ABSTRACT
To evaluate the suitability of the new nutritional composition of renewed commercial Formula A (protein reduced to 2.2 g/100 kcal, arachidonic acid increased to 13.2 mg/100 kcal, and docosahexaenoic acid maintained at 20 mg/100 kcal), we examined whether the growth of Formula A-fed infants was equivalent to that of breastfed infants. In this observational study, 1,053 infants were followed-up to 12 months. Growth, stool consistency, and the health condition of 99 infants fed with Formula A and 295 breastfed infants were compared. Body weight, body mass index, and head circumference of Formula A-fed infants were similar to those of breastfed infants. Additionally, there were no differences in the stool consistency and the health condition (infection and allergy prevalence) between the two groups. Formula A-fed infants grew as well as breastfed infants, suggesting the appropriate nutritional composition of Formula A. The findings may contribute to further improvements in infant formulas.
Graphical abstract
The equivalent growth for Formula A-fed and breastfed infants.
To confirm the appropriateness of the nutritional composition of an infant formula, the growth pattern of infants fed with the formula should be evaluated [Citation1–Citation4]. However, only a limited number of products have been assessed for suitability through evaluating infant growth.
Protein intake during infancy can directly affect growth and later health outcomes. For infants to grow appropriately, protein intake must comprise neither excess nor insufficiency. Insufficient intake of protein could affect the growth and development of infants [Citation3]. Excessive intake of protein during infancy has been reported to affect future health and contribute to diseases such as obesity [Citation5,Citation6]; therefore, it is highly desirable to assess infant growth whenever the protein content in an infant formula is changed.
There has been much focus on the importance of docosahexaenoic acid (DHA) and arachidonic acid (ARA) intake for the growth and development of infants. During infancy, DHA and ARA rapidly accumulate in the brain and the retina [Citation7,Citation8]. A correlation between head circumference (HC) and brain volume [Citation9–Citation11] and an association between DHA and ARA intake during infancy and HC growth [Citation12] has been observed. Furthermore, DHA and ARA intake [Citation13,Citation14] and HC growth [Citation9,Citation10] can both affect visual and cognitive functions. Due to low biosynthesis of DHA and ARA in infancy [Citation15], a suitable DHA and ARA composition of infant formula is important.
In the renewed commercial infant formula A (Formula A), the protein concentration was reduced to 2.2 g/100 kcal, and the ARA concentration was increased to 13.2 mg/100 kcal. Here, we evaluated the suitability of Formula A via a 12-month follow-up study, assessing growth parameters, stool consistency, and health conditions of Formula A-fed infants compared to breastfed infants.
Methods
Study participants and design
This observational follow-up study, registered in the University Hospital Medical Information Network (UMIN) Clinical Trial Registry (UMIN000023110) (http://www.umin.ac.jp/), was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Meiji Institutional Review Board. Written informed consent was obtained from the parents prior to study entry. Infants aged 1 month, who had been breastfed or fed using commercial infant Formula A (Meiji Co., Ltd., Tokyo, Japan) purchased by parents, were recruited between September 1,, 2014, and March 31, 2016, throughout Japan (n = 1,053). The infants were followed-up at 1 month (15–44 days), 4 months (90–149 days), 7 months (180–239 days), and 12 months (330–389 days) of age.
Formulation of formula A
In Formula A (), the protein concentration was reduced from 2.3 to 2.2 g/100 kcal (less than the mean plus the standard deviation (SD) in breast milk) to bring them closer to the mean concentration in breastmilk (1.9 g/100 kcal) [Citation16]; this enables the nutritional intake to be close to that of breastfed infants. The ARA concentration was increased from 5.1 to 13.2 mg/100 kcal (= the mean minus the SD in breast milk). The DHA concentration was maintained at 20 mg/100 kcal (= the mean minus the SD in breast milk) [Citation16]. Therefore, concentrations of both DHA and ARA in Formula A were formulated to be equivalent to their respective concentrations in breast milk.
Table 1. Nutritional composition of renewed commercial infant formula.
Data collection and outcomes
We used a questionnaire to collect the data. Demographic data comprised sex, gestational age at birth, delivery mode, and anthropometric data. Daily volume of formula intake and anthropometric measurements (body weight, body length, and HC) was collected at 1, 4, 7, and 12 months of age. We only used the body length and HC data measured by medical staff. Infant body mass index (BMI) was calculated as weight in kg divided by body length in meters squared. Stool consistency was recorded at 1, 4, and 7 months of age. Stool consistency was assessed based on an infant stool scale [Citation17]; each stool was ranked according to 3 grades (watery, soft, and formed to hard). Fever (>38°C) and a diagnosis of otitis media, influenza virus and rotavirus infections, eczema, food allergy, or atopic dermatitis were recorded every month. To encourage continual participation in the study, a questionnaire form was sent to participants along with baby care goods.
