ABSTRACT
Although not fully investigated, 8-HEPE, 8-HETE, and 10-HDHA have potentially beneficial effects for human health. Euphausia pacifica (North Pacific krill) is unique in containing several ppm level of 8R-HEPE, and sub-ppm levels of 8R-HETE and 10R-HDHA. Obtaining sufficient quantities of these compounds is a major bottleneck for conducting in vivo experiments to evaluate their biological activities. In this study, we examined an efficient way of obtaining 8R-HEPE, 8R-HETE, and 10R-HDHA by enzymatic production in E. pacifica. We devised a novel method to purify 199.4 mg of 8R-HEPE, 2.1 mg of 8R-HETE and 5.6 mg of 10R-HDHA from 1 kg of E. pacifica. We identified the stereochemistry of the hydroxy group at C-8 of HEPE and HETE and C-10 of HDHA as the R configuration by chiral column chromatography analysis using LC/QTOFMS.
Abbreviations: 8-HEPE: 8-hydroxy-eicosapentaenoic acid; 8-HETE: 8-hydroxy-eicosatetraenoic acid; 10-HDHA: 10-hydroxy-docosahexaenoic acid; EPA: eicosapentaenoic acid; TLC-FID, thin layer chromatograph-Flame Ionization Detector; LC/QTOFMS: liquid chromatography/hybrid quadrupole time of flight mass spectrometry.
GraphicalAbstract
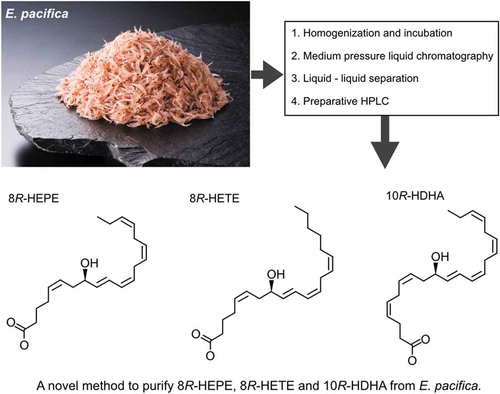
A novel method to pulify 8R-HEPE, 8R-HETE, and 10R-HDHA from E. pacifica.
Euphausia pacifica (North Pacific krill) is the most common species of krill found in the northern Pacific Ocean and is one of the few commercially caught Euphausiids. In Japan, more than 20,000 tons of E. pacifica were caught in 2019. Uniquely, E. pacifica contains 8-hydroxy-eicosapentaenoic acid (8-HEPE), 8-hydroxy-eicosatetraenoic acid (8-HETE) and 10-hydroxy-docosahexaenoic acid (10-HDHA) () [Citation1]. We previously showed that 8-HEPE has a higher ligand activity for peroxisome proliferate-activated receptors (PPARs) than eicosapentaenoic acid (EPA) [Citation2]. Moreover, 8-HEPE consumption was found to suppress the level of plasma and hepatic triacylglycerol in high-fat diet-induced obese mice [Citation3]. These studies suggest 8-HEPE is a potential anti-obese compound for people struggling with obesity, although this has not been investigated. Moreover, 8-HETE displays an anti-tumor effect in carcinoma cells [Citation4], but its effectiveness has not been examined in vivo. Previous studies showed that hydroxy-DHA has an anti-inflammatory effect, suggesting 10-HDHA could be a candidate drug for preventing lifestyle-related diseases. However, there are a paucity of studies focusing on the bioactivity of 10-HDHA. Further investigations are hampered by the difficulty of obtaining sufficient quantities of 8-HEPE, 8-HETE, and 10-HDHA for in vivo experiments. Thus, the development of an efficient method to purify 8-HEPE, 8-HETE, and 10-HDHA from E. pacifica is an important step toward the future assessment of these compounds for human health.
In a previous study, we showed that 8-HEPE could be produced enzymatically from EPA using protein extracted from E. pacifica [Citation1]. In this study, we examined an efficient means to purify 8-HEPE, 8-HETE, and 10-HDHA from E. pacifica that uses its inherent enzyme activity. Moreover, we identified the stereochemical structure of 8-HEPE, 8-HETE, and 10-HDHA from E. pacifica by chiral column chromatography analysis using LC/QTOFMS.
Results
Examination of conditions that increase the levels of 8-HEPE, 8-HETE, and 10-HDHA
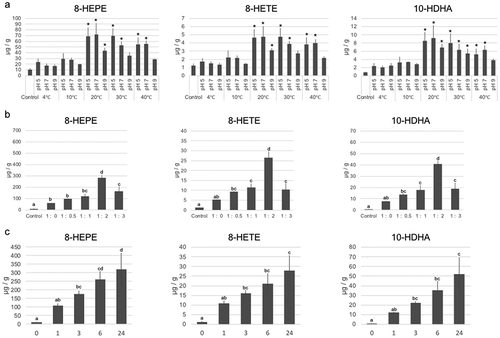
In a previous study, we demonstrated the potential enzymatic production of 8-HEPE in E. pacifica [Citation1]. Here, we confirmed enzymatic production of 8-HEPE in E. pacifica using d5-EPA (Supplemental Figure S1). To establish a method to increase the level of 8-HEPE, 8-HETE and 10-HDHA, we analyzed the incubation conditions. Firstly, we examined incubation temperature and pH using Tris-buffer. The concentration of 8-HEPE, 8-HETE, and 10-HDHA reached the maximum values at 20°C and pH 7.4 ()). Next, we examined a suitable volume of buffer to add for incubation. These experiments showed adding twice the volume E. pacifica weight of buffer was best for increasing the yield of 8-HEPE, 8-HETE, and 10-HDHA ()). We also examined the ideal incubation time. The concentration of 8-HEPE, 8-HETE and 10-HDHA increased as the incubation time increased. After 6-h incubation, the concentration of 8-HEPE, 8-HETE, and 10-HDHA were 20-fold higher than before incubation ()). To examine changes in the lipid profile of E. pacifica during incubation, we analyzed different classes of lipids by TLC-FID and specific lipid molecules by LC/QTOFMS. The level of phosphatidylcholine decreased with incubation time, while the level of free fatty acids increased ( and ).
Table 1. Proportion of lipid class in incubated E. pacifica oil.
Table 2. LC/QTOF analysis of PC and fatty acid in incubated E. pacifica oil.
Figure 2. Examination of the conditions of incubation to increase the levels of 8-HEPE, 8-HETE, and 10-HDHA.
Examination of incubation temperature, pH (a) and buffer volume (b). The value corresponding to E. pacifica extract prior to incubation was used as a control. B. The ratio shown in the x-axis refers to the weight of E. pacifica: water volume. A,B. Samples were incubated for 3 h. (c) Examination of incubation time. (a-c) Values are the mean ± s.d. of four samples. * indicate a significant difference compared with the control (p < 0.05). Values marked by different letters were significantly different (p < 0.05).
Methods to concentrate 8-HEPE, 8-HETE, and 10-HDHA
We previously concentrated 8-HEPE from methanol extracts of dried E. pacifica using HP-20: DIAION synthetic adsorbent beads [Citation2]. Suitable beads to concentrate 8-HEPE, 8-HETE and 10-HDHA were investigated and HP-20, SP70, and SP700 were found to capture and release more than 95% of 8-HEPE. The recovery rate of 8-HEPE using SP850, SP207, and HP2MGL was 55%, 80%, and 13%, respectively. The proportions of 8-HEPE in the eluted fraction from HP-20, SP70, SP700 beads were 0.58%, 1.18%, and 5.28%, respectively (). SP700 were the most suitable DIAION beads to concentrate 8-HEPE, 8-HETE and 10-HDHA from E. pacifica.
Table 3. Examination of the concentration of 8-HETE, 8-HEPE, and 10-HDHA using synthetic adsorbent beads. Values are the mean of three examinations.
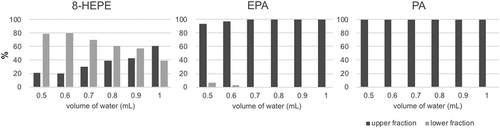
Next, we examined the methods of liquid-liquid separation using water, ethanol, and hexane to separate fatty acids and hydroxy-fatty acids. We used 10 mg of the SP700 eluted fraction that contained 509 µg of 8-HEPE, 326 µg of EPA and 181 µg of palmitic acid (PA). We examined the relationship between additional volume of water and distribution of 8-HEPE, EPA, and PA in the upper or lower fractions. More than 90% of EPA and PA were distributed in the upper fraction in any of the conditions we examined. The distribution of 8-HEPE was changed by the volume of water added. Using a solution with a ratio of water: ethanol: hexane of 0.5: 1: 1, the proportion of 8-HEPE distributed in the upper and lower layers was 20% and 80%, respectively. As the amount of water increased, the proportion of 8-HEPE in the lower fraction decreased ().
Development of a procedure to isolate 8-HEPE, 8-HETE, and 10-HDHA
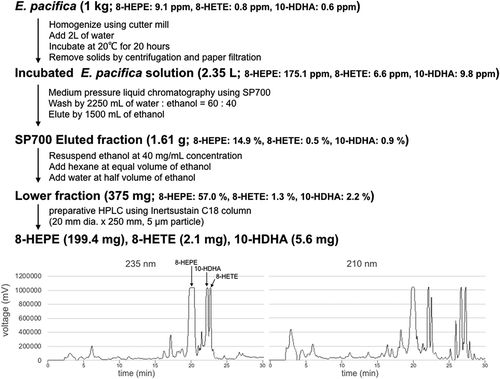
We developed an efficient method to purify 8-HEPE, 8-HETE, and 10-HDHA from E. pacifica in only four steps (). The first step is the homogenization and incubation of E. pacifica. The second step involves a separation procedure using SP700 beads. The third step is liquid-liquid separation using a water, ethanol and hexane mixture. The fourth step is purification by HPLC. The concentration of 8-HEPE, 8-HETE, and 10-HDHA in each step is summarized in . Using this procedure we obtained 199.4 mg of 8-HETE, 2.1 mg of 8-HEPE and 5.6 mg of 10-HDHA from 1 kg of E. pacifica. The degree of purification of 8-HEPE, 8-HETE, and 10-HDHA after preparative HPLC exceeded 95%.
Table 4. Summary of the purification of 8-HEPE, 8-HETE, and 10-HDHA from 1 kg of E. pacifica.
Figure 4. Scheme outlining the purification of 8-HEPE, 8-HETE, and 10-HDHA from 1 kg of E. pacifica.
Values are the mean of six examinations. Chromatograms of preparative HPLC are shown at the bottom. The peaks of 8-HEPE, 8-HETE and 10-HDHA are annotated in the 235 nm chromatogram. The chromatogram shows the peak corresponding to 8-HEPE. An expanded range around the peaks for 10-HDHA and 8-HETE is shown in supplemental Figure S2.
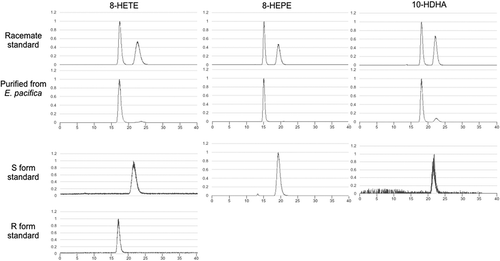
Stereochemical structures of 8-HETE, 8-HEPE, and 10-HDHA isolated from E. pacifica
8-HETE, 8-HEPE, and 10-HDHA contain an asymmetric carbon atom. Stereochemical analysis of 8-HETE, 8-HEPE, and 10-HDHA isolated from E. pacifica has not been reported. Here, we analyzed the stereochemical structures of 8-HETE, 8-HEPE, and 10-HDHA purified from E. pacifica using a CHIRALPAK ID column. Under suitable conditions, racemate standards of 8-HETE, 8-HEPE, and 10-HDHA could be resolved as two peaks using this column. 8-HEPE from E. pacifica corresponded with the first peak of the racemate. The major peak of 8-HETE and 10-HDHA from E. pacifica corresponded with the first peak of the racemate. The ratio of 8R-HETE: 8S-HETE in 8-HETE from E. pacifica is 98.86: 1.14. The ratio of 10R-HDHA: 10S-HDHA in 10-HDHA from E. pacifica is 98.02: 1.98 (). The 8S-HETE standard, 8S-HEPE standard and 10S-HDHA produced by Alox8 (Supplemental Figure S3) corresponded to the second peak of the racemate standards. Therefore, 8-HEPE, 8-HETE, and 10-HDHA from E. pacifica were identified as 8R-HEPE, 8R-HETE and 10R-HDHA, respectively.
