resulted in ChlR super-activators. Interestingly, most substitutions were located in the N-terminal region, with one being in the C-terminal region. Structural modeling of ChlR and comparison with known structures of other MarR-type regulators suggested that amino acid residues involved in the dimerization contribute to the stabilization of the active form of ChlR.
Materials and methods
Growth conditions
Synechocystis 6803 (strain YF [Citation11];) was cultivated in BG-11 buffered with 20 mM HEPES-KOH pH 8.2, under 50 µmolphoton m–2 s–1 (FLR40SW, Hitachi, Japan) [Citation20,Citation24], which was defined as normal light conditions for this study. For low-oxygen conditions, we used an anaerobic jar (BBL GasPak anaerobic systems, BD Biosciences; gas-generating kit anaerobic system, Oxoid, Basingstoke, Hants, UK). When necessary, we added 1.5% (w/v) BactoAgar (Difco), 15 µg mL–1 kanamycin and 10 µg mL–1 chloramphenicol.
Construction of plasmids
chlAI (sll1214) and chlAII (sll1874) DNA fragments were amplified by PCR from pASK3-ChlAI and pASK3-ChlAII (Minamizaki, Kawajiri and Fujita, unpublished) that contain the coding regions of chlAI and chlAII genes with primer pairs pNdeI1214f1 and pBgLII3plusStrep-r1, and pNdeI1874f1 and pBgLII3plusStrep-r1, respectively. These fragments were digested with NdeI and BglII, and cloned into pTCRH2031 [Citation25], in which chlAI and chlAII are expressed under the control of the trc promoter.
Transformation of ∆chlAI to obtain chlAI and chlAII overexpressing mutants
∆chlAI was grown under low-oxygen conditions. A 400-µL aliquot of ∆chlAI cell suspension and 5 µL of the plasmid solution were mixed and spread onto a nylon membrane (Hybond-N+) on BG-11 agar plate. The agar plate was incubated under normal light and low-oxygen conditions for 1 day of recovery. The nylon membrane was transferred onto a new BG-11 agar plate supplemented with 10 µg mL–1 chloramphenicol, and colonies were selected under normal light and low-oxygen conditions for two weeks. The chloramphenicol-resistant colonies were inoculated twice to complete the segregation of the mutant genome copy from the WT genome copy. The segregation was confirmed by colony PCR.
Preparation of epPCR DNA fragments for screening
To amplify the chlR gene the first cycle of PCR was performed with primers sll1512-f2 and sll1512-r2 [Citation11] using the ∆chlAI genomic DNA as a template. The resultant PCR fragment was used for epPCR. epPCR was performed with the same primers as above for 20 cycles (GeneMorph II Randam Mutagenasis Kit, Agilent Technologies). The fragment library obtained was used for screening pseudorevertants.
Screening of pseudorevertants from ∆chlAI with chlR amplified by epPCR
∆chlAI cells grown under low-oxygen conditions were suspended in 4 mL water (OD730 = 3.0). A 3.7-mL aliquot of the cell suspension was mixed with 1.9 µg of the DNA fragment library and spread onto 12 BG-11 agar plates (about 0.3 mL for each). The remaining 0.3 mL aliquot of the cell suspension was also spread onto an agar plate as a negative control to monitor the appearance of pseudorevertant colonies by spontaneous mutations. These 12 agar plates were incubated under low-oxygen conditions for 1 day and shifted to aerobic conditions for screening. During the screening for 10 days, the initial light intensity was maintained at 50 µmolphoton m–2 s–1 for 3 days and then increased to 65 µmolphoton m–2 s–1. Colonies appeared after 10 days ()). In total, 282 colonies were isolated as pseudorevertants by epPCR.
Figure 2. Isolation of pseudorevertants of ∆chlAI by transformation with the epPCR-amplified chlR gene fragments.