Inclusion criteria for analysis and assessment of the mode of feeding
Inclusion criteria for analysis required infants to have no disease, a gestational age of not less than 37 weeks, a birth weight from 2,500 to 4,000 g, and no missing body weight data until 7 months of age. The mode of feeding was assessed, based on energy intake from the formula. “Breastfed infants” were defined as infants with no formula intake. “Formula A-fed infants” were defined as infants who received at least 123 kcal/kg/day of formula at 0.5–1 months, 115 kcal/kg/day of formula at 1–2 months, 82 kcal/kg/day of formula at 3–4 months, and 76 kcal/kg/day of formula at over 4 months, equivalent to 90% of the average energy intake in exclusively formula-fed infants at the ages of 0.5–1 months, 1–2 months, 3–4 months, 4–5 months, respectively [Citation18].
Statistical analysis
The sample size determination was based on previously published studies on the evaluation of body weight [Citation19–Citation21]. In those studies, a sample size of ≥55 infants per group has been shown to give sufficient statistical power for a non-inferiority study. According to the Food and Drug Administration guideline, 28 infants of each sex per group would have to be enrolled for a body weight evaluation [Citation22]. Therefore, a minimum of 56 infants per feeding group should be assessed for body weight. In our observational follow-up study, 134 Formula A-fed infants at 1 month of age were enrolled to allow a 40% loss to follow-up and a 20% change in the mode of feeding during follow-up. The values are expressed as means ± standard deviation or as medians. We compared differences between groups through performing a Student’s t-test, a two-way ANOVA with repeated measures, a chi-squared test, or a Fisher’s exact test, using the Bell Curve for Excel (Social Survey Research Information Co., Ltd., Japan). A P-value <.05 was considered statistically significant.
Results
Participant characteristics
We obtained informed consent from 1,053 participants. In total, 394 (295 breastfed, 99 Formula A-fed) infants were included (). Baseline characteristics are shown in .
Table 2. Baseline characteristics of Formula A-fed and breastfed infants.
Growth parameters
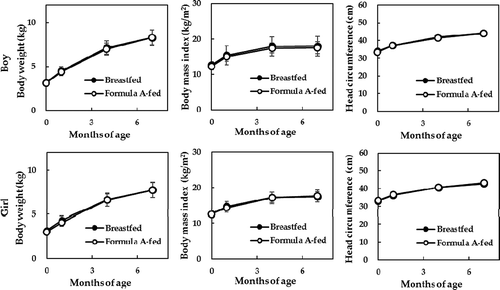
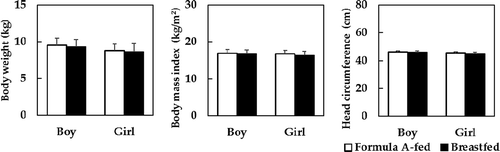
The results of growth parameters from birth to 7 months of age are shown in –). There was a significant time-dependent effect (P < .05), but no group-dependent effects or group-by-time interaction effects in any of the growth measurements. Similarly, no differences were observed in body weight gains between Formula A-fed and breastfed boys (25.6 ± 4.0 and 24.9 ± 4.1 g/day, respectively) or girls (22.9 ± 5.3 and 22.3 ± 3.7 g/day, respectively). At the 12-month follow-up, there were no differences in growth measurements between Formula A-fed and breastfed groups (). Overall, body weight in boys was 9.5 ± 1.0 and 9.3 ± 1.0 kg; body weight in girls was 8.8 ± 0.9 and 8.7 ± 1.1 kg; BMI in boys was 16.9 ± 1.4 and 16.9 ± 1.5 kg/m2; BMI in girls was 16.8 ± 1.7 and 16.3 ± 1.9 kg/m2; HC in boys was 46.1 ± 1.7 and 46.2 ± 1.5 cm, and; HC in girls was 45.1 ± 1.6 and 45.1 ± 2.0 cm, respectively.
Figure 2. (a) Body weight, (b) body mass index, (c) head circumference, and (d) stool consistency up to 7 months of age. Two-way ANOVA with repeated measures showed a significant time-dependent effect (P < .05), but no group-dependent effects or group-by-time interaction effects in any of the growth measurements. Stool consistency was rated on 3 grades (watery, soft, and formed to hard). No differences between groups were observed at 1, 4, and 7 months of age (using a chi-squared test).
