?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Fish cell lines are widely used for the studies of developmental biology, virology, biology of aging, and nutrition physiology. However, little is known about their physicochemical properties. Here, we report the phospholipid compositions and mechanical properties of cell membranes derived from freshwater, anadromous and marine fish species. Biophysical analyses revealed that fish cell lines have highly deformable cell membranes with significantly low membrane tensions and Young’s moduli compared with those of mammalian cell lines. The induction of cellular senescence by DNA demethylation using 5-Aza-2ʹ-deoxycytidine significantly reduced the deformability of fish cell membrane, but hydrogen peroxide-induced oxidative stress did not affect the deformability. Mass spectrometry analysis of phospholipids revealed that the level of phosphatidylethanolamine molecules containing polyunsaturated fatty acids significantly increased during the 5-Aza-2ʹ-deoxycytidine-induced cellular senescence. Fish cell lines provide a useful model system for studying the changes in the physicochemical properties of cell membranes during cellular senescence.
Abbreviations: 2D-TLC: two-dimensional thin layer chromatography; 5-Aza-dC: 5-Aza-2ʹ-deoxycytidine; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; FBS: fetal bovine serum; PC: phosphatidylcholine; PE: phosphatidylethanolamine; PI: phosphatidylinositol; PS: phosphatidylserine; PUFA: polyunsaturated fatty acid; SA-β-gal: senescence-associated beta-galactosidase; SM: sphingomyelin
Graphic abstract
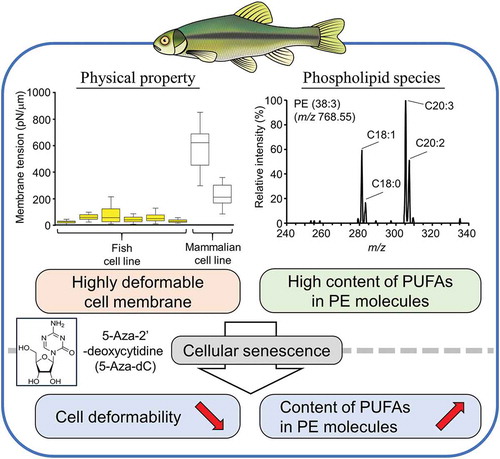
Fish cells have highly deformable cell membranes and induction of cellular senescence significantly affected the deformability and phospholipid species of the membrane.
Fish are the most diverse vertebrate class and comprise 50% of the extant vertebrate species on Earth [Citation1]. Fish provide essential nutrients for human development and health, which include polyunsaturated fatty acids (PUFAs), such as eicosapentaenoic acid (EPA, C20:5) and docosahexaenoic acid (DHA, C22:6) [Citation2]. Fish live in diverse habitat with different temperatures and salinities ranging from freshwater to hypersaline pools. Some euryhaline and anadromous fish species are tolerant to changes in osmotic pressures, where changes in environmental salinities have direct effects on the lipid metabolism of the fish cells: a significant increase in the content of C22:6 in membrane phospholipids following increased salinity has been reported in several fish species, such as rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar) [Citation3].
Another unique feature of fish is their indeterminate growth. The size of many fish species is not fixed; they grow continuously throughout their lifespan [Citation4–Citation6]. Although zebrafish (Danio rerio) have been used as a model organism for studying aging in vertebrates [Citation7], they are not ideal for studying the indeterminate growth of fish [Citation5]. Primary cultured cells from various fish species have also been shown to maintain their proliferation capacity and do not show signs of replicative senescence [Citation8–Citation10]. It has been suggested that the indeterminate growth of fish and fish cells is due to the high activity of telomerase, an enzyme which maintains the length of the telomeres at the ends of chromosomes, thereby attenuating the age-dependent decline of proliferative capacity [Citation11]. A recent study revealed that DNA demethylation induces senescence-associated phenotypes in a Pimephales promelas-derived immortal cell line (EPC); however, it does not cause further telomere shortening [Citation12]. This result suggests that the telomerase activity is not critical for the avoidance of cellular senescence in fish cell lines.
Aging is accompanied by progressive changes in the mechanical integrity and impaired responses of cells to external mechanical stimuli [Citation13,Citation14]. It has also been shown that the unsaturation of fatty acids in membrane lipids exacerbates the aging of animal species through the modulation of the basal metabolic rate and the production of reactive oxygen species that induce lipid peroxidation, oxidation of respiratory proteins, and DNA damage in mitochondria [Citation15,Citation16]. In this study, we analyzed the physicochemical properties of cell membranes derived from freshwater and saltwater fish species. We also examined whether cellular senescence caused by DNA hypomethylation affects the physicochemical properties of cell membranes. From these analyses, we demonstrated that fish cells have highly deformable cell membranes compared with mammalian cells. Furthermore, cellular senescence induced by 5-Aza-2ʹ-deoxycytidine (5-Aza-dC) impairs the deformability of fish cell membranes.
Materials and methods
Cell culture
EPC, EK1, and HINAE cells were derived from the following species: cyprinid fathead minnow (Pimephales promelas) [Citation17], Japanese eel (Anguilla japonica) [Citation18], Japanese flounder (Paralichthys olivaceus) [Citation19]. OMF cell was established in this study from a fish species produced by the crossbreeding of the Sakhalin sturgeon (Acipenser medirostis mikadoi) and the Russian sturgeon (Acipenser gueldenstaedtii). These cells were maintained in Leibovitz’s L-15 medium (Sigma-Aldrich) supplemented with 2 mM L-glutamine (Thermo Fisher Scientific), penicillin–streptomycin (Wako), and 10% (v/v) fetal bovine serum (FBS; Sigma) at 25°C. GEM-81 cells derived from the goldfish (Carassius auratus) [Citation20] were maintained in Leibovitz’s L-15 medium supplemented with 2 mM L-glutamine, penicillin–streptomycin, and 20% (v/v) FBS at 25°C. BRF-41 cells derived from zebrafish (Danio rerio) [Citation21] were maintained in Leibovitz’s L-15 medium (Sigma-Aldrich) supplemented with 2 mM L-glutamine, 10 mM HEPES, penicillin–streptomycin, and 15% (v/v) FBS at 33°C. HeLa and PC3 cells were maintained in Dulbecco’s Modified Eagle Medium (Thermo Fisher Scientific) and RPMI-1640 medium (Sigma-Aldrich) supplemented with 10% (v/v) FBS and penicillin–streptomycin at 37°C with 5% CO2, respectively.
Measurement of phospholipid composition and cholesterol content
Cellular total lipids were extracted using the Bligh and Dyer method [Citation22] and dissolved in chloroform. Phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI), and sphingomyelin (SM) were separated from the total lipid extract using two-dimensional thin-layer chromatography (2D-TLC). For the 2D-TLC, a first solvent system of chloroform/methanol/acetic acid (65:25:10, v/v/v) was used followed by a second solvent system of chloroform/methanol/formic acid (65:25:10, v/v/v) on a silica plate (Merk). The amount of phospholipid in each spot was determined by inorganic phosphate quantification [Citation23]. Cholesterol content was measured using the Cholesterol E-Test Kit (Wako).
Measurements of membrane tension and Young’s modulus
Micropipettes were manufactured from borosilicate glass capillaries using a PC-10 Puller (Narishige) and were polished using Microforge (Narishige). Cells were washed with PBS and incubated in 0.01% BSA in PBS for 10 min and then suspended in PBS containing 0.01% BSA. The cell suspension was maintained on the FluoroDish (World Precision Instruments) coated with agarose (Iwai Chemicals) to prevent cell adhesion.
Cells were aspirated with a micropipette under a series of increasing hydrostatic pressures created using adjustable water reservoirs. The normalized deformation (Lp/Rp), the ratio of the inner radius of the pipette (Rp) to the protrusion length (Lp), was plotted against each suction pressure (ΔP) and fitted to a curve using linear regression with the OriginPro software (OriginLab).
Young’s modulus (E) was calculated using Equation 1 employing the elastic model from Theret et al. [Citation24]. Phi (Φ) indicates the wall function, and a typical value for Φ is Φ = 2.1.
Membrane tension values were calculated using Equation 2 employing the law of Laplace [Citation25]. The values Tc, ∆Pc, and Rc indicate the membrane tension, critical pressure, and cell radius, respectively. Outlier values were identified using Smirnov–Grubbs’ outlier test.
Induction of the cellular senescence
Cells were incubated with 10 μM 5-Aza-2ʹ-deoxycytidine (5-Aza-dC; Tokyo Chemical Industry) at 25°C for 5 days to induce hypomethylation of DNA [Citation12]. Cells were incubated with culture medium containing 40 μM H2O2 for 2 h to induce oxidative stress.
To detect senescence-associated beta-galactosidase (SA-β-gal) activity, cells were fixed for 3 min in 2% formaldehyde/0.2% glutaraldehyde in PBS and incubated at 37°C for 3 days in a staining solution containing 40 mM citric acid/Na phosphate buffer, 5 mM K4[Fe(CN)6] 3H2O, 5 mM K3[Fe(CN)6], 150 mM NaCl, 2 mM MgCl2, and 1 mg/mL X-gal (Promega) [Citation26]. Cells were observed with fluorescence microscope Observer. Z1 (Zeiss). Nuclei structure of EPC cells was observed using confocal microscope LSM800 (Zeiss) after staining with Hoechst33342 dye.
Cells were digested in lysis buffer (20 mM Tris-HCl, pH 8.0, 5 mM EDTA, 1% SDS, 400 mM NaCl) containing 0.2 mg/mL Proteinase K (Sigma-Aldrich). Genomic DNA was isolated using ethanol precipitation, and extracted DNA was treated with RNase A to remove RNA contaminants. The proportion of methylated cytosines in the genomic DNA was determined using the MethylampTM Global DNA Methylation Quantification Kit (Epigentek) and a SPARK 10M multimode microplate reader (TECAN). The GC content in EPC cells was estimated to be 40.6% based on reported values for Pimephales promelas [Citation27].
