ABSTRACT
Alveolar and bronchial epithelial cells have critical functions in acute respiratory distress syndrome progress. Genistein could protect the lungs from acute lung injury, however, whether genistein protects the alveolar epithelial cells from LPS-induced injury was less studied. Spectrophotometric method 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) and enzyme-linked immunosorbent assay (ELISA) were performed to detect cell viability and levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6. Flow cytometry and western blot assay were performed to detect cells apoptosis and protein levels. In LPS-induced model of mouse lung epithelial (MLE)-12 cells, PBEF (proinflammatory cytokine) expression, and cell apoptosis were increased and cell viability was decreased, whereas NF-κB was activated and expression levels of TNF-α, IL-1β, and IL-6 were increased. However, genistein partly reversed the effect of LPS, and it plays a protective role in lung injury by reducing expression of PBEF, inhibiting the activation of NF-κB and alleviating inflammatory response of cells.
Graphical abstract
LPS increased the alveolar epithelial cell injury, and GEN suppressed the activation of NF-κB by reducing the of PBEF expression, reducing the inflammatory response of cell.
Inflammatory pulmonary disease could lead to severe lung failures such as acute lung injury, respiratory distress syndrome, and alveolar edema [Citation1]. Acute pulmonary inflammation is characterized by increased endothelial cell permeability, alveolar damage, pulmonary edema, and aggregation of inflammatory cells into lung tissue, moreover, tissue injuries will be aggravated under server inflammatory conditions [Citation2]. However, the mechanism of pulmonary epithelial barrier rupture during inflammation remains unclear. In recent years, it has been found that bronchial epithelial and alveolar epithelial cells play critical roles in the pathological development of pulmonary fibrosis and acute respiratory distress syndrome [Citation3,Citation4], and that normal prompt repair of epithelial structure and function could be decisive to the recovery from lung injury [Citation5].
Pre-B cell colony enhancing factor (PBEF) is synthesized and secreted by activating lymphocytes, neutrophils, and epithelial cells, and its pro-inflammatory effect occurs by promoting the production of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6 and pro-inflammatory cytokines [Citation6], moreover, these inflammatory factors have important functions in the diagnosis and evaluation of inflammatory diseases [Citation7]. It was demonstrated that PBEF can inhibit apoptosis of pulmonary neutrophils, promote release of inflammatory mediators, increase cell permeability, and could cause pulmonary edema and lung injury [Citation8]. Furthermore, previous study [Citation9] showed that PBEF promotes apoptosis of endothelial progenitor cells through increasing the expressions of pro-inflammatory mediators via nuclear factor kappa B (NF-κB) pathway. Another study confirmed that in pneumococcal pneumonia, the antimicrobial defense mechanism is initiated by alveolar macrophages, which partly depends on the transcription of NF-κB [Citation10].
It is widely known that genistein has anti-oxidative, anti-inflammatory, and anti-cancer effects and could protect the human body from lungs injury [Citation11,Citation12]. According to Vidya Mukund et al. [Citation13], genistein has a positive effect on inhibiting breast cancer, and the expression of NF-κB in the nuclear of breast cancer cell lines was observed inhibited by genistein. Yuzhen Zheng et al. [Citation14] also found that genistein can inhibit the growth of gliosarcoma cells and induce apoptosis through the inhibition of NF-κB-mediated signaling. However, it is rarely reported whether genistein affects the repair of alveolar epithelial cells. Therefore, the current study conducted in vitro experiments to investigate the repairing effect of genistein on alveolar epithelial cells and its mechanism. Moreover, possible mechanism of genistein on pulmonary inflammatory response was explored, so as to improve the current treatment of pulmonary inflammatory response.
Materials and methods
Cell culture and group intervention
Murine lung epithelial (MLE-12) cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in DMEM medium (Gibco, BRL, USA) containing 10% fetal bovine serum (FBS) (Gibco, BRL, USA), 100 U/mL penicillin and 100 µg/mL streptomycin, and incubated with 5% CO2 at 37°C. The cells at logarithmic growth phase were used for further experiments.