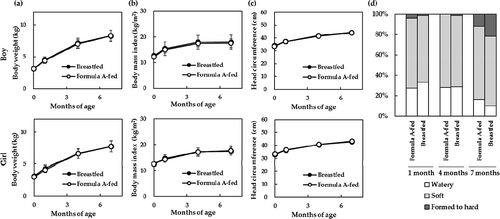
Figure 3. A follow-up of body weight, body mass index, and head circumference at 12 months of age. The numbers of Formula A-fed and breastfed infants are as follows: body weight in boys (n = 40 and n = 121) and in girls (n = 34 and n = 114), BMI in boys (n = 28 and n = 81) and in girls (n = 22 and n = 76), and health status in boys (n = 21 and n = 58) and in girls (n = 15 and n = 51), respectively. No differences were observed in any growth measurements at the 12-month follow-up based on a Student’s t-test.
Stool consistency
No differences were observed in the stool consistency between Formula A-fed and breastfed infants at 1, 4, and 7 months of age ()).
Health condition
Infection and allergy prevalence is presented in . Based on the results of Fisher’s exact test, no differences were observed in prevalence.
Table 3. The number of infants who experienced fever, infections and allergies.
Discussion
The present study indicated that the growth of Formula A-fed infants was equivalent to that of breastfed infants. In renewed Formula A, changes were made to the concentrations of protein and ARA, while maintaining DHA concentration, which can affect growth, development, and later health outcomes, to approximate these concentration levels in breast milk. The present study provides valuable evidence indicating that the nutritional composition of Formula A is appropriate.
The protein concentration of Formula A (2.2 g/100 kcal) was shown to be appropriate through evaluation of the body weight and BMI of infants. The body weight and BMI of Formula A-fed infants were equivalent to those of breastfed infants ( and ). Moreover, changes in body weight were in accordance with World Health Organization (WHO) and Japan standard growth curves for infants [Citation23,Citation24]. Although there was a significant difference in the body weight at birth among girls between the two groups, no differences in the rates of body weight gain were observed. These findings suggest that there is little risk of protein insufficiency for Formula A-fed infants. Although previous studies have shown that when protein intake was notably high, BMI was elevated at ≥6 months of age, resulting in pre-peritoneal fat tissue accumulation at the age of 5 years [Citation6,Citation25], our previous cross-sectional study showed that infants fed with Formula B, which is the previous formulation of Formula A with protein content of 2.3 g/100 kcal, grow appropriately for the first 6 months [Citation26]. In the present follow-up study, there was no difference in BMI between Formula A-fed infants and breastfed infants at 7 and 12 months of age. These findings provide evidence that the new formulation of Formula A, including reduced protein content, is with no excess for infants.
That appropriate growth was achieved despite the reduction in the “amount” of protein in Formula A may mean that the improved quality of protein in Formula A partly contributed to this result. One of the most important factors in protein quality design concerns ensuring an appropriate amount of individual amino acids, and there should be no deficiencies in the required amount of each essential amino acid. Formula A was designed so that essential amino acids would be present at equivalent levels to or above those in breastmilk (). To improve the digestibility of protein to amino acids, the amount of casein was adjusted, which affects physical properties such as viscosity in the stomach, to approximate the amount of casein found in breastmilk. Beta-lactoglobulin, which is not found in breast milk and is relatively hard to digest, was also reduced and the ratio of easily digestible alpha-lactalbumin was increased. Furthermore, the concentration of large molecular proteins was set to a similar level to that of breast milk, because large molecular proteins can promote the development of digestive functions [Citation27]. It is, therefore, essential to consider the protein quality in infant formula when reducing the amount of protein. If the protein concentration was to be further reduced, it would be important to further improve the quality of the protein.
The present results also suggest that the protein formulation of Formula A was appropriate in terms of digestion and absorption. In infants receiving breast milk or infant formula, 30% of ingested protein is excreted [Citation28]. However, if the amount or quality of protein drastically differs between infant formula and breast milk, stool consistency may change. In the present study, stool consistency of Formula A-fed infants was comparable to that of breastfed infants (). This is in contrast to our previous study, which showed a possibility for relatively harder stool in infants fed with Formula B, having a protein content of 2.3 g/100 kcal, than in breastfed infants. It is necessary to consider the difference in the method of evaluation, these findings further suggest that the amount and quality of protein in the renewed Formula A was appropriate.
An evaluation of HC showed that the DHA and ARA composition of Formula A was also appropriate. HC growth, a potential indicator of brain volume [Citation9–Citation11], was found in Formula A-fed infants to be equivalent to that of breastfed infants ( and ) and was in accordance with the WHO and Japan standard growth curves for infants [Citation23,Citation24,Citation28]. Several studies have indicated that, in terms of DHA and ARA, there should not be a notably higher intake of one compared to the other [Citation12,Citation13,Citation29]. These findings support the appropriateness of Formula A, in which both DHA and ARA concentrations were within the ranges found in breast milk.