(a) Gene arrangement of chlR (slr1598-sll1512-sll1513) in the genome of Synechocystis 6803. To prepare DNA fragments to isolate pseudorevertants carrying super-activator mutations in chlR, a 2.3 kb fragment was amplified by epPCR (shown as a thick orange bar). Arrows indicate primers (sll1512-f2 and sll1512-r2 [Citation11]) used in epPCR. (b) Colonies of pseudorevertants appeared on an agar plate after transformation of ∆chlAI with epPCR DNA fragments (left). No colonies appeared without epPCR DNA fragments (right). (c) Photoautotrophic growth of pseudorevertants. Pseudorevertants were incubated under low-oxygen (lane 1) and aerobic (lane 2) conditions as were WT and ∆chlAI cells.
![Figure 2. Isolation of pseudorevertants of ∆chlAI by transformation with the epPCR-amplified chlR gene fragments.(a) Gene arrangement of chlR (slr1598-sll1512-sll1513) in the genome of Synechocystis 6803. To prepare DNA fragments to isolate pseudorevertants carrying super-activator mutations in chlR, a 2.3 kb fragment was amplified by epPCR (shown as a thick orange bar). Arrows indicate primers (sll1512-f2 and sll1512-r2 [Citation11]) used in epPCR. (b) Colonies of pseudorevertants appeared on an agar plate after transformation of ∆chlAI with epPCR DNA fragments (left). No colonies appeared without epPCR DNA fragments (right). (c) Photoautotrophic growth of pseudorevertants. Pseudorevertants were incubated under low-oxygen (lane 1) and aerobic (lane 2) conditions as were WT and ∆chlAI cells.](/cms/asset/b462fc8a-a668-4a20-8211-2d503fe1fddb/tbbb_a_1687281_f0002_oc.jpg)
Sequencing of chlR genes of ∆chlAI
The chlR genes in the pseudorevertants were amplified by colony PCR with sll1512-f1 and sl111512-r1 primers [Citation11] and their nucleotide sequences were determined by the Sanger method with seq-sll1512 as the sequence primer.
Growth test of ∆chlAI-derived pseudorevertants
Cells of the ∆chlAI-derived pseudorevertants were pre-cultured under low-oxygen conditions; these cells as well as WT and ∆chlAI cells were suspended in water, and diluted to adjust to 1.0 at OD730. The cell suspensions were spotted onto BG-11 agar plates and incubated under low-oxygen conditions and aerobic conditions under 50 µmolphoton m–2 s–1 for 3 days.
RNA extraction and determination of RNA concentration
Total RNA was extracted from cells grown on BG-11 agar plates under low-oxygen conditions for 4 days (for low-oxygen samples) and under aerobic conditions where cells grown under low-oxygen conditions for 3 days were shifted to aerobic conditions 1 day before RNA extraction (total 4 days for aerobic samples). Cells were harvested by centrifugation and 1 mL boiled TRIzol reagent (Life Technologies) was added to the cell pellets. The samples were vortexed vigorously and incubated for 2 min at room temperature. Chloroform (200 µL) was added to the samples, which were vortexed and centrifuged at 15,000 g for 10 min. The supernatant was collected to a new tube, and 320 µL ethanol was added. For further purification, the SV Total RNA Isolation System (Promega) was used. The samples were transferred to spin columns in collection tubes and centrifuged to discard the flow-through solution. The spin columns were washed with 600 µL RNA Wash Solution. A mixture of 40 µLYellow Core Buffer, 5 µL MnCl2 (0.09 M), and 5 µL DNaseI (2–4 U µL–1) was added to the spin columns. The spin columns were then incubated at 30ºC for 45 min, and 200 µL DNase Stop Solution was added. After centrifugation, the flow-through was discarded, and the spin columns were washed twice with 600 and 250 µL RNA Wash Solution. RNA was eluted from the columns with 20 µL nuclease-free water. Concentrations of extracted RNA samples were determined using the QuantiFluor™ RNA System (Promega).
Quantitative reverse transcription PCR
To synthesize cDNA, the extracted RNA was reverse-transcribed with ReverTra Ace and random primer (TOYOBO). The synthesized cDNAs were amplified using SYBR Premix Ex Taq II (Takara Bio) with primer sets for each target gene (Table S2). qPCR reaction was analyzed by StepOneTM Plus Real-Time PCR System (Life technologies). As an internal control, the house-keeping gene rrn16Sa that encodes 16S ribosomal RNA was used. Based on the comparative CT values, relative expression levels were calculated.