The cells (3 × 105 cells/well) were inoculated into a 6-well plate. To investigate the cytotoxic effects of genistein at different concentrations on the cells, the cells were divided into five groups according to treated genistein concentrations (0, 1, 5, 10, and 25 µM) (No. 446-72-0, Shanghai Ronghe Medical Science and Technology Development Co., Ltd., Shanghai, China). To explore the possible mechanism of genistein in cell repair, the cells were divided into six groups, namely, control group (untreated cells), lipopolysaccharide group (cells treated by 500 ng/mL LPS (Sigma, USA), LPS + FK866 group (cells treated by LPS and FK866 (PBEF inhibitor, 10 nM, Sigma, USA)), LPS + L-GEN group (cells treated by LPS and genistein (1 µM)); LPS + H-GEN group (cells treated by LPS and genistein (10 µM)), H-GEN group (10 µM). In order to detect the transfection rate, the cells were divided into three groups, namely, control group, negative control (NC) group, PBEF group (cells transfected with pCAGGS-PBEF).
Cell transfection
The cells (3 × 105 cells/well) were inoculated into a 6-well plate. After cell fusion reached 80% fusion, the cells were transfected with pCAGGS empty vector as NC, while those transfected with the recombinant plasmid pCAGGS-PBEF, which was constructed by directional cloning of PBEF into pCAGGS vector as PBEF group. PBEF was overexpressed in MLE-12 cells by a transient transfection of pCAGGS-PBEF into MLE-12 cells. The cell transfection was carried out using Lipofectamine 2000 Kit (Invitrogen, Carlsbad, CA). Finally, the cells were cultured in an incubator with 5% CO2 at 37°C for 4 days for further experiment.
MTT assay
The cells (1 × 104 cells/well) were inoculated in 96-well plates. LPS, FK866, and different concentrations of genistein (1, 5, 10, and 25 µM) were added into the cell after cell adherence; meanwhile, corresponding control groups were set up. Five multiple wells were set in each group, with the final volume of 200 µL. After incubation for 24 h, 20 µL MTT (5 g/L) was added into each well, incubated for 4 h until the liquid in the well was absorbed, DMSO was then added in to the wells. After crystallization was completely dissolved, the absorbance of each well was determined by ELISA reader (Micro-plate Reader, µQuant-MQX 200, USA).
ELISA assay
The amount of inflammatory cytokines of TNF-α, IL-1β, IL-6, and KC were detected using ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. All detections were performed in triplicate.
Apoptosis assay
The cells (0.5 × 106) were collected and transplanted into an EP tube, and the cells were resuspended in 500 µL binding buffer containing 5 µL Annexin V-fluorescein isothiocyanate and 5 µL propidium iodide. Then the solution was cultured for 15 min in the dark. Cell apoptosis was then detected by flow cytometry using Annexin V-fluorescein isothiocyanate Apoptosis Detection kit (BD Biosciences) and flow cytometric analysis (Moflo XDP; Beckman Coulter, Brea, CA, USA).
Western blotting (WB) analysis
The cells were lysed on ice for 10 min and maintain in a 1.5 mL centrifugal tube. The lysates were centrifuged at 10,000 g at 4°C for 15 min. Then, a BCA kit (Beyotime) was used to determine protein concentration. Equal amounts of proteins were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), which were then transferred onto polyvinylidene difluoride membranes (Immobilon, IPVH00010). The membranes were incubated with 5% nonfat milk for 1.5 h, and then with primary antibodies PBEF (ab45890; 1/250; 56 kD, abcam), p65 antibody (#8242; 1/1000; 65 kD; CST, USA), β-actin antibody (ab8226, 1/1000, 42kD, abcam), Histon3 antibody (ab1791, 1/1000, 17kD, abcam). After washed by TBS-T for three times, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Beyotime Institute of Biotechnology) at room temperature for 1 h. The relative protein expression was measured using Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
The data were analyzed by SPSS 20.0 software (SPSS Inc., Chicago, IL), and shown as mean ± SD. Student’s t-test was used to analyze the differences between two groups, while differences among three or more groups were analyzed by one-way analysis of variance (ANOVA). A p < 0.05 was considered as statistically significant. Each experiment in the current study was performed in triplicate.