The prevalence of infections and allergies in Formula A-fed infants did not differ from that in breastfed infants. In previous reports, the prevalence of otitis media was higher in formula-fed infants [Citation30], and the cause was considered to be due to bottle usage [Citation31]. However, there was no difference between the two groups in our study (). This could be because most infants in the present study were likely to have been vaccinated against Streptococcus pneumonia [Citation32]. In terms of the prevalence of food allergies and atopic dermatitis, there was also no difference between the two groups (). The diagnoses were revised in accordance with the Japanese Pediatric Guideline for Food Allergy in 2016 [Citation33]; therefore, it is possible that the diagnostic criteria were not consistent during the study period. Nevertheless, this result was consistent with reports indicating there is insufficient evidence to support the hypothesis that breast milk feeding prevents allergies [Citation33,Citation34]. These findings also suggest that the composition of Formula A was appropriate.
There are some limitations in this study. First, the observational study design will always have the possibility of unknown confounders, which cannot be taken into account. Second, data were collected by using questionnaire. Third, this study had selection bias due to loss to follow-up and lack of data. These methodological limitations might have results in over- or underestimation of the impact of feeding mode on the measured outcomes. Nevertheless, there have been few reports published to-date on the growth of term infants fed with a commercial infant formula. Therefore, our findings will contribute to improving infant nutrition in future.
The equivalent growth for Formula A-fed and breastfed infants shown in the present study indicated that the nutritional composition of the renewed Formula A was appropriate, which suggests that the renewed Formula A may be used as a sole source of nutrition for infants. When changing the composition of infant formulas, its suitability must be confirmed based on the growth of infants, considering that formulas can affect growth and development. In future, through acquiring multifaceted and linked data for future studies on infant growth, more targeted findings are likely to be obtained that may contribute to further improvements in infant formulas.
Author contributions
S. J. and T. K. designed the study; S. J., K. Y. and T. K. conducted the study; S. J., K.Y. and Y. N. analyzed the data, and S. J., Y.N. and T. K. wrote the paper. All authors read and approved the final manuscript.
Study Identification No
UMIN000023110 (http://www.umin.ac.jp/)
Acknowledgments
The authors are extremely grateful to the infants and their families for their participation. We also thank the registered dietitians and dietitians at the head and branch offices of Meiji Co., Ltd. throughout Japan for the recruitment of infants to the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Jinno S. Infant growth evaluation: essential for appropriate design of infant formula (in Japanese). Milk Sci. 2015;64(1):35–42.
- SCF (Scientific Committee on Food). Report of the Scientific Committee on Food on the Revision of Essential Requirements of Infant Formulae and Follow-on Formulae. Brussels (Belgium): EUROPIAN COMMISSION;2003:11.
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific opinion on the essential composition of infant and follow-on formulae. Efsa J. 2014;12:3760.
- WHO/FAO. Standard for infant formula and formulas for special medical purposes intended for infants. Rome (Itary): CODEX Alimentarius Commission; 1981:72.
- Weber M, Grote V, Closa-Monasterolo R, et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am J Clin Nutr. 2014;99(5):1041–1051.
- Koletzko B, Kries V, Closa R, et al. Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr. 2009;89(6):1836–1845.
- Hadley K, Ryan A, Forsyth S, et al. The essentiality of arachidonic acid in infant development. Nutrients. 2016;8(4):216.
- Martinez M. Tissue levels of polyunsaturated fatty acids during early human development. J Pediatr. 1992;120(4):S129–S138.
- Cheong JLY, Hunt RW, Anderson PJ, et al. Head growth in preterm infants: correlation with magnetic resonance imaging and neurodevelopmental outcome. Pediatrics. 2008;121(6):e1534–e1540.
- Gale CR, O’Callaghan FJ, Bredow M, et al. The influence of head growth in fetal life, infancy, and childhood on intelligence at the ages of 4 and 8 years. Pediatrics. 2006;118(4):1486–1492.
- Cooke RW, Lucas A, Yudkin PL, et al. Head circumference as an index of brain weight in the fetus and newborn. Early Hum Dev. 1977;1(2):145–149.
- Xiang M, Alfvén G, Blennow M, et al. Long-chain polyunsaturated fatty acids in human milk and brain growth during early infancy. Acta Paediatr. 2000;89(2):142–147.