Structural modeling of ChlR
The putative structure of ChlR was generated by the SWISS-MODEL [Citation26], and we selected Enterococcus feacalis V583 EF0647 as the best model by using GMQE [Citation27] and QMEAN [Citation28] values as criteria.
Results
Overexpression of chlAII recovered aerobic growth of ∆chlAI
The chlAI-disrupted mutant ∆chlAI is lethal under light and aerobic conditions, where the gene chlAII encoding the hypoxic isoform ChlAII is not induced [Citation20]. To examine whether ChlAII is able to replace the role of ChlAI in vivo under aerobic conditions, we isolated a ChlAII overexpression transformant, ΔchlAI/A2-ox, from ∆chlAI as well as a ChlAI overexpression transformant, ΔchlAI/A1-ox ()). In these transformants, ΔchlAI/A1-ox and ΔchlAI/A2-ox, the chlAI and chlAII genes are constitutively expressed under the control of the trc promoter, respectively. Both transformants, ΔchlAI/A1-ox and ΔchlAI/A2-ox, recovered aerobic growth, although the growth was slightly slower than that of the wild type (WT) under aerobic conditions ()). This result clearly indicated that the hypoxic isoform ChlAII is able to replace the function of ChlAI when it is constitutively expressed under the trc promoter, although chlAII at the original operon is expressed only under low-oxygen conditions in WT cells.
Screening of ∆chlAI mutants with mutation(s) in the chlR gene by random epPCR
In the previous study, through genomic analysis of the pseudorevertant ∆ho1R, we identified a D35H variant of ChlR that constitutively activated the chlAII-ho2-hemN operon, which restored the photosynthetic growth of ∆ho1 under aerobic conditions [Citation11]. The phenotype of ∆ho1R indicated that the hypoxic isoform HO2 can replace the function of HO1. Given that the constitutive expression of chlAII is able to complement the lethal phenotype of chlAI ()), we expected that similar pseudorevertants would appear from ∆chlAI incubated under aerobic conditions by spontaneous mutation. Such pseudorevertants may have a mutation(s) in chlR for amino acid substitution(s) such as D35H, which makes ChlR a super-activator variant.
We isolated five pseudorevertants through aerobic incubation of ∆chlAI. Sequencing of the chlR gene in the pseudorevertants indicated that four of them carried the same mutation as D35H (G-to-C transversion at the 35th codon). Interestingly, one of them carried a new mutation in chlR, which was a G-to-T transversion causing an Asp-to-Ala amino acid substitution at the 16th codon (D16A). This result indicated that super-activator variants could be generated by amino acid substitutions other than D35H.
To further assess amino acid substitutions responsible for super-activator ChlR variants, we applied epPCR to the chlR gene. If amino acid substitutions convert ChlR to super-activator variants, colonies of ∆chlAI carrying such mutations should appear as pseudorevertants on an agar plate incubated under aerobic conditions. A DNA fragment (2,322 bp) covering of the chlR coding regions with upstream (slr1598) and downstream (sll1513) sequences was amplified by epPCR ()) to use for the transformation of ∆chlAI. ∆chlAI cells were mixed with the DNA fragments and exposed to aerobic conditions for the positive selection of pseudorevertants. We successfully isolated 282 colonies of ∆chlAI pseudorevertants ()). We confirmed by colony PCR that the original chlAI locus in the pseudorevertants remained as in the parental strain ∆chlAI, strongly suggesting that aerobic growth of these pseudorevertants was restored by the constitutive expression of chlAII caused by super-activator ChlR variants rather than by regaining the chlAI gene.