Results
Effects of genistein on viability of MLE-12 cells
To determine the cytotoxicity of genistein, MTT assay was performed to investigate the effects of genistein at different concentrations on MLE-12 cells viability. The results revealed that cell viability was not affected by low concentration of genistein (1–10 µM), while 25 µM genistein showed a significant inhibitory effect on MLE-12 cells viability (p < 0.001, ). Next, we explored the effect of genistein on LPS-induced injury in MLE-12 cells, and the results showed that LPS-inhibited MLE-12 cells viability, FK866 (PBEF inhibitor, 10 nM) increased the viability of cells, which treated with LPS and genistein concentration-dependent enhanced LPS-induced low cell viability, compared with that in LPS group (p < 0.001, ).
Figure 1. Effects of genistein on viability of MLE-12 cells.
(a) Low concentration of genistein (1–10 µM) did not affect cell viability, while 25 µM genistein inhibited cell viability by spectrophotometric method 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) experiment. **p < 0.001 vs. genistein (0 µM). (b) High concentration of genistein did not affect cell viability, lipopolysaccharide (LPS) inhibited cell viability, PBEF inhibitor FK866 induces increased cell viability and genistein enhanced LPS-induced low cell viability in a concentration-dependent manner by MTT experiment. **p < 0.001 vs. Control; ##p < 0.001 vs. LPS; ^^p < 0.001 vs. LPS + L-GENISTEIN; ΦΦP<0.001 vs. LPS + H-GENISTEIN.
![Figure 1. Effects of genistein on viability of MLE-12 cells.(a) Low concentration of genistein (1–10 µM) did not affect cell viability, while 25 µM genistein inhibited cell viability by spectrophotometric method 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) experiment. **p < 0.001 vs. genistein (0 µM). (b) High concentration of genistein did not affect cell viability, lipopolysaccharide (LPS) inhibited cell viability, PBEF inhibitor FK866 induces increased cell viability and genistein enhanced LPS-induced low cell viability in a concentration-dependent manner by MTT experiment. **p < 0.001 vs. Control; ##p < 0.001 vs. LPS; ^^p < 0.001 vs. LPS + L-GENISTEIN; ΦΦP<0.001 vs. LPS + H-GENISTEIN.](/cms/asset/5ca6cee9-b2b4-495b-bf02-b8c9749d0202/tbbb_a_1697197_f0001_oc.jpg)
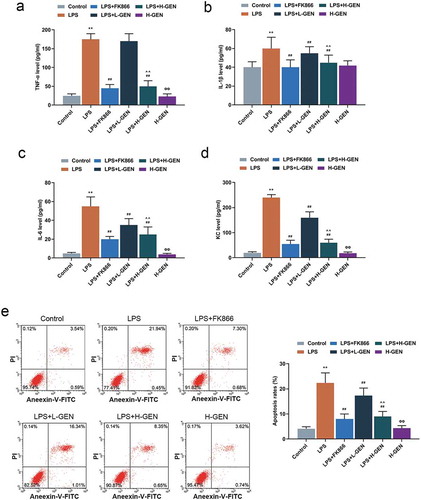
Effects of genistein on inflammatory factors and apoptosis of MLE-12 cells
We further explored the role of genistein in repairing LPS-induced cell injury by examining conditions of inflammation and cell apoptosis. After treating the cells by LPS for 12 h, the results of ELISA revealed that LPS increased the contents of TNF-α, IL-1β, IL-6, and KC, which were reduced by FK866, moreover, as compared with LPS group, genistein decreased the contents of above factors in a concentration-dependent manner(p < 0.001, ). In addition, the data from flow cytometry revealed that LPS increased MLE-12 cell apoptosis, and FK866 partially reversed such an effect, and genistein decreased MLE-12 cell apoptosis in a concentration-dependent manner compared with LPS group (p < 0.001, ).