- Birch EE, Garfield S, Hoffman DR, et al. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev Med Child Neurol. 2000;42(3):174–181.
- Birch EE, Garfield S, Castañeda Y, et al. Visual acuity and cognitive outcomes at 4 years of age in a double-blind, randomized trial of long-chain polyunsaturated fatty acid-supplemented infant formula. Early Hum Dev. 2007;83(5):279–284.
- Salem N, Wegher B, Mena P, et al. Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc Natl Acad Sci USA. 1996;93(1):49–54.
- Yamawaki N, Yamada M, Kan-no T, et al. Macronutrient, mineral and trace element composition of breast milk from Japanese women. J Trace Elem Med Biol. 2005;19(2–3):171–181.
- Bekkali N, Hamers SL, Reitsma JB, et al. Infant stool form scale: development and results. J Pediatr. 2009;154(4):521–526.
- Jinno S, Nakamura Y, Kanno T, et al. Intake of energy and nutrients in exclusively formula-fed infants (in Japanese). Milk Sci. 2014;63(2):63–69.
- Gibson RA, Barclay D, Marshall H, et al. Safety of supplementing infant formula with long-chain polyunsaturated fatty acids and Bifidobacterium lactis in term infants: a randomised controlled trial. Br J Nutr. 2009;101(11):1706–1713.
- Rozé JC, Barbarot S, Butel MJ, et al. An α-lactalbumin-enriched and symbiotic-supplemented v. a standard infant formula: a multicentre, double-blind, randomised trial. Br J Nutr. 2012;107(11):1616–1622.
- Fleddermann M, Demmelmair H, Grote V, et al. Infant formula composition affects energetic efficiency for growth: the BeMIM study, a randomized controlled trial. Clin Nutr. 2014;33(4):588–595.
- American Academy of Pediatrics. Clinical testing of infant formulas with respect to nutritional suitability for term infants. Report to the FDA. Committee on Nutrition. Silver spring (MD); 1988. Available from: https://wayback.archive-it.org/7993/20170722090324/https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/InfantFormula/ucm170649.htm
- World Health Organization. The WHO child growth standards. [ cited 2019 June]. Available from: www.who.int/childgrowth/standards/en
- Yokoyama T, Kato N, Takimoto H, et al. Guidelines for the issuance and practical use of the maternal and child health handbook. Tokyo (Japan): National Institute of Public Health; 2012. in Japanese. Available from: https://www.niph.go.jp/soshiki/07shougai/hatsuiku/index.files/katsuyou.pdf
- Gruszfeld D, Weber M, Gradowska K, et al. Association of early protein intake and pre-peritoneal fat at five years of age: follow-up of a randomized clinical trial. Nutr Metab Cardiovasc Dis. 2016;26(9):824–832.
- Kanno T, Jinno S, Kaneko T. Survey of the anthropometric growth, nutritional intake, fecal properties, and morbidity of infants, in relation with feeding methods (XI). J Child Heal. 2013;72: 253–260. Japanese.
- Kinouchi T, Koyama S, Harada E, et al. Large molecule protein feeding during the suckling period is required for the development of pancreatic digestive functions in rats. Am J Physiol Integr Comp Physiol. 2012;303(12):R1268–R1276.
- WHO/FAO/UNU Expert Consultation. Protein and amino acid requirements in human nutrition. World Health Organ Tech Rep Ser 2007:1–265.23. WHO Multicentre Growth Reference Study Group. WHO child growth standards: head circumference-for-age, arm circumference-forage, triceps skinfold-for-age and subscapular skinfold-for-age: methods and development.Geneva: World Health Organisation; 2007.
- Hoffman DR, Birch EE, Birch DG, et al. Impact of early dietary intake and blood lipid composition of long-chain polyunsaturated fatty acids on later visual development. J Pediatr Gastroenterol Nutr. 2000;31(5):540–553.
- Sabirov A, Casey JR, Murphy TF, et al. Breast-feeding is associated with a reduced frequency of acute otitis media and high serum antibody levels against NTHi and outer membrane protein vaccine antigen candidate P6. Pediatr Res. 2009;66(5):565–570.
- Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131(3):e964–e999.
- Taylor S, Marchisio P, Vergison A, et al. Impact of pneumococcal conjugate vaccination on otitis media: a systematic review. Clin Infect Dis. 2012;54(12):1765–1773.
- Ebisawa M, Ito K, Fujisawa T. Japanese guidelines for food allergy 2017. Allerg Int. 2017;66(2):248–264.
- Greer FR, Sicherer SH, Burks AW. The effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, hydrolyzed formulas, and timing of introduction of allergenic complementary foods. Pediatrics. 2019;143(4):e20190281.