chlR super-activator variants that enable ∆chlAI to grow under aerobic conditions
Out of the 282 ∆chlAI pseudorevertant colonies, 108 colonies were selected; chlR gene from these colonies was then amplified by PCR and sequenced (Table S1). Most colonies (95) carried single mutations in the chlR coding region, and more than two mutations were detected in 12 colonies. One colony had no mutation in chlR. The following eight amino acid substitutions were found in the 95 colonies: D16Y, D32G, D35H, D35A, N36K, L41V, L41S, and I104L (). For more than half, 67 pseudorevertants carried the N36K substitution, which was caused by a T-to-A substitution. Such base-substitution bias may be caused by the DNA polymerase (Mutazyme II DNA polymerase) used in the epPCR mutagenesis, or it may indicate that the N36K super-activator is the most effective in activating the chlAII operon for the restoration of aerobic photosynthetic growth of ∆chlAI. There were three other amino acid substitutions (H29Y, R43Q, and L34I) in two colonies, 1–22 (H29Y and R43Q) and 1–34 (H29Y and L34I; and Table S1), that were not included in the eight amino acid substitutions. We did not determine which substitution was responsible for the restoration of the aerobic growth of ∆chlAI. However, since H29Y was the common substitution, H29Y might be important for the conversion of ChlR into a super-activator.
Table 1. List of mutations in the chlR gene in pseudorevertants from ∆chlAI.
We also isolated one mutant (No. 3–27) that grew very slowly but significantly under aerobic conditions without any mutations in the chlR gene ()). We analyzed the nucleotide sequence of the chlAII operon promoter region based on the assumption that a mutation in this region might trigger constitutive transcription of the chlAII operon, even under aerobic conditions. We found a C-to-T conversion 108 bp upstream from the initial codon of the chlAII gene (Figure S1(a)). This mutation suggested that the pseudorevertant 3–27 produces ChlAII even under aerobic conditions to support the photosynthetic growth of ∆chlAI; it does so by the leaky expression of the chlAII operon by a mutated promoter, independent of ChlR.
We performed transformation analysis [Citation11] to confirm that mutations in the chlR gene are responsible for aerobic growth of these pseudorevertants (Figure S2). The chlR gene fragments were amplified by PCR from colonies of the pseudorevertants, and spotted onto lawn of ∆chlAI on an agar plate followed by aerobic incubation [Citation11]. Colonies appeared only on the areas where the mutated chlR gene fragments were spotted except for WT and 3–27, which is consistent with the idea the mutations in the chlR gene are responsible for the restoration of aerobic growth of chlAI.
Growth of ∆chlAI epPCR mutants
We compared the growth of nine pseudorevertants: 3–4, 6–35, 6–5, 1–11, 6–3, 6–7, 6–21, 6–23, and 3–27, possessing amino acid substitutions D16Y, D32G, D35H, D35A, N36K, L41V, L41S, I104L, and the – 108 mutation in the chlAII promoter, respectively, under aerobic and low-oxygen conditions ()). All the strains grew comparably under low oxygen conditions, and the growth was comparable to that of WT and ∆chlAI. Under aerobic conditions, there was no growth of ∆chlAI, but all mutants with mutations in chlR showed growth that was comparable to that of WT. Only 3–27, which has the chlAII promoter mutation, showed very slow but significant aerobic growth, suggesting that the expression level of chlAII in 3–27 is not high enough to support aerobic growth comparable to that of WT.
Expression levels of chlAII and chlR
We compared the transcript levels of chlAII and chlR in these pseudorevertants grown under aerobic and low-oxygen conditions by quantitative RT-PCR (). In the WT, the transcript level of chlAII in cells grown under low-oxygen conditions was about 10 times higher than that of cells grown under aerobic conditions, and this induction rate was almost maintained in ∆chlAI. In contrast, in the pseudorevertants, the transcript levels of chlAII in cells grown under aerobic conditions increased 40- to 200-fold over that of the WT. The enhanced level of the chlAII transcript under aerobic conditions is in good agreement with the hypothesis that the aerobic growth of the pseudorevertants is restored by the constitutive expression of chlAII, which replaces the function of chlAI. When comparing the chlAII transcript levels under low-oxygen and aerobic conditions in the individual pseudorevertants, the difference was within about 10-fold (0.3–7.0), indicating that chlAII is constitutively transcribed in the pseudorevertants irrespective of the oxygen levels ()).