Figure 2. Effects of genistein on inflammatory factors and apoptosis of MLE-12 cells.
(a–d) High concentration of genistein treatment alone had no significant effect on the levels of inflammatory factors. LPS increased the contents of inflammatory factors, FK866 induced decreased contents of inflammatory factors and genistein decreased LPS-induced high levels of inflammatory factors in a concentration-dependent manner by enzyme-linked immunosorbent assay (ELISA). (e) High concentration of genistein alone had no significant effect on apoptosis of MLE-12 cells. LPS increased the apoptotic rate, FK866 induced decreased cell apoptotic rate and genistein decreased the apoptotic rate of MLE-12 cells in a concentration-dependent manner by flow cytometry test. **p < 0.001 vs. Control; ##p < 0.001 vs. LPS; ^^p < 0.001 vs. LPS + L-GENISTEIN; ΦΦP<0.001 vs. LPS + H-GENISTEIN.
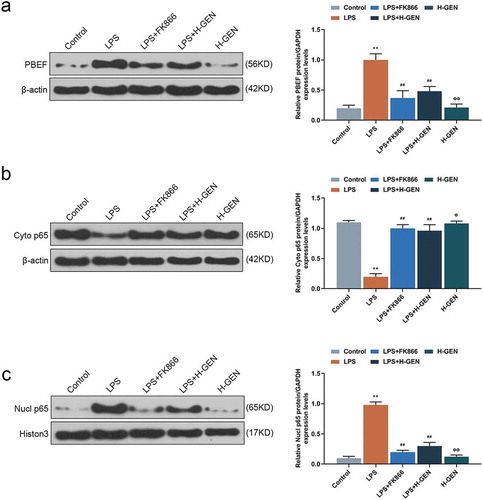
Genistein regulated the expressions of PBEF and NF-κB pathway proteins
In order to explore the potential mechanism of genistein on LPS-induced cell injury, we studied the effects of genistein on PBEF and p65 by WB analysis. The results showed that LPS up-regulated the expressions of PBEF and nuclear p65, but down-regulated the expression of cytoplasm p65. Moreover, genistein and FK866 reversed effects of LPS on cell injury ().
Figure 3. Effects of genistein on PBEF and NF-κB pathway protein.
(a) High concentration of genistein treatment alone had no significant effect on PBEF expression. LPS up-regulated the expression of PBEF, while high concentration of genistein and FK866 decreased PBEF expression by western blot (WB) analysis. (b–c) High concentration of genistein treatment alone had no significant effect on p65 expression. LPS up-regulated nuclear p65 and down-regulated cytoplasmic p65, H-GENISTEIN and FK866 down-regulated nuclear p65 and up-regulated cytoplasmic p65 western blotting analysis. **p < 0.001 vs. Control; ##p < 0.001 vs. LPS; ΦP<0.05, ΦΦP<0.001 vs. LPS + H-GENISTEIN.