Figure 5. Stereochemical analysis of 8-HETE, 8-HEPE, and 10-HDHA purified from E. pacifica.
The chromatograph of 8-HETE was an extracted ion chromatogram of product ion (m/z = 155.071) generated from precursor ion (m/z = 319.2). The chromatograph of 8-HEPE was an extracted ion chromatogram of product ion (m/z = 155.071) generated from precursor ion (m/z = 317.2). The chromatograph of 10-HDHA was an extracted ion chromatogram of product ion (m/z = 153.092) generated from precursor ion (m/z = 343.2).
Discussion
This study describes a novel method to purify 8-HEPE, 8-HETE, and 10-HDHA from E. pacifica. Starting from 1 kg of E. pacifica, we isolated 199.4 mg of 8-HEPE, 2.1 mg of 8-HETE and 5.6 mg of 10-HDHA. Moreover, this method is more efficient at purifying 8-HEPE than a previously reported procedure [Citation2]. In addition, the new method is also suitable for isolating 8-HETE and 10-HDHA from E. pacifica. The concentration levels of the three compounds in E. pacifica was as follows: 8-HEPE, several ppm to several tens of ppm; 8-HETE, several hundreds of ppb to several ppm; 10-HDHA, several hundreds of ppb to several ppm (Supplemental Table S1). Interestingly, these concentration levels of 8-HEPE, 8-HETE and 10-HDHA corresponded to those of E. pacifica caught in 2017. Taken together, our findings indicate that E. pacifica caught near Iwate, Japan in Spring are likely to be a reliable source of 8-HEPE, 8-HETE, and 10-HDHA.
Moreover, the levels of 8-HEPE, 8-HETE and 10-HDHA could be increased over 20-fold by incubation at 20°C for 24 h. After incubation, the proportion of phosphatidylcholine (PC) in the E. pacifica lipid fraction decreased and the proportion of free fatty acid increased (). These observations indicate that the hydrolysis of PC was promoted during the incubation period. The level of PC decreased regardless the molecular species, and the level of fatty acids, excluding EPA (FA 20:5), increased upon incubation (). These results suggest that EPA liberated from PC is metabolized to 8-HEPE during the incubation period.
SP700 and SP70 were suitable DIAION synthetic beads to concentrate 8-HEPE, 8-HETE and 10-HDHA from incubated E. pacifica solution. HP-20 also gave a recovery rate of more than 95% 8-HEPE. However, significant amounts of impurity were also eluted from HP-20. Therefore, the concentration of 8-HEPE in the eluted fraction of HP-20 was lower than the SP700 or SP70 eluted fractions (). Liquid-liquid fractionation using water, ethanol, and hexane could separate 8-HEPE and fatty acids (). 8-HEPE, 8-HETE, and 10-HDHA were purified from the lower fraction of liquid-liquid separation by preparative HPLC. Using this procedure, we could achieve >95% purity of 8-HEPE, 8-HETE, and 10-HDHA.
The stereochemistry of 8-HEPE, 8-HETE, and 10-HDHA from E. pacifica had not previously been determined. Here, we identified the stereochemistry of 8-HEPE, 8-HETE, and 10-HDHA as the R configuration (). In an earlier study, hydroxy-fatty acids had to be converted to the methyl ester derivative to analyze their stereochemistry [Citation5]. The method of LC/QTOFMS analysis using a CHIRALPAK ID column makes it possible to identify the stereochemistry of hydroxy fatty acids without the need for derivatization. Hydroxy fatty acids are derivatives of unsaturated fatty acids produced via oxidation catalyzed by a lipoxygenase. The mouse genome includes the 8S-lipoxygenase gene, and 8S-HETE is produced in phorbol ester stimulated mouse skin [Citation5]. Most lipoxygenase activity identified in mammals and plants convert unsaturated fatty acids to the S form of hydroxy fatty acids. The R forms of 8-HEPE, 8-HETE and 10-HDHA are unusual in mammals and plants. Some studies have suggested 8S-HETE and 8R-HETE have a distinct biological activity [Citation6,Citation7]. However, the stereochemical specific biological activity of 8-HEPE, 8-HETE and 10-HDHA has not been fully investigated. The availability of 8R-HEPE, 8R-HETE and 10R-HDHA will facilitate the study of the stereochemical specific activity of 8-HEPE, 8-HETE, and 10-HDHA.
In summary, to eliminate the bottleneck of in vivo experiments involving 8-HEPE, 8-HETE, and 10-HDHA, we have developed a novel and efficient method to purify these compounds from E. pacifica. This method could also be used to purify other hydroxy fatty acids from natural sources. We hope that this study will encourage research focused on the use of hydroxy fatty acids for human health.
Materials and methods
Materials
Pacific krill was a kind gift from Kokuyou Inc. (Iwate, Japan). The krill was originally caught in the Pacific Ocean near Iwate, Japan in 2019. Fatty acids, hydroxyeicosapentaenoic acids (HEPEs), hydroxyeicosatetraenoic acids (HETEs) and hydroxy docasahexaenoic acids (HDHAs) were purchased from Cayman Chemical (Ann Arbor, MI). HP-20, SP850, SP70, SP700, SP207, and HP2MGL were purchased from Mitsubishi Chemical Corp. (Tokyo, Japan)
Lipid extraction and quantification of 8-HEPE, 8-HETE, and 10-HDHA
Lipids were extracted according to the Bligh-Dyer method [Citation8]. 8-HEPE, 8-HETE and 10-HDHA were separated on an InertSustain AQ-C18 column (2.1 mm dia. × 50 mm; GL Science Inc., Fukushima, Japan) with gradient elution (water containing 0.1% formic acid/acetonitrile containing 0.1% formic acid, 55/45 to 0/100 in 10 min) at a flow rate of 0.3 mL/min. The temperature of the column was maintained at 40°C. The peaks were identified and quantified by liquid chromatography/hybrid quadrupole time-of-flight mass spectrometry (LC/QTOFMS: Triple TOF 5600, SCIEX) using SCIEX OS Software. The pressure of nebulizer gas, turbo gas, and curtain gas were 50, 50 and 30 psi, respectively. The temperature of the turbo gas was 500°C. The voltage of the ion spray was −4000 V. 8-HEPE, 8-HETE and 10-HDHA were ionized by the electron spray ionization (ESI) method and detected as M-H ions. We used the multiple reaction monitoring (MRM) method to quantify 8-HEPE, 8-HETE, and 10-HDHA. The m/z of precursor ion and product ion of 8-HEPE was 317.21 and 155.071, respectively. The m/z of the precursor ion and product ion of 8-HETE was 319.22 and 155.071, respectively. The m/z of the precursor ion and product ion of 10-HDHA was 343.22 and 153.092, respectively. We quantified the amount of 8-HEPE, 8-HETE and 10-HDHA from a calibration curve generated using 8-HEPE, 8-HETE and 10-HDHA purchased from Cayman Chemical.
Examination of incubation conditions
We homogenized 50 g of E. pacifica using a mortar and pestle. Next, we prepared 200 microtubes containing 100 mg of homogenized E. pacifica, which were stored at −60°C until required. We used a Tris-HCl buffer (pH 5.0, 7.4 or 9.0) to examine the most suitable incubation conditions. For examination of incubation temperature and pH ()), we added 100 µL of Tris-HCl buffer to microtubes containing 100 mg of homogenized E. pacifica. To examine the incubation volume ()), we added 0, 50, 100, 200, 300 µL of Tris-HCl buffer. For investigating different incubation times ()), we added 200 µL of Tris-HCl buffer. As a control, samples were extracted immediately after Tris-HCl buffer addition. The mixtures were incubated in an aluminum block bath (TAITEC; Saitama, Japan) at 4°C, 10°C, 20°C, 30°C, or 40°C.
Analysis of TLC-FID
The lipid extract was resuspended at 10 mg/mL in chloroform. A 0.1 µg aliquot of lipid was applied to a Chromarod (LSI Medience Corp., Tokyo, Japan). A chloroform/methanol/water (42:24:2.5) mix was drawn 5 cm up the Chromarod. After drying, hexane/diethyl ether (50:30) was drawn 10 cm up the Chromarod and lipids were detected and measured by TLC-FID (LSI Medience Corp.). Dipalmitoyl phosphotidylcholine, 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine, glyceryl tripalmitate, DL-alpha-palmitin and 1-palmitoyl-sn-glycero-3-phosphocholine were used as lipid standards to identify the lipid class from the Rf value.