Figure 3. Transcript levels of chlAII (a) and chlR (b) in the pseudorevertants. Transcript levels of chlAII and chlR in pseudorevertant cells grown under aerobic (blue) and low oxygen (pink) conditions were determined by real-time PCR. Relative transcript levels to those of rrn16 in the wild type grown under aerobic conditions were set to 1.0. Bars are SDs in technical triplicates. The relative transcript levels are shown on a logarithmic scale and a linear scale for chlAII (a) and chlR (b), respectively.
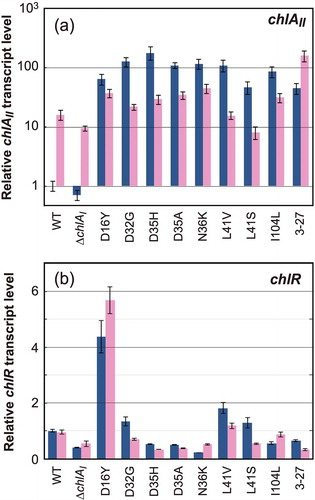
The transcript levels of chlR were almost the same in all pseudorevertants as the WT except D16Y ()). This finding supported the idea that the constitutive expression of chlAII is caused by the action of a ChlR super-activator, a qualitative effect, rather than an increase in the amount of ChlR. Only the transcript level of chlR in the pseudorevertant D16Y was enhanced to about 4 to 6 times higher than that of the WT ()), which was probably due to a second mutation (A-to-T) 14 bp upstream of the initial codon of chlR in addition to the G-to-T mutation for D16Y in the chlR coding region (Figure S1(b)). This second mutation in the promoter region caused the increase in chlR transcript levels, which may have the increased content of ChlR and compensated for insufficient constitutive activation of ChlR-D16Y. For future work, it will be essential to evaluate the effect of D16Y-ChlR as a super-activator without the promoter mutation.
Discussion
In this study, through epPCR and positive screening for pseudorevertants that restored photosynthetic growth under aerobic conditions in ∆chlAI, we identified new seven additional amino acid substitutions (D16Y, D32G, D35A, D36K, L41V, L41S, and I104L) that resulted in ChlR super-activators in addition to D35H.
The amino acid substitutions identified in this study were plotted on the ChlR amino acid sequence, as a histogram of the numbers of pseudorevertants ()). All substitutions except for I104L are located in the N-terminal region. To evaluate structurally the amino acid substitutions in the super-activator variants of ChlR, we perform calculation for building of a putative ChlR structural model. The best model (residues 20–123) was generated using a MarR homologous protein EF0647 from E. faecalis V583 (PDB ID:1Z7U) as a template structure (sequence identity: 33%; ). The resulting structural model of ChlR was constructed as in a dimeric state because homodimer structure is one of the common features of MarR-type transcriptional regulators. The function of EF0647 used as the template is still unknown. Therefore, to validate structural feasibility of the ChlR model, we compared it with the structure of OhrR from B. subtilis, a well-characterized MarR family protein (sequence identity: 24%; ). The superposition confirmed high similarity between them. Namely, the arrangement of secondary structure of ChlR model is almost same to that of OhrR ()) and the monomeric structures are superimposable (), its r.m.s. deviation is 1.5 Å). Particularly, the structures of the helix α3-α4 and strands β2-β3, which directly interact with the major groove of the target DNA [Citation7,Citation15], are almost identical. Two notable structural differences are found; one is the presence of an additional helix α6 in the C-terminal region of OhrR, and the other is the configuration of the helix α1. These structural differences may reflect differences in environmental signals that they sense.
Figure 4. Mapping of the amino acid residues responsible for super-activator variants of ChlR.