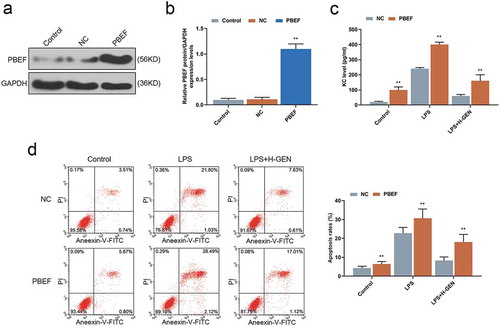
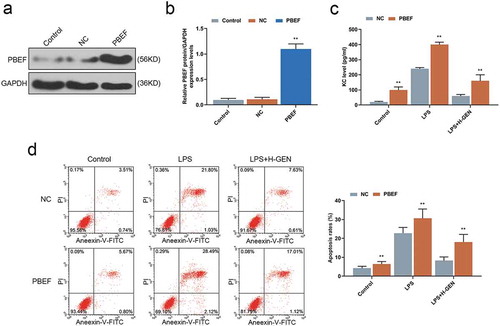
Genistein affected the level of KC and apoptosis of MLE-12 cells through regulating the expression of PBEF
The transfection efficiency of PBEF was detected by WB assay, and the result showed that PBEF expression was increased in the PBEF group as compared with NC group (). Finally, we further verified that the repairing effect of genistein on LPS-induced cell injury was achieved through regulating PBEF. ELISA test results showed that up-regulation of PBEF reduced the inhibitory effect of genistein on KC release after cells had been treated by LPS as compared with NC group (). Furthermore, flow cytometry test results revealed that PBEF attenuated the inhibitory effect of genistein on apoptosis of MLE-12 cells treated by LPS compared with NC group ().
Figure 4. Effects of genistein on keratinocyte-derived chemokine (KC) and MLE-12 cells apoptosis via regulating PBEF.
(a–b) WB test showed that PBEF transfection was successful. (c) Overexpression of PBEF attenuated the inhibitory effect of genistein on KC release. (d) Overexpression of PBEF attenuated the inhibitory effect of GENISTEIN on MLE-12 cell apoptosis. **p < 0.001 vs. NC.
Discussion
Acute respiratory distress syndrome and acute lung injury, which are severe clinical symptoms around the world, result in high morbidity and mortality in critically ill patients [Citation15]. As a biological response in the immune system, inflammation can be triggered by pathogens, toxic compounds, and some other factors [Citation16]. In recent years, studies have increasingly found that alveolar epithelial cells play an active role in inducing fibroblast proliferation and activation, which could lead to the destruction of lung structure and pulmonary inflammation [Citation17]. Therefore, whether the structure of alveolar epithelial barrier can be promptly repaired may play an important role in successful regression of pulmonary inflammation.
LPS was recognized as one of the important factors leading to lung injury, and it can cause pulmonary inflammation [Citation18]. It has been found that LPS-induced inflammatory response and tissue damage can be effectively reduced through inhibiting NF-κB signaling pathway [Citation19–Citation21]. Furthermore, in LPS-induced acute lung injury, NF-kB and mitogen-activated protein kinase play important roles in regulating the productions of pro-inflammatory cytokines and chemokines [Citation22]. Study showed that NF-κB can promote the proliferation, metastasis, inflammation, and resistance to chemotherapy and radiotherapy of cancer cells, and the expression of p65 and p-p105 was found as adverse prognostic factors for non-small cell lung cancer [Citation23]. Cytokines such as TNF-α could act as stimuli to activate NF-κB, and p65 plays an important role in the activation of NF-κB [Citation24]. Activation of NF-κB causes p65 phosphorylation, transfer of p65 into the nucleus and acetylation [Citation25]. These findings suggest that finding effective interventions to regulate NF-κB signaling pathway could be one of the therapeutic strategies for treating inflammatory diseases and tissue damages.
By performing in vivo experiments, previous study [Citation26] showed that genistein intake can improve the response of breast cancer rats to treatment through the enhancement of anti-tumor immunity. According to Islam Ibrahim Hegab et al. [Citation27] that genistein had protective effect on indomethacin-induced gastric injury, and its mechanism may be the interaction among anti-oxidation, anti-inflammation, and anti-apoptosis. Genistein was also found to significantly reduce inflammatory and apoptotic markers in ovariectomized diabetic rats, and it was shown to have protective effects on lung structure [Citation28]. However, there are relatively few reports on the role and mechanism of alveolar epithelial cells in repairing injury. In this study, we observed the effect of genistein on LPS-induced injury in MLE-12 cells, and found that genistein may contribute to the inhibition of NF-κB activation through inhibiting p65 nuclear translocation and alleviating inflammatory reaction of alveolar epithelial cells by reducing the expression of PBEF.
It has been reported that emodin can protect severe acute pancreatitis-induced acute lung injury through reducing the expression of PBEF and promoting the apoptosis of polymorphonuclear neutrophils [Citation29]. Genistein has anti-inflammatory effect and can significantly inhibit the expressions of pro-inflammatory factors [Citation30,Citation31]. Genistein can modulate humoral and cellular immunity at multiple levels and inhibit the exudation of granule enzyme via the stimulation of macrophages and neutrophils [Citation32,Citation33]. PBEF is regarded as a pro-inflammatory cytokine [Citation34] and is synthesized and secreted by many types of cells, such as activated lymphocytes and neutrophils [Citation6,Citation35]. Genistein could reduce the expression of PBEF by regulating the cells from which it was secreted. In our study, in LPS-induced inflammatory model of alveolar epithelial cells, the viability of MLE-12 cells decreased significantly, meanwhile, NF-κB was activated and the expressions of TNF-α, IL-1β, and IL-6 inflammatory factors were increased, and cell apoptosis was induced. KC, which plays a key role in regulating the migration and activation of neutrophils in LPS-induced pulmonary inflammation [Citation36], was found increased in cells treated by LPS. Furthermore, in the cells treated by PBEF inhibitor FK866, the cell activity increased, the nuclear translocation of NF-κB was suppressed, the levels of TNF-α, IL-1β, IL-6, and KC and the apoptotic rate were reduced. These results suggested that PBEF inhibitor shown an effect in repairing LPS-induced inflammatory model of alveolar epithelial cells through regulating NF-κB. Study [Citation37] also found that NF-κB p65 signaling plays a key role in acute inflammation, while sirtulin 1 activation can inhibit NF-κB p65 signaling, thereby enhancing oxidative metabolism and reducing the expression of inflammatory and fibrotic factors, including TNF-α. Such findings are similar to our results, and moreover, a regulatory relationship between PBEF and NF-κB is demonstrated in our study.
The current study observed that genistein reduced PBEF expression, activity of NF-κB, the expressions of inflammatory factors, and the apoptotic rate of alveolar epithelial cells stimulated by LPS. Therefore, genistein contributes to the repair of LPS-induced injury of alveolar epithelial cells through partly regulating NF-κB pathway.
In conclusion, the repairing effect of genistein on LPS-induced injury of alveolar epithelial cells was closely related to the inhibition of NF-κB activation and the alleviation of inflammatory reaction through reducing the expression of PBEF. However, our research still had some limitations, for example, such a mechanism determined in the current study should be further verified through in vivo experiments, moreover, whether other possible mechanisms and other family members of NF-κB were involved remain to be clarified.
Author contribution
Substantial contributions to conception and design: QZ, WZ.
Data acquisition, data analysis and interpretation: DM, HZ, SW, HZ.
Drafting the article or critically revising it for important intellectual content: QZ, WZ.
Final approval of the version to be published: All authors.
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved: All authors.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Wittekindt OH. Tight junctions in pulmonary epithelia during lung inflammation. Pflugers Arch. 2017;469(1):135–147.
- Ngamsri K-C, Müller A, Bösmüller H, et al. The pivotal role of CXCR7 in stabilization of the pulmonary epithelial barrier in acute pulmonary inflammation. J Cell Immunol. 2017;198(6):2403–2413.
- Lehmann M, Korfei M, Mutze K, et al. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur Respir J. 2017;50(2):1602367.
- Laffey JG, Matthay MA. Fifty years of research in ARDS. Cell-based therapy for acute respiratory distress syndrome. Biology and potential therapeutic value. Am J Respir Crit Care Med. 2017;196(3):266–273.
- McClendon J, Jansing NL, Redente EF, et al. Hypoxia-inