ABSTRACT
Reg3β, a lectin, displays antibacterial activity. This study investigated Reg3β-expressing cells using IL-22-stimulated enteroids. IL-22 stimulation elevated the mRNA and protein levels of Reg3β. IL-22 also increased the mRNA levels of CD133 (a transit-amplifying cell marker) and lysozyme (a Paneth cell marker). Immunohistochemistry showed partial colocalization of Reg3β- and lysozyme-positive cells, suggesting that Paneth cells are one of Reg3β-producing cells.
KEYWORDS:
The intestine not only has a nutritional function by absorbing nutrients and water after consumption and digestion of foods but also has a barrier function by protecting the body from microorganisms, viruses, and toxic substances. The barrier function involves immune cells and various factors (IgA, mucus, and antimicrobial peptides). Regenerating islet-derived (Reg) proteins, which are C-type lectins, are among these antimicrobial peptides and are classified into four subtypes: I, II, III, and IV [Citation1]. Reg3 is expressed in the epithelial cells of the stomach, small intestine, and colon. It is secreted into the gut lumen in response to bacterial gut colonization and pathogenic infection or inflammation-elicited IL-22 [Citation2]. The murine intestine expresses two Reg3 family members (Reg3γ and Reg3β), which are similar in terms of structure, expression pattern, and regulation but have different bactericidal activities [Citation3]. Reg3γ binds to a peptidoglycan layer of gram-positive bacteria but not gram-negative bacteria and exerts bactericidal activity by oligomerizing to form hexameric transmembrane pores [Citation1,Citation2,Citation4]. Conversely, Reg3β has bactericidal activity against gram-positive and gram-negative bacteria. Against gram-negative bacteria, Reg3β recognizes the carbohydrate moiety of lipid A, a component of lipopolysaccharide, thereby eliciting outer membrane permeabilization and ultimately cell death [Citation2,Citation4].
Reg3γ is thought to be secreted from Paneth cells [Citation5–Citation8], enterocytes [Citation5–Citation8], and goblet cells [Citation5], and Cash et al. [Citation9] confirmed that Reg3γ is present in the secretory granules of Paneth cells using immunogold electron microscopy. Conversely to Reg3γ, information about Reg3β-producing cells is limited, although Paneth cells [Citation5,Citation6,Citation8], enterocytes [Citation5,Citation6,Citation10], goblet cells [Citation5], and transit-amplifying (TA) cells [Citation11] have been suggested as Reg3β-producing cells. Because the above studies were performed using small intestinal tissues, we attempted to use an intestinal organoid (enteroid) culture system to identify Reg3β-producing cells. In 2009, Sato et al. [Citation12] established a three-dimensional intestinal organoid culture system that mimics villi-crypt structures. The enteroids comprise stem cells and mature intestinal epithelial cells (goblet cells, enteroendocrine cells, Paneth cells, enterocytes, etc.), which replicate the in vivo functions of these cell types. Here we report that Paneth cells are one of Reg3β-producing cells and demonstrate the potential of using enteroids to identify cells that secrete functional substances.
Materials and methods used in this study is described in Supplemental materials. Initially, we investigated the presence of Reg3β protein and Reg3β mRNA in the jejunum using immunohistochemistry and in situ hybridization, respectively (Supplemental data 1). Immunohistochemistry results showed that the Reg3β protein was mainly present in the crypt region and villi base (Supplemental data 1A). Similarly, in situ hybridization results showed that the Reg3β mRNA was present in the crypt region and villi base (Supplemental data 1B). Burger-van Paassen et al. [Citation5] also showed a similar staining pattern for the Reg3β protein and mRNA in the jejuna of wild-type mice, and their further experiments suggested that Reg3β is produced by Paneth cells, enterocytes, and goblet cells. Other reports have also implied that Reg3β production is associated with Paneth cells [Citation6,Citation8], enterocytes [Citation6,Citation10], and TA cells [Citation11]. Our finding that the Reg3β protein was present in the crypt region and villi base suggests that Paneth, TA, and stem cells are involved in Reg3β production because these cells are also localized in crypt region and villi base. However, the broad expression pattern of the Reg3β protein, ranging from crypt to villi, made specific Reg3β-producing cells difficult to identify.
Therefore, we attempted to use enteroids to identify Reg3β-producing cells. However, unlike intestinal tissues, enteroids do not produce a detectable amount of Reg3β because purely cultured enteroids have no opportunity to be stimulated by immune cells or bacteria. IL-22 and bacterial stimulation can induce the expression of Reg3 [Citation2,Citation7,Citation16]. Therefore, we investigated whether IL-22 treatment induces the mRNA and protein expressions of Reg3β in enteroids. IL-22 treatment (10 ng/mL) significantly enhanced the mRNA levels of Reg3β after incubation for 1 h in a time-dependent manner (Supplemental data 2A). IL-22 stimulation at 1.0 or 10 ng/mL for 24 h significantly increased the mRNA levels of Reg3β (Supplemental data 2B). Very recently, Zha et al. [Citation17] showed that IL-22 stimulation (5 ng/mL for 3 days) increased the mRNA levels of Reg3β in jejunum enteroids. Immunohistochemistry () showed that the Reg3β protein was hardly detectable in the enteroids without IL-22 stimulation. Conversely, IL-22 treatment (10 ng/mL for 24 h) strongly induced the protein levels of Reg3β. The immunofluorescence staining of Reg3β-positive cells using enteroids was clearer than that using jejunum tissues (Supplemental data 1A). Particularly, the Reg3β-positive cells in the crypt region could be identified.
Figure 1. Induction of Reg3β protein in enteroids after IL-22 stimulation.
Representative confocal images of immunohistochemistry (Reg3β, green; nucleus, blue) for enteroids treated in the absence of IL-22 (control) and the presence of 10 ng/mL IL-22 [(IL-22 (+)] for 24 h. White arrow heads indicate Reg3β-producing cells. Scale bar is 50 μm.
![Figure 1. Induction of Reg3β protein in enteroids after IL-22 stimulation.Representative confocal images of immunohistochemistry (Reg3β, green; nucleus, blue) for enteroids treated in the absence of IL-22 (control) and the presence of 10 ng/mL IL-22 [(IL-22 (+)] for 24 h. White arrow heads indicate Reg3β-producing cells. Scale bar is 50 μm.](/cms/asset/e604e1f1-c1ac-41bd-b1cf-f87b5e484d89/tbbb_a_1695575_f0001_oc.jpg)
Next, to understand the effects of IL-22 on intestinal epithelial cells, the mRNA levels of marker proteins representing intestinal epithelial cells were measured after IL-22 stimulation (10 ng/mL for 24 h) (). IL-22 did not affect the mRNA levels of intestinal trefoil factor (Itf), chromogranin A (ChgA), sodium glucose co-transporter 1 (Sglt1), doublecortin-like kinase 1 (Dclk1), or leucine-rich orphan G-protein-coupled receptor 5 (Lgr5), which are markers for goblet cells, endocrine cells, enterocytes, tuft cells, and stem cells, respectively. Conversely, IL-22 treatment increased the mRNA levels of CD133 (a TA cell marker) and significantly increased the mRNA levels of lysozyme 1 (Lyz 1; a Paneth cell marker). These results suggest that TA cells and Paneth cells may be Reg3β-producing cells, but there is a possibility that IL-22 may have just increased the mRNA levels. Thus, the immunohistochemical staining of cell markers in enteroids after the treatment of IL-22 was performed. The immunohistochemical staining for CD133 covered the entire inside of the enteroids, and it was not clear whether CD133-positive and Reg3β-positive cells overlapped (data not shown). Therefore, we could not determine if TA cells expressed Reg3β in the present study. Moreover, staining for Lgr5 (the stem cell marker), ChgA (the endocrine cell marker), and Dclk1 (the tuft cell marker) did not overlap with Reg3β-positive cells (data not shown). Conversely, lysozyme-positive cells partly overlapped with Reg3β-positive cells (), strongly suggesting that Paneth cells are one of Reg3β-producing cells. However, some Reg3β-positive cells did not overlap with Paneth cells, suggesting that other cells are also associated with Reg3β production. Interestingly, Zha et al. [Citation17] found that IL-22 promoted TA cell proliferation. Our results also showed that IL-22 tended to elevate the mRNA levels of CD133 (the TA cell marker) (). Therefore, we expect TA cells to be Reg3β-producing cells. Further studies on this aspect are warranted.
Figure 2. The mRNA levels of marker of intestinal epithelial cells in enteroids after IL-22 stimulation.
The mRNA levels of Itf, ChgA, Dclk1, Sglt1, Lgr5, CD133, and lysozyme in enteroids after IL-22 stimulation (10 ng/mL) for 24 h were measured by qPCR. The mRNA level of Reg3β was normalized with that of β2 microglobuin, the expression level of the control group was set to 1, and that of the IL-22-treated group was relatively expressed. Bars represent means ± SE (n = 4). The comparison of means was performed with Student’s t-test. Asterisks represent a significant difference (p < 0.05).
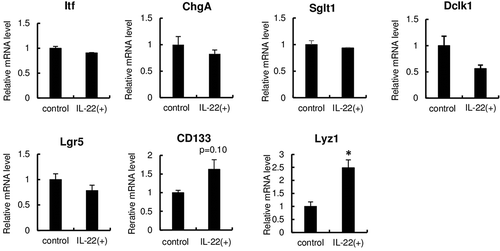
Figure 3. Immunohistochemical detection of Reg3β and lysozyme in enteroids after IL-22 stimulation.
Representative confocal images of immunohistochemistry (Reg3β, green; lysozyme, red; nucleus, blue) for enteroids treated in the absence of IL-22 (control) and the presence of 10 ng/mL IL-22 [IL-22 (+)] for 24 h. Green and red arrowheads indicate Reg3β-producing cells and Paneth cells, respectively. Yellow arrowheads indicate the overlapping of Reg3β-producing and Paneth cells. Scale bar is 50 μm.
![Figure 3. Immunohistochemical detection of Reg3β and lysozyme in enteroids after IL-22 stimulation.Representative confocal images of immunohistochemistry (Reg3β, green; lysozyme, red; nucleus, blue) for enteroids treated in the absence of IL-22 (control) and the presence of 10 ng/mL IL-22 [IL-22 (+)] for 24 h. Green and red arrowheads indicate Reg3β-producing cells and Paneth cells, respectively. Yellow arrowheads indicate the overlapping of Reg3β-producing and Paneth cells. Scale bar is 50 μm.](/cms/asset/6ec47217-a660-4ea7-92e8-20406b36a442/tbbb_a_1695575_f0003_oc.jpg)
In conclusion, our study is the first to directly show that Paneth cells are one of Reg3β-producing cells using IL-22-stimulated enteroids. Our study also demonstrated the potential of using enteroids as a tool to identify cells that secrete functional substances. Additionally, because enteroids can be passaged, their use can lead to a reduction or replacement of some experimental animal studies.
Author contribution
Mika Sato, Ken Iwatsuki, Miki Tadaishi, Makoto Shimizu, and Kazuo Kobayashi-Hattori designed the experiments. Mika Sato, Akihiko Inaba, and Yuki Saito performed the experiments. Mika Sato, Ken Iwatsuki, Makoto Shimizu, and Kazuo Kobayashi-Hattori wrote the paper.
revised_supplemental_materials_YT.pdf
Download PDF (48.1 KB)Supplemental_data_2_final__Sato_et_al_.pdf
Download PDF (93 KB)Supplemental_data_1_final__Sato_et_al_.pdf
Download PDF (114.6 KB)Acknowledgments
We are grateful to Dr. Tokiyoshi Ayabe (Hokkaido University) for the kind gift of anti-lysozyme antibody.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Shin JH, Seeley RJ. Reg3 proteins as gut hormones? Endocrinology. 2019;160:1506–1514.
- Miki T, Okada N, Hardt WD. Inflammatory bactericidal lectin RegIIIβ: Friend or foe for the host? Gut Microbes. 2018;9:179–187.
- Narushima Y, Unno M, Nakagawara K, et al. Structure, chromosomal localization and expression of mouse genes encoding type III Reg, RegIII alpha, RegIII beta, RegIII gamma. Gene. 1997;185:159–168.
- Miki T, Holst O, Hardt WD. The bactericidal activity of the C-type lectin RegIIIβ against Gram-negative bacteria involves binding to lipid A. J Biol Chem. 2012;287:34844–34855.
- Burger-van Paassen N, Loonen LM, Witte-Bouma J, et al. Mucin Muc2 deficiency and weaning influences the expression of the innate defense genes Reg3β, Reg3γ and angiogenin-4. PLoS One. 2012;7:e38798.
- Wells JM, Brummer RJ, Derrien M, et al. Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Gastrointest Liver Physiol. 2017;312:G171–G193.
- Mukherjee S, Hooper LV. Antimicrobial defense of the intestine. Immunity. 2015;42:28–39.
- Vaishnava S, Behrendt CL, Ismail AS, et al. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008;105:20858–20863.
- Cash HL, Whitham CV, Behrendt CL, et al. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130.
- Stelter C, Käppeli R, König C, et al. Salmonella-induced mucosal lectin RegIIIβ kills competing gut microbiota. PLoS One. 2011;6:e20749.
- Li B, Lu Y, Srikant CB, et al. Intestinal adaptation and Reg gene expression induced by antidiabetic duodenal-jejunal bypass surgery in Zucker fatty rats. Am J Physiol Gastrointest Liver Physiol. 2013;304:G635–G645.
- Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265.
- Saito Y, Iwatsuki K, Hanyu H, et al. Effect of essential amino acids on enteroids: methionine deprivation suppresses proliferation and affects differentiation in enteroid stem cells. Biochem Biophys Res Commun. 2017;488:171–176.
- Petersen N, Reimann F, Bartfeld S, et al. Generation of L cells in mouse and human small intestine organoids. Diabetes. 2014;63:410–420.
- Ke MT, Fujimoto S, Imai T. Optical clearing using SeeDB. Bio-protocol. 2014;4:e1042.
- Hou Q, Ye L, Liu H, et al. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death Differ. 2018;25:1657–1670.
- Zha JM, Li HS, Lin Q, et al. Interleukin 22 expands transit-amplifying cells while depleting Lgr5+ stem cells via inhibition of wnt and notch signaling. Cell Mol Gastroenterol Hepatol. 2019;7:255–274.