(a) ChlR super-activator substitutions in pseudorevertants generated by transformation with epPCR chlR fragments are plotted against the horizontal axis of the ChlR amino acid sequence. Red asterisks indicate amino acid residues that turn ChlR into a super-activator form via a single substitution. H29 indicated with a gray asterisk is accompanied with other substitutions in ChlR. Color of bars and amino acid residues in the horizontal axis indicate property of the substituted amino acid residues; red, acidic (D and E); blue, basic (H, K, and R); gray, aromatic (F, W, and Y); green, polar (C, N, Q, S, and T); and black, nonpolar (A, G, I, L, M, P, and V). (b) Multiple alignments of ChlR amino acid sequences, 13 probable ChlR orthologs from other cyanobacteria, and EF0647 from E. feacalis V583, and OhrR (B.subtilis). Secondary structure of EF0647, OhrR, and predicted ChlR structure are shown: orange bars, α helices 1–6; green arrows, β sheets 1–3. In the structures of EF0647 and ChlR the β1 sheet is missing but for consistency in the names of β2 and β3 sheets are adopted. Syn_6803, Synechocystis sp. PCC 6803; Leptol_7376, Leptolyngbya sp. PCC 7376; Syn_7002, Synechococcus sp. PCC 7002; L_boryana, Leptolyngbya boryana dg5; Oscil_7112, Oscillatoria nigro-viridis PCC 7112; Psanab_6802, Pseudoanabaena sp. PCC 6802; Acaryoc_MBIC11017, Acaryochloris marina MBIC11017; Acaryoc_CCMEE5410, Acaryochloris marina CCMEE5410; Oscil_6506, Oscillatoria sp. PCC 6506; Tricho_IMS101, Trichodesmium erythraeum IMS101; Arthr_NIES-39, Arthrospira platensis NIES-39; Arthr_8005, Arthrospira sp. PCC 8005; Leptol_6406, Leptolyngbya sp. PCC 6406; LeptolHoron; Leptolyngbya sp. Heron Island J; EnterocEF0647, E. feacalis V583; and BsOhrR, B. subtilis. (c) Superposition of ChlR putative model (light yellow) and the BsOhrR crystal structure (light blue) in the protomer. (d, e) Structural comparison between OhrR (d) and the ChlR putative dimeric model (e) in dimeric state. Six amino acid residues of OhrR (K27, K30, L33, D34, T39, and L107) corresponding to H29, D32, D35, D36, L41 and I104 of ChlR, respectively, are shown in red as the amino acid residues in the OhrR protein (d). The six amino acid residues responsible for the super-activation variants are shown in red (e).
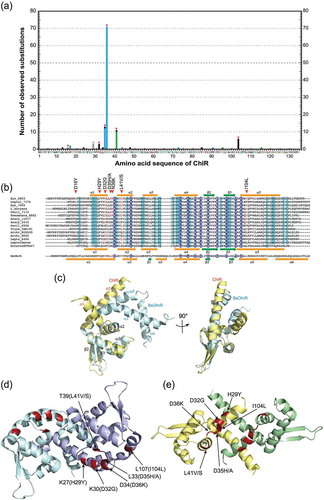
Comparison of the dimer structures between the ChlR model ()) and OhrR ()) demonstrates that configuration of the dimer is different, probably due to the configurational differences in the helix α1 ()). The N-terminal helices α1–α2 and the connecting loop are mainly responsible for the dimer interaction in both structures. As shown in the histogram ()), the amino acid substitutions in the super-activator variants are mainly distributed in the N-terminal domain in ChlR; the substitutions of H29Y and D32G are in the helix α1, those of D35H/A and N36K are in the connecting loop, and L41V/S in helix α2 ()). Interestingly, in this model the substitution I104L in the C-terminal region is located close to the helix α1. These substitutions may affect the interaction for the dimer formation, resulting in stabilization of the active-dimeric structure of ChlR. Of course, further biochemical experiments are needed including the contribution of the Fe-S cluster to the conformational change in response to the oxygen level.
In MarR of E. coli, amino acid substitutions D26N, G95S, V132M, and L135F result in the constitutive active forms, “super-repressors”, which bind constitutively to the operator even in the presence of the effector [Citation5]. The constitutive repression of MarR and the constitutive activation of ChlR may share the common features in which the structures are fixed as the DNA binding form irrespective of their effectors. In particularly, D26N substitution in the MarR super-repressor variants is located in the helix α1 [Citation12], suggesting that this substitution corresponds to D35H/A and D36K in the super-activator variants of ChlR.