Lipid analysis using LC/QTOFMS
Lipids were separated on an InertSustain AQ-C18 column (2.1 mm dia. × 50 mm; GL Science Inc.) with gradient elution (water: methanol: acetonitrile (6:2:2) containing 5 mM ammonium acetate and 10 nM EDTA/isopropanol containing 5 mM ammonium acetate and 10 nM EDTA, 100/0 to 0/100 in 25 min) at a flow rate of 0.2 mL/min. The MSMS data were annotated using MSDial software (http://prime.psc.riken.jp/Metabolomics_Software/MS-DIAL/). Lipids were ionized by the ESI method. Phospholipids were detected as M + H ions. Triacylglycerols were detected as M+ NH4 ions. Free fatty acids were detected as M-H ions.
Column chromatography using synthetic adsorbent beads
Ten grams of synthetic adsorbent beads (HP-20, SP850, SP70, SP700, SP207, and HP2MGL) were packed in empty reservoirs with a capacity of 20 mL (GL Science Inc.). The beads were washed with 30 mL of ethanol and equilibrated in 30 mL of an water: ethanol (75: 25) mix. Ten grams of homogenized E. pacifica were incubated with 20 mL of Tris-HCl (pH 7.4) buffer at 20°C for 24 h and then the mixture was centrifuged at 8,000 g for 10 min. The supernatant was corrected and applied to the beads, which were then washed in 30 mL of water: ethanol (75: 25) and 30 mL of water: ethanol (60: 40). Finally, 8-HETE, 8-HEPE, and 10-HDHA were eluted from the beads using ethanol.
Examination of liquid-liquid separation using water, ethanol, and hexane
The solvent was removed from the eluted fraction of SP700 and the residue resuspended at 10 mg/mL in ethanol. One milliliter of hexane was added to 1 ml of eluted fraction (10 mg/mL in ethanol) and the mixture was sonicated for 30 s. Next, we added 0.5, 0.6, 0.7, 0.8, 0.9 or 1.0 mL of pure water to the ethanol and hexane mixture solution, which was sonicated for 30 s, and centrifuged at 3000 g for 5 min. The upper fraction was collected, and 1 mL of hexane added to the lower fraction, which was sonicated and centrifuged as before. Solvent was removed from the upper solution and the residue resuspended in 2 mL of ethanol. The 8-HEPE, EPA and palmitic acid content in each fraction was quantified by LC/QTOFMS.
Purification of 8-HEPE, 8-HETE, and 10-HDHA from E. pacifica
E. pacifica caught near Iwate was stored at −50°C. One kg of frozen E. pacifica was homogenized using a cutter mill (Verder Scientific, Tokyo Japan). Homogenized E. pacifica was incubated with 2 L of water at 20°C for 20 h with 160 rpm shaking in a constant temperature incubator shaker (TAITEC, Saitama Japan). Incubated E. pacifica solution was collected by centrifugation at 8,000 g for 10 min and further clarified by filtration using a paper filter. E. pacifica solution was applied to 125 g of SP700 packed into a Sepacore glass column using the Sepacore easy purification system (Buchi, Tokyo Japan). The clarified E. pacifica solution was applied to the column at a flow rate of 10 mL/min, which was then washed with 2250 mL of water: ethanol (60: 40) solution. 8-HEPE, 8-HETE, and 10-HDHA were eluted from SP700 in ethanol. The solvent was removed from the eluted fraction of SP700 using an evaporator (Buchi) and freeze dryer (EYELA, Tokyo Japan). The eluted fraction was then resuspended in ethanol (one volume) at a concentration of 100 mg/mL. Next, one volume of hexane was added to the eluted fraction and mixed by sonication. Finally, a half volume of water was added to the eluted fraction, mixed, and centrifuged at 1,000 g for 3 min. The upper fraction was removed. One volume of hexane added to the lower fraction again, mixed, and centrifuged. The lower fraction was collected, and the solvent in the lower fraction was removed by evaporation and freeze-drying. The lower fraction was resuspended at 20 mg/mL in water: ethanol (50: 50) solution. Twenty mg of lower fraction at a time was applied to preparative HPLC. 8-HEPE, 8-HETE, and 10-HDHA were separated on an InertSustain ODS-3 column (20 mm dia. × 250 mm, GL Science Inc.) by gradient elution (water containing 0.1% formic acid/acetonitrile containing 0.1% formic acid, 55/45 to 0/100 in 20 min) at a flow rate of 15 mL/min. Compounds in the eluate were detected at 235 nm.
LC/QTOFMS analysis using a chiral column
Enantiomers of 8-HETE, 8-HEPE and 10-HDHA were separated on a CHIRALPAK ID (4.6 mm dia. × 250 mm column; DAICEL, Osaka, Japan) with isocratic elution (formic acid solution pH 2.0/methanol, 20/80) at a flow rate of 0.4 mL/min. The temperature of the column was maintained at 30°C. The compounds were identified by QTOFMS using SCIEX OS Software. The pressure of nebulizer gas, turbo gas, and curtain gas were 50, 50 and 30 psi, respectively. The temperature of the turbo gas was 500°C. The voltage of the ion spray was −4000 V. 8-HEPE, 8-HETE and 10-HDHA were ionized by the ESI method and detected as M-H ions. The ratio of S and R isomers of 8-HETE and 10-HDHA isolated from E. pacifica was calculated according to peak area.
10S-HDHA production using the mouse alox8 gene
The Alox8 gene was cloned from mouse skin cDNA using specific primers (5ʹ- AACTGGTCAGGAGGATGGCG −3ʹ and 5ʹ- AGGAGAAGCATACGGGGACT −3ʹ). The Alox8 gene, which included a poly-histidine (6xHis) tag, was engineered into the pFastBac vector. Alox8 protein was expressed using a bac expression system (Thermo Fisher Scientific, Waltham, MA) in Sf9 cells (Supplemental Figure S3). The Sf9 cells expressing Alox8 were incubated with 125 pmol DHA in 25 µL of Tris-HCl buffer (pH 7.4) at 27°C for 1 h. Then, 75 µL of acetonitrile was added that contained 1% of formic acid and the mixture centrifuged at 20,000 g for 10 min. The clarified liquid fraction was applied to an LC/QTOFMS analysis.
Statistical analysis
Statistically significant differences between the experimental groups were identified using one-way ANOVA and Tukey’s post-hoc tests.
Author contribution
H.Y. designed the research; H.Y., K.K., A.U., and S.Y. conducted the research; H.Y. analyzed the data; H.Y. wrote the manuscript and had primary responsibility for the final content. All authors read and approved the final manuscript.
08_Supplemental_data_YamadaH_BBB_P1.docx
Download MS Word (493.6 KB)Acknowledgments
This work was supported by funds from the Basic Biotechnology Project of Iwate Prefecture, Japan, and Technology Agency and the Project of the NARO Bio-oriented Technology Research Advancement Institution (the special scheme project on vitalizing management entities of agriculture, forestry and fisheries).
Data availability statement
The data that support the findings of this study are available from the corresponding author, H.Y., upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Yamada H, Yamazaki Y, Koike S, et al. Lipids, fatty acids and hydroxy-fatty acids of Euphausia pacifica. Sci Rep. 2017;7:9944.
- Yamada H, Oshiro E, Kikuchi S, et al. Hydroxyeicosapentaenoic acids from the Pacific krill show high ligand activities for PPARs. J Lipid Res. 2014;55:895–904.
- Yamada H, Kikuchi S, Hakozaki M, et al. 8-hydroxyeicosapentaenoic acid decreases plasma and hepatic triglycerides via activation of peroxisome proliferator-activated receptor alpha in high-fat diet-induced obese mice. J Lipids. 2016;2016:7498508.
- Kim E, Rundhaug JE, Benavides F, et al. An antitumorigenic role for murine 8S-lipoxygenase in skin carcinogenesis. Oncogene. 2005;24:1174–1187.
- Nanney LB, Brash AR, Jisaka M, et al. Molecular cloning and functional expression of a phorbol ester-inducible 8S-lipoxygenase from mouse skin. J Biol Chem. 1997;272:24410–24416.
- Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A. 1997;94:4312–4317.
- Meijer L, Brash AR, Bryant RW, et al. Stereospecific induction of starfish oocyte maturation by (8R)-hydroxyeicosatetraenoic acid. J Biol Chem. 1986;261:17040–17047.
- EG BLIGH, WJ DYER. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917.