Mass spectrometric analysis of phospholipids
LC-MS/MS analysis was conducted according to a previously reported protocol [Citation28]. Analysis of PC and PE was performed using the LC-30AD high-performance liquid chromatography system (Shimadzu) coupled to a triple quadrupole mass spectrometer LCMS-8040 (Shimadzu) equipped with an electrospray source [Citation2]. The separation was performed on a Kinetex C8 column (2.6 μm, 2.1 × 150 mm) (Phenomenex). The binary mobile phase had the following composition: 10 mM NH4HCO2 in water (mobile phase A) and 10 mM NH4HCO2 in 2-propanol/acetonitrile/water (45:45:10, v/v/v) (mobile phase B). The pump controlling the gradient of mobile phase B was programmed as follows: an initial isocratic flow at 20% B for 1 min, a linear increase to 40% B for 1 min, an increase to 92.5% B using a curved gradient for 23 min, a linear increase to 100% B for 1 min, and a hold at 100% B for 4 min. The total flow rate was 0.3 mL/min, the column temperature was 45°C, and the sample temperature was 4°C. The parameters for the spectrophotometer were as follows: nebulizer gas flow 2 L/min, drying gas flow 15 L/min, interface voltage 4.5 kV, DL temperature 250°C, and heating block temperature 400°C. The multiple reaction monitoring transition was [M + H]+ → [184.1]+ for PC and [M + H]+ → [M + H – 141.0]+ for PE. The fatty acid compositions of PC and PE were determined via product scan analyses of [M + HCOO]− and [M – H]− as precursor ions, respectively. PC (12:0–13:0) (Avanti Polar Lipids) and PE (12:0–13:0) (Avanti Polar Lipids) were used as internal standard. The peak area of each molecule was normalized by the peak area of internal standard and the total phospholipid content.
Statistical analysis
Statistically significant differences between the mean values were analyzed using the non-paired t-test. For datasets containing more than two groups, ANOVA was performed followed by Tukey’s test. A P-value less than 0.05 was considered statistically significant.
Results
Phospholipid composition of fish cell membranes
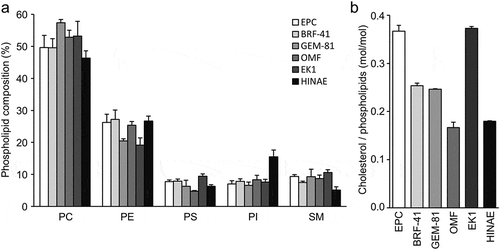
We used five cell lines originating from the Neopterygii group: three Cyprinidae cell lines [EPC (Pimephales promelas), BRF-41 (Danio rerio), GEM-81 (Carassius auratus)], one Anguillidae cell line [EK1 (Anguilla japonica)], and one Paralichthyidae cell line [HINAE (Paralichthys olivaceus)]. We also used one cell line originating from the Chondrostei group: Acipenseridae cell line [OMF (Acipenser species)]. These six cell lines are also categorized by their habitats: EPC, BRF-41, GEM-81, and OMF cells are derived from freshwater species, EK1 cells are derived from an anadromous species, and HINAE cells are derived from a marine species.
Since the composition of the cell membrane directly affects the physicochemical properties of the bilayer [Citation29,Citation30], we measured the phospholipid composition of the membranes from six fish cell lines by separating phospholipids via 2D-TLC and quantifying inorganic phosphate. PC was the most abundant phospholipid, which constituted more than 45% of the total phospholipids ()). PE was the second most abundant phospholipid in the cell membrane, which comprised between 15.1%–26.7% of the total phospholipid among the six cell lines. The proportions of PS, PI, and SM of each cell line were within the ranges of 4.8%–8.2%, 6.6%–15.5%, and 5.1%–9.6%, respectively. The cholesterol to phospholipid molar ratio in each cell line was within the range of 0.17–0.37 ()). The phospholipid compositions and cholesterol contents of the cell membranes were similar to those reported for mammalian cell lines, such as HeLa and PC3 cells [Citation31–Citation33]. These results raised the possibility that cells from fish have similar physicochemical properties to the cell membranes of mammalian cells.
Deformability of fish cell membranes
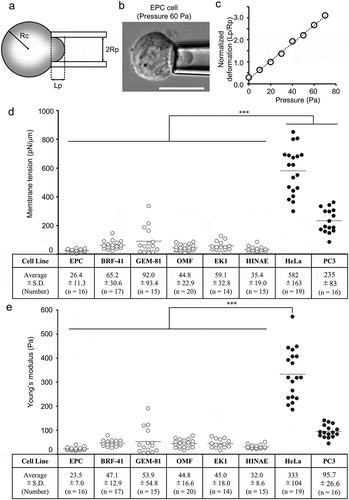
We evaluated the deformability of the cell membranes by measuring the membrane tension, a force per unit length acting on a cross section of the membrane, and Young’s modulus, a parameter for cellular stiffness, using the micropipette aspiration assay (). The values for the membrane tensions of the EPC, BRF-41, GEM-81, OMF, EK1, and HINAE cell lines were 26.4 ± 11.3, 65.2 ± 30.6, 92.0 ± 93.4, 44.8 ± 22.9, 59.1 ± 32.8, and 35.4 ± 19.0 pN/μm, respectively. We also measured the membrane tensions of two mammalian tumor cell lines, HeLa and PC3, which have rigid and flexible cell membranes, respectively [Citation34,Citation35]. The membrane tension measurements for HeLa and PC3 cells were 582 ± 163 and 235 ± 82.8 pN/μm, respectively. These values were significantly higher than those for any of the fish cell lines ()). The Young’s moduli for the membranes from fish cells ranged from 23.5 to 53.9 pN/μm. These measurements were significantly lower than those of HeLa and PC3 cells (333 ± 104 and 95.7 ± 26.6 Pa, respectively) ()). These results clearly demonstrate that cell membranes of fish cells are more deformable than those of mammalian cells.
Figure 2. Membrane deformability of fish cell lines.
(a) Schematic of the micropipette aspiration assay. Lp, Rp, and Rc indicate protrusion length, inner radius of the pipette, and cell radius, respectively. (b) EPC cells were aspirated using cylindrical micropipettes at 60 Pa hydrostatic pressure. Scale bar = 10 μm. (c) Representative data from the micropipette aspiration assay for the EPC cell line. Membrane tensions (d) and Young’s moduli (e) for EPC, BRF-41, GEM-81, OMF, EK1, HINAE, HeLa, and PC3 cells were calculated from the linear function using the law of Laplace and the theoretical model. Values are expressed as Means ± SDs, and the number of analyzed cells are shown in parentheses. *** P < 0.001 by Tukey’s test.
Effect of 5-Aza-dC on the deformability of cell membranes in EPC cells
Changes in the mechanical properties of cell membranes are known as a hallmark of cellular senescence [Citation13]. 5-Aza-dC is an inhibitor of DNA methyltransferase and is reported to induce DNA hypomethylation and cellular senescence in mammalian tumor cells [Citation36]. Recently, 5-Aza-dC was also reported to induce cellular senescence in EPC cells [Citation12]. Because EPC is an immortal fish cell line that has the most deformable cell membrane among the analyzed cell lines, we evaluated the effect of 5-Aza-dC on the deformability of EPC cell membranes to examine the relationship between cellular senescence and the physicochemical properties of cell membranes in fish cells.
First, we measured the proportion of methylated cytosines in the genomic DNA of EPC cells. The proportion of methylated cytosines in the total cytosine bases of genomic DNA of EPC cells was 3.3 ± 0.3% under normal culture conditions but decreased to 1.6 ± 0.2% after 5 days of 10 µM 5-Aza-dC treatment ()). The induction of cellular senescence after 5-Aza-dC treatment was confirmed by measuring the activity of SA-β-Gal [Citation37], using X-gal-staining. Similar to previous report [Citation12], SA-β-Gal activity in EPC cells was robustly induced by 5-Aza-dC treatment for 5 days [Citation38] ()). Given that 5-Aza-dC treatment induces mitotic arrest and apoptosis in mammalian cells [Citation39,Citation40], we analyzed the nuclear morphology of EPC cells using confocal microscopy after staining with Hoechst33342 dye. Aberrant nuclei structures, including lobulated nuclei and enlarged nuclei, were observed in most of the EPC cells treated with 5-Aza-dC (), arrows). Moreover, fragmented nuclei, a sign of apoptosis, was also observed in some of the 5-Aza-dC-treated EPC cells (), arrowheads). Consistent with a previous report by Futami et al [Citation12], the senescence-associated phenotype could be induced by 5-Aza-dC treatment in EPC cells.
Figure 3. Cellular senescence induced by 5-Aza-dC.
(a) DNA methylation levels in EPC cells treated with 10 μM 5-Aza-dC or vehicle were measured using the MethylampTM Global DNA Methylation Quantification Kit (n = 3). Values are expressed as Means ± SDs. ** P < 0.01 by Student’s t-test. (b) EPC cells were stained with X-gal to detect the activity of SA-β-gal. Scale bar = 100 μm. (c) Cell nuclei were stained with Hoechst 33342 dye. Arrows indicate lobulated nuclei and enlarged nuclei, and arrowheads indicate fragmented nuclei. Scale bar = 50 μm. (b, c) Representative results from three independent experiments are shown. Membrane tensions (d) and Young’s moduli (e) for EPC cells treated with vehicle, 10 μM 5-Aza-dC, and 40 μM H2O2. Values are expressed as Means ± SDs, and the number of analyzed cells are shown in parentheses. n.s. = not significant, *** P < 0.001 by Tukey’s test.
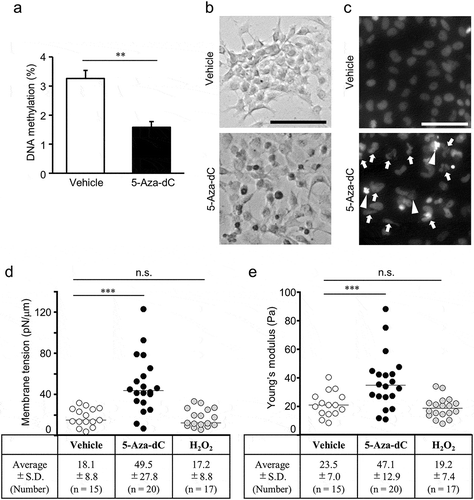
To evaluate the relationship between cellular senescence and the deformability of cell membranes, we analyzed the effect of 5-Aza-dC treatment on the membrane tension and Young’s modulus in the cell line. The membrane tension of EPC cells treated with 5-Aza-dC was 49.5 ± 27.8 pN/μm, which was 2.7-fold higher than that of vehicle-treated cells ()). Young’s modulus of EPC cells treated with 5-Aza-dC was 47.1 ± 12.9 Pa, which was 2.0-fold higher than that of vehicle-treated cells ()). In contrast, the membrane tension and Young’s modulus of EPC cells treated with H2O2, an agent inducing cellular senescence through oxidative stress, were comparable to those of vehicle-treated cells. These results revealed that the deformability of cell membranes was decreased by 5-Aza-dC-induced cellular senescence in EPC cells.
Effect of 5-Aza-dC on the fatty acid composition of phospholipids
It has been reported that the fatty acid unsaturation of membrane lipids is closely linked with aging in human, mouse, insects, and fish [Citation41,Citation42]. Moreover, the composition of acyl chains in phospholipids affects the deformability of the lipid bilayer [Citation43]. Thus, the fatty acid compositions of PC and PE in EPC cells were analyzed using LC-MS/MS. In our LC-MS/MS system, 20 and 26 molecular species with different total acyl chain lengths and numbers of double bonds were detected for PC and PE, respectively. The predominant PC molecule in EPC cells was PC (34:1) followed by PC (36:2) ()). PC molecules containing zero, one, or two double bond(s) comprised 86.1% of the total PC molecules. In contrast, the predominant PE molecule in EPC cells was PE (36:2) followed by PE (34:1) ()). PE molecules containing three or more double bonds comprised 52.7% of the total PE molecules.
Figure 4. Changes in phospholipid molecules after 5-Aza-dC treatment.
PC (a) and PE (b) molecules in EPC cells treated with vehicle or 10 μM 5-Aza-dC were analyzed by LC-MS/MS. Phospholipid molecules were presented in the format PC (X:Y) or PE (X:Y), where X denotes the total number of acyl chain carbons and Y denotes the total number of double bonds in acyl chains (n = 3). Values are expressed as Means ± SDs. *P < 0.05, **P < 0.01, by Student’s t-test.
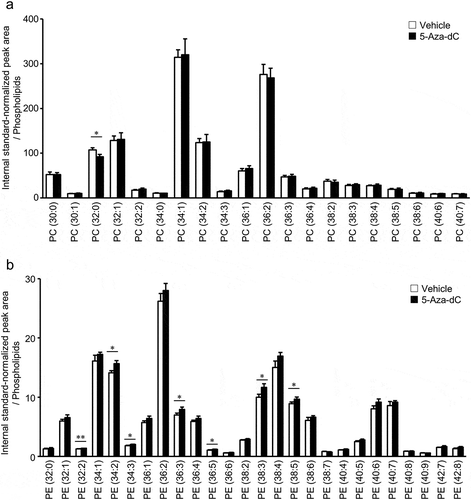
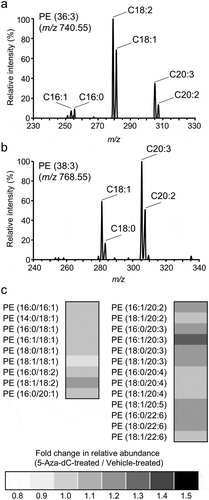
Next, the effects of 5-Aza-dC treatment on the fatty acid composition of PC and PE in EPC cells were analyzed. As presented in ), the amount of PC molecules, except for PC (36:0), was not significantly changed by 5 days of 5-Aza-dC treatment. On the other hand, the levels of several PE molecules containing two or more double bonds [PE (32:2), PE (34:2), PE (34:3), PE (36:3), PE (36:5), PE (38:3), and PE (38:5)] were significantly increased by 5-Aza-dC treatment ()). In order to determine the composition of fatty acid in each PE molecule, product ion scan analysis was conducted on 13 major molecules ( and Sup. Table. 1). For instance, C22:6, which was expected to be derived from the culture medium, was detected in PE (38:6), PE (40:6), and PE (40:7) (Sup. Table. 1). As previously reported, C20:2 and C20:3, which do not exist in the culture medium were also detected in PE molecules in EPC cells (Sup. Table. 1) [Citation44]. Especially, C20:3 was enriched in PE (36:3) ()) and PE (38:3) ()), whose contents were increased by 5-Aza-dC treatment ()). As presented in ), several types of PE species containing culture medium-derived PUFAs and de novo-synthesized PUFAs were increased following treatment with 5-Aza-dC. These results demonstrated that the fatty acid composition of PE, rather than PC, is modulated during the course of cellular senescence induced by 5-Aza-dC treatment in EPC cells.
Figure 5. Changes in molecular species of PE after 5-Aza-dC treatment.
Components of fatty acids in PE (36:3) (a) and PE (38:3) (b) were analyzed using product ion scan analysis with LC-MS/MS. (c) The fold changes of relative peak areas were described using a heat map. PE molecules were presented in the format PE (X/Y), where X and Y denote fatty acid species. The order of fatty acids does not represent the position of acyl chains in the PE molecule.
Discussion
Although fish cell lines are widely used for toxicology, developmental biology, virology, and nutritional physiology [Citation45–Citation47], little is known about the physicochemical properties of their cell membranes. In this study, we demonstrated that fish cells have highly deformable membranes, with significantly lower membrane tensions and Young’s moduli compared with those of mammalian cells (). Although the fish cell lines were derived from various tissues, such as the embryo (HINAE), kidney (EK1), fin (BRF-41), skin (EPC), and cutaneous tumor (GEM-81), no significant differences in membrane deformability were observed among the different fish cell lines. This finding suggests that increased deformability is a general feature of fish cell membranes. It has been demonstrated that cells with the ability to grow continuously, such as mammalian stem cells and cancer cells, have highly deformable cell membranes, and their deformability drastically decreases during differentiation [Citation48–Citation50]. Therefore, it is plausible to speculate that increased deformability of fish cell membranes is associated with the continuous proliferative capacity of fish cells, as described in the Introduction.
The mechanical properties of cell membranes are closely related to aging [Citation13]. It has been reported that cellular elasticities of human cardiac myocytes and epithelial cells are significantly decreased with aging [Citation51,Citation52], and numerous aging-related diseases, such as vascular degeneration are caused by defects in cellular stiffness [Citation53]. We demonstrated that the membrane deformability of EPC cells was significantly decreased following treatment with an inhibitor of DNA methyltransferase, 5-Aza-dC ()). Although 5-Aza-dC supposedly induces oxidative stresses [Citation54], the membrane deformability of EPC cells was not affected by H2O2-induced oxidative stress. This finding suggests that the change in membrane deformability by 5-Aza-dC treatment is not dependent on oxidative stress in EPC cells. Currently, 5-Aza-dC is a clinically approved DNA methyltransferase (DNMT) inhibitor that causes DNA hypomethylation and induces p53-dependent cell senescence in mammalian tumor cells [Citation36]. In the present assay conditions, EPC cells also exhibited several senescence-associated phenotypes after 5-Aza-dC treatment: (i) hypomethylation of genomic DNA ()), (ii) enhancement of SA-β-Gal activity ()) [Citation12], and (iii) aberrant nuclei structures induced by mitotic arrest and apoptosis ()) [Citation12]. These results suggest that the stiffness of the cell membrane is also increased by cellular senescence in fish cells. It was reported that aging causes remodeling of the actin cytoskeleton, which plays a major role in the regulation of cell membrane deformation [Citation55]. Moreover, 5-Aza-dC is reported to cause cytoskeletal remodeling in mammalian cells [Citation56]. Thus, further studies focusing on the construction and remodeling of the cytoskeleton are required to reveal the molecular mechanisms underlying the decreased deformability of the cell membrane in 5-Aza-dC-treated EPC cells.
Since the unsaturation of fatty acyl chains of membrane phospholipids is closely related to longevity and aging [Citation42], we also examined whether the 5-Aza-dC-induced cellular senescence affects the composition of fatty acyl chains in phospholipids of EPC cells. Our study revealed that 52.7% of PE molecules contained PUFAs, including C20:2, C20:3, C20:4, C20:5, and C22:6. By contrast, 86.1% of PC molecules were composed of zero, one, or two double bond(s)-containing species. Thus, it is likely that EPC cells may have acyltransferases that preferentially incorporate PUFAs into PE molecules. Tocher et al. [Citation44] reported that several fish cell lines have characteristic patterns of n-9 PUFAs which do not exist in the culture medium. EPC cells can de novo synthesize n-9 PUFAs, including C20:2 and C20:3 using ∆6- and ∆5-desaturases and C18-20- and C20-22-elongases [Citation57]. Moreover, the levels of C20:2 and C20:3 in fish cell lines are increased by cultivating cells in culture medium that is deficient in essential fatty acids [Citation44], thus indicating that C20:2 and C20:3 are actively synthesized in fish cells. We demonstrated that the proportions of PE molecules containing C20:2 and C20:3 were increased by 5-Aza-dC treatment in EPC cells. In mammals, aging alters the fatty acid composition of phospholipids and therefore the membrane fluidity [Citation58,Citation59]. Moreover, the dysregulation of lipid metabolism is one cause of aging-related diseases [Citation16]. It has been demonstrated that aging-associated DNA methylation induces epigenetic reprogramming of lipid metabolism [Citation60]. For example, hypomethylation of genes involved in regulating the metabolism of long-chain fatty acids, such as fatty acid elongase 5 (Elovl5) and Elovl6, which catalyzes the initial and rate-limiting steps in fatty acid elongation to produce long-chain PUFA, is associated with aging in mice. It is intriguing to speculate that changes in the PE molecular species of EPC cells are caused by the aging-related remodeling of DNA methylation specific to certain genome loci. Moreover, it is interesting to evaluate whether the acyl chain remodeling in PE molecules affects the physicochemical properties of the cell membranes during cellular senescence in EPC cells. Because increased PUFA contents are preferred for cell membrane deformation [Citation61], increased PUFA content in PE molecules is unlikely to cause a decrease in the deformability of the bilayer membrane itself in EPC cells. Alternatively, actin dynamics regulated by PE molecules [Citation62,Citation63] might be involved in the decreased deformability of the cell membrane in 5-Aza-dC-treated EPC cells.
In this study, we demonstrated that the deformability of fish cells is higher than that of mammalian cells, and it is decreased by cellular senescence induced by 5-Aza-dC treatment. Fish cell lines provide a useful model system for analyzing the physicochemical properties of cell membranes during cellular senescence.
Authors contribution
M.U. conceived and designed the project. M.U. and K.N. supervised the research. A.S. performed most experiments with assistance from H.K. and Y.H. H.K. provided fish cell lines. A.S., K.N., and M.U. interpreted data and wrote the manuscript.
Supplemental_Table1.pdf
Download PDF (56.1 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Zhang Z-Q. Animal biodiversity: an update of classification and diversity in 2013. Zootaxa. 2013;3703(1):5–11.
- Suito T, Nagao K, Hatano M, et al. Synthesis of omega-3 long-chain polyunsaturated fatty acid-rich triacylglycerols in an endemic goby, gymnogobius isaza, from Lake Biwa, Japan. J Biochem. 2018 Aug 1;164(2):127–140.
- Tocher DR, Castell JD, Dick JR, et al. Effects of salinity on the fatty acid compositions of total lipid and individual glycerophospholipid classes of Atlantic salmon (Salmo salar) and turbot (Scophthalmus maximus) cells in culture. Fish Physiol Biochem. 1995;14(2):125–137.
- Sebens KP. The ecology of indeterminate growth in animals. Annu Rev Ecol Syst. 1987;18(1):371–407.
- Mommsen TP. Paradigms of growth in fish. Comp Biochem Physiol Part B Biochem Mol Biol. 2001;129(2–3):207–219.
- Johnston IA, Bower NI, Macqueen DJ. Growth and the regulation of myotomal muscle mass in teleost fish. J Exp Biol. 2011;214(10):1617–1628.
- Anchelin M, Murcia L, Alcaraz-Pérez F, et al. Behaviour of telomere and telomerase during aging and regeneration in zebrafish. PloS One. 2011;6(2):e16955.
- Fryer JL, Lannan C. Three decades of fish cell culture: a current listing of cell lines derived from fishes. J Tissue Culture Methods. 1994;16(2):87–94.
- Servili A, Bufalino MR, Nishikawa R, et al. Establishment of long term cultures of neural stem cells from adult sea bass, dicentrarchus labrax. Comp Biochem Physiol Part A. 2009;152(2):245–254.
- Lakra W, Swaminathan TR, Joy K. Development, characterization, conservation and storage of fish cell lines: a review. Fish Physiol Biochem. 2011;37(1):1–20.
- Klapper W, Heidorn K, Kühne K, et al. Telomerase activity in ‘immortal’fish 1. FEBS Lett. 1998;434(3):409–412.
- Futami K, Maita M, Katagiri T. DNA demethylation with 5-aza-2ʹ-deoxycytidine induces the senescence-associated secretory phenotype in the immortal fish cell line, EPC. Gene. 2019 May 20;697:194–200.
- Phillip JM, Aifuwa I, Walston J, et al. The mechanobiology of aging. Annu Rev Biomed Eng. 2015;17:113–141.
- Harris MJ, Wirtz D, Wu P-H. Dissecting cellular mechanics: implications for aging, cancer, and immunity. Semin Cell Dev Biol. 2019;93:16–25.
- López-Otín C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell. 2013;153(6):1194–1217.
- Gonzalez-Covarrubias V. Lipidomics in longevity and healthy aging. Biogerontology. 2013;14(6):663–672.
- Fijan N, Sulimanović D, Bearzotti M, et al. Some properties of the epithelioma papulosum cyprini (EPC) cell line from carp Cyprinus carpio. Annales de l’Institut pasteur/Virologie. 1983;134(2):207–220.
- Chen S-N. A cell line derived from Japanese eel (Anguilla japonica) kidney. Proc Natl Sci Counc ROC. 1982;6:93–100.
- Kasai H, Yoshimizu M. Establishment of two Japanese flounder [Paralichthys olivaceus] embryo cell lines. Japan: Bulletin of Fisheries Sciences, Hokkaido University; 2001.
- Matsumoto J, Ishikawa T, Masahito P, et al. Permanent cell lines from erythrophoromas in goldfish (Carassius auratus). J Natl Cancer Inst. 1980;64(4):879–890.
- Saito T, Hascilowicz T, Ohkido I, et al. Two zebrafish (Danio rerio) antizymes with different expression and activities. Biochem J. 2000;345(Pt 1):99.
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917.
- Rouser G, Siakotos AN, Fleischer S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids. 1966. 1. Jan(1):85–86.
- Theret DP, Levesque M, Sato M, et al. The application of a homogeneous half-space model in the analysis of endothelial cell micropipette measurements. J Biomech Eng. 1988;110(3):190–199.
- Hochmuth RM. Micropipette aspiration of living cells. J Biomech. 2000 Jan;33(1):15–22.
- Debacq-Chainiaux F, Erusalimsky JD, Campisi J, et al. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc. 2009;4(12):1798–1806.
- Symonová R, Howell WM. Vertebrate genome evolution in the light of fish cytogenomics and rDNAomics. Genes (Basel). 2018;9(2):96.
- Matsuo N, Nagao K, Suito T, et al. Different mechanisms for selective transport of fatty acids using a single class of lipoprotein in Drosophila. J Lipid Res. 2019;60(7):1199–1211.
- Evans E, Needham D. Physical properties of surfactant bilayer membranes: thermal transitions, elasticity, rigidity, cohesion and colloidal interactions. J Phys Chem. 1987;91(16):4219–4228.
- Garnier M, Attali J, Valensi P, et al. Erythrocyte deformability in diabetes and erythrocyte membrane lipid composition. Metabolism. 1990;39(8):794–798.
- Llorente A, Skotland T, Sylvänne T, et al. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim Biophys Acta (BBA)-Mol Cell Biol Lipids. 2013;1831(7):1302–1309.
- Litvak V, Dahan N, Ramachandran S, et al. Maintenance of the diacylglycerol level in the Golgi apparatus by the Nir2 protein is critical for Golgi secretory function. Nat Cell Biol. 2005;7(3):225.
- Van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9(2):112.
- Hu S, Wang R, Tsang CM, et al. Revealing elasticity of largely deformed cells flowing along confining microchannels. RSC Adv. 2018;8(2):1030–1038.
- Li Y-J, Yang Y-N, Zhang H-J, et al. A microfluidic micropipette aspiration device to study single-cell mechanics inspired by the principle of wheatstone bridge. Micromachines. 2019;10(2):131.
- Venturelli S, Berger A, Weiland T, et al. Differential induction of apoptosis and senescence by the DNA methyltransferase inhibitors 5-azacytidine and 5-aza-2′-deoxycytidine in solid tumor cells. Mol Cancer Ther. 2013;12(10):2226–2236.
- Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Nat Acad Sci. 1995;92(20):9363–9367.
- Vo NT, Mikhaeil MS, Lee LE, et al. Senescence-associated β-galactosidase staining in fish cell lines and primary cultures from several tissues and species, including rainbow trout coelomic fluid and milt. In Vitro Cell Dev Biol-Anim. 2015;51(4):361–371.
- Galluzzi L, Vitale I, Abrams J, et al. Molecular definitions of cell death subroutines: recommendations of the nomenclature committee on cell death 2012. Cell Death Differ. 2012;19(1):107.
- Palii SS, Van Emburgh BO, Sankpal UT, et al. DNA methylation inhibitor 5-Aza-2′-deoxycytidine induces reversible genome-wide DNA damage that is distinctly influenced by DNA methyltransferases 1 and 3B. Mol Cell Biol. 2008;28(2):752–771.
- Almaida-Pagán PF, Lucas-Sanchez A, Tocher DR. Changes in mitochondrial membrane composition and oxidative status during rapid growth, maturation and aging in zebrafish, Danio rerio. Biochim Biophys Acta (BBA)-Mol Cell Biol Lipids. 2014;1841(7):1003–1011.
- Calhoon EA, Ro J, Williams JB. Perspectives on the membrane fatty acid unsaturation/pacemaker hypotheses of metabolism and aging. Chem Phys Lipids. 2015;191:48–60.
- Rawicz W, Olbrich K, McIntosh T, et al. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys J. 2000;79(1):328–339.
- Tocher DR, Dick JR, Sargent JR. Development of an in vitro model of essential fatty acid deficiency in fish cells. Prostaglandins, Leukotrienes Essent Fatty Acids. 1995;53(5):365–375.
- Takano R, Nishizawa T, Arimoto M, et al. Isolation of viral haemorrhagic septicaemia virus (VHSV) from wild Japanese flounder, Paralichthys olivaceus. Bull Eur Assoc Fish Pathol. 2000;20(5):186–192.
- Hashimoto H, Takeuchi K, Matsuo Y, et al. Cell growth of fish cultures in hyper-and hypoosmotic media. Fish Sci. 1998;64(2):350–351.
- Ikemoto K, Yoshimizu M, Furihata M, et al. Pathogenicity of a rainbow trout isolate RtNa-0010 of Oncorhynchus masou virus (Salmonid herpesvirus 2) in Salmonid and Cyprinid Fish. Fish Pathol. 2016;51(2):60–63.
- Sliogeryte K, Thorpe SD, Lee DA, et al. Stem cell differentiation increases membrane-actin adhesion regulating cell blebability, migration and mechanics. Sci Rep. 2014;4:7307.
- Zhang W, Kai K, Choi DS, et al. Microfluidics separation reveals the stem-cell–like deformability of tumor-initiating cells. Proc Nat Acad Sci. 2012;109(46):18707–18712.
- Uzer G, Fuchs RK, Rubin J, et al. Concise review: plasma and nuclear membranes convey mechanical information to regulate mesenchymal stem cell lineage. Stem Cells. 2016;34(6):1455–1463.
- Lieber SC, Aubry N, Pain J, et al. Aging increases stiffness of cardiac myocytes measured by atomic force microscopy nanoindentation. Am J Physiol Heart Circ Physiol. 2004;287(2):H645–H651.
- Berdyyeva TK, Woodworth CD, Sokolov I. Human epithelial cells increase their rigidity with ageing in vitro: direct measurements. Phys Med Biol. 2004;50(1):81.
- Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932–943.
- Shin DY, Park Y-S, Yang K, et al. Decitabine, a DNA methyltransferase inhibitor, induces apoptosis in human leukemia cells through intracellular reactive oxygen species generation. Int J Oncol. 2012;41(3):910–918.
- Pourati J, Maniotis A, Spiegel D, et al. Is cytoskeletal tension a major determinant of cell deformability in adherent endothelial cells? Am J Physiol Cell Physiol. 1998;274(5):C1283–C1289.
- Logan PC, Ponnampalam AP, Rahnama F, et al. The effect of DNA methylation inhibitor 5-Aza-2′-deoxycytidine on human endometrial stromal cells. Hum Reprod. 2010;25(11):2859–2869.
- Tocher DR, Dick JR. Polyunsaturated fatty acid metabolism in a cell culture model of essential fatty acid deficiency in a freshwater fish, carp (Cyprinus carpio). Fish Physiol Biochem. 1999;21(3):257–267.
- Alvarez E, Ruiz‐Gutiérrez V, Sobrino F, et al. Age‐related changes in membrane lipid composition, fluidity and respiratory burst in rat peritoneal neutrophils. Clin Exp Immunol. 2001;124(1):95–102.
- Hulbert AJ, Pamplona R, Buffenstein R, et al. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev. 2007;87(4):1175–1213.
- Hahn O, Grönke S, Stubbs TM, et al. Dietary restriction protects from age-associated DNA methylation and induces epigenetic reprogramming of lipid metabolism. Genome Biol. 2017;18(1):56.
- Pinot M, Vanni S, Pagnotta S, et al. Polyunsaturated phospholipids facilitate membrane deformation and fission by endocytic proteins. Science. 2014;345(6197):693–697.
- Emoto K, Inadome H, Kanaho Y, et al. Local change in phospholipid composition at the cleavage furrow is essential for completion of cytokinesis. J Biol Chem. 2005;280(45):37901–37907.
- Kato U, Inadome H, Yamamoto M, et al. Role for phospholipid flippase complex of ATP8A1 and CDC50A proteins in cell migration. J Biol Chem. 2013;288(7):4922–4934.