It remains unclear why 3–27 showed a slow growth phenotype under aerobic conditions, though the mRNA level of 3–27 was almost comparable to those of other pseudorevertants (). The C-to-T transition 108 bp upstream of the initial codon of chlAII could be responsible for the constitutive expression of chlAII (Figure S1). However, this mutation is outside the motif TTCCCN4GGAAA, which we proposed previously as the putative cis element recognized by ChlR [Citation11]. Given that the wild-type ChlR does not bind to operator DNA under aerobic conditions [Citation11], the constitutive expression of chlAII in 3–27 may be caused by RNA polymerase or some other unknown proteins, independent ChlR.
Further study is needed to clarify the expression of chlAII in 3–27. In addition, the cis-element recognized by ChlR should be identified using an appropriate reporter system such as CnfR [Citation29]. Such information on ChlR and the cis element is indispensable for understanding the molecular mechanism by which ChlR activates transcription of the chlAII operon under low-oxygen conditions. The mutant ∆chlAI provides another unique and efficient system to glean insights into the structure-function relationship of ChlR via isolation of pseudorevertants under aerobic conditions, in addition to the ∆ho1 mutant [Citation11].
Author contributions
YF conceived and supervised the study; YH and YF designed experiments; YK performed experiments of ∆chlAI/A1-ox and ∆chlAI/A2-ox experiments; YH performed the isolation of ChlR super-activator variants experiments; HaY performed determination of transcript levels of chlAII and chlR and transformation analysis; KW constructed the ChlR model and structural comparison; YH, HiY, HaY, KW, and YF wrote the manuscript.
HiraideY_SI_R1x.docx
Download MS Word (1.7 MB)Acknowledgments
We thank Masahiko Ikeuchi and Rei Narikawa (University of Tokyo) for generous gift of pTCRH2031. We thank Tatsuo Omata, Takafumi Yamashino, members of the Laboratories of Molecular and Functional Genomics and Photosynthesis Research (formerly Laboratory of Molecular Plant Physiology) (Nagoya University), and Kazuki Terauchi (Ritsumeikan University) for their valuable discussions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementry material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Seoane AS, Levy SB. Characterization of MarR, the repressor of the multiple antibiotic resistance (mar) operon in Escherichia coli. J Bacteriol. 1995;177:3414–3419.
- Ellison DW, Miller VL. Regulation of virulence by members of the MarR/SlyA family. Curr Opin Microbiol. 2006;9:153–159.
- Perera IC, Grove A. Molecular mechanisms of ligand-mediated attenuation of DNA binding by MarR family transcriptional regulators. J Mol Cell Biol. 2010;2:243–254.
- Deochand DK, Grove A. MarR family transcription factors: dynamic variations on a common scaffold. Crit Rev Biochem Mol Biol. 2017;52:595–613.
- Alekshun MN, Levy SB. Characterization of MarR superrepressor mutants. J Bacteriol. 1999;181:3303–3306.
- Alekshun MN, Levy SB, Mealy TR, et al. The crystal structure of MarR, a regulator of multiple antibiotic resistance, at 2.3 Å resolution. Nat Struct Biol. 2001;8:710–714.
- Hong M, Fuangthong M, Helmann JD, et al. Structure of an OhrR-ohrA operator complex reveals the DNA binding mechanism of the MarR family. Mol Cell. 2005;20:131–141.
- Newberry KJ, Fuangthong M, Panmanee W, et al. Structural mechanism of organic hydroperoxide induction of the transcription regulator OhrR. Mol Cell. 2007;28:652–664.
- Saridakis V, Shahinas D, Xu X, et al. Structural insight on the mechanism of regulation of the MarR family of proteins: high-resolution crystal structure of a transcriptional repressor from Methanobacterium thermoautotrophicum. J Mol Biol. 2008;377:655–667.
- Poor CB, Chen PR, Duguid E, et al. Crystal structures of the reduced, sulfenic acid, and mixed disulfide forms of SarZ, a redox active global regulator in Staphylococcus aureus. J Biol Chem. 2009;284:23517–23524.
- Aoki R, Takeda T, Omata T, et al. MarR-type transcriptional regulator ChlR activates expression of tetrapyrrole biosynthesis genes in response to low-oxygen conditions in cyanobacteria. J Biol Chem. 2012;287:13500–13507.
- McMurry LM, Levy SB. Amino acid residues involved in inactivation of the Escherichia coli multidrug resistance repressor MarR by salicylate, 2,4-dinitrophenol, and plumbagin. FEMS Microbiol Lett. 2013;349:16–24.
- Duval V, McMurry LM, Foster K, et al. Mutational analysis of the multiple-antibiotic resistance regulator MarR reveals a ligand binding pocket at the interface between the dimerization and DNA binding domains. J Bacteriol. 2013;195:3341–3351.
- Pagliai FA, Gonzalez CF, Lorca GL. Identification of a ligand binding pocket in LdtR from Liberibacter asiaticus. Front Microbiol. 2015;6:1314.
- Kim Y, Joachimiak G, Bigelow L, et al. How aromatic compounds block DNA binding of HcaR catabolite regulator. J Biol Chem. 2016;291:13243–13256.
- Guo J, Zhang X, Lu X, et al. SAV4189, a MarR-family regulator in Streptmyces avermitilis, activates avermectin biosynthesis. Front Microbiol. 2018;9:1358.
- Chi BK, Gronau K, Mäder U, et al. S-bacillithiolation protects against hypochlorite stress in Bacillus subtilis as revealed by transcriptomics and redox proteomics. Mol Cell Proteomics. 2011;10:M111.009506.
- Di Fiore A, Fiorentino G, Vitale RM, et al. Structural analysis of BldR from Sulfolobus solfataricus provides insights into the molecular basis of transcriptional activation in archaea by MarR family proteins. J Mol Biol. 2009;388:559–569.
- Fujita Y, Tsujimoto R, Aoki R. Evolutionary aspects and regulation of tetrapyrrole biosynthesis in cyanobacteria under aerobic and anaerobic environments. Life (Basel). 2015;5:1172–1203.
- Minamizaki K, Mizoguchi T, Goto T, et al. Identification of two homologous genes, chlAI and chlAII, that are differentially involved in isocyclic ring formation of chlorophyll a in the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem. 2008;283:2684–2692.
- Aoki R, Goto T, Fujita Y. A heme oxygenase isoform is essential for aerobic growth in the cyanobacterium Synechocystis sp. PCC 6803: modes of differential operation of two isoforms/enzymes to adapt to low oxygen environments in cyanobacteria. Plant Cell Physiol. 2011;52:1744–1756.
- Goto T, Aoki R, Minamizaki K, et al. Functional differentiation of two analogous coproporphyrinogen III oxidases for heme and chlorophyll biosynthesis pathways in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2010;51:650–663.
- Ludwig M, Pandelia ME, Chew CY, et al. ChlR protein of Synechococcus sp. PCC 7002 is a transcription activator that uses an oxygen-sensitive [4Fe-4S] cluster to control genes involved in pigment biosynthesis. J Biol Chem. 2014;289:16624–16639.
- Yamazaki S, Nomata J, Fujita Y. Differential operation of dual protochlorophyllide reductases for chlorophyll biosynthesis in response to environmental oxygen levels in the cyanobacterium Leptolyngbya boryana. Plant Physiol. 2006;142:911–922.
- Ishizuka T, Shimada T, Okajima K, et al. Characterization of cyanobacteriochrome TePixJ from a thermophilic cyanobacterium Thermosynechococcus elongatus strain BP-1. Plant Cell Physiol. 2006;47:1251–1261.
- Bienert S, Waterhouse A, de Beer TA, et al. The SWISS-MODEL repository-new features and functionality. Nucleic Acids Res. 2017;45:D313–D319.
- Waterhouse A, Bertoni M, Bienert S, et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–W303.
- Benkert P, Biasini M, Schwede T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics. 2011;27:343–350.
- Tsujimoto R, Kamiya N, Fujita Y. Identification of a cis-acting element in nitrogen fixation genes recognized by CnfR in the nonheterocystous nitrogen-fixing cyanobacterium Leptolyngbya boryana. Mol Microbiol. 2016;101:411–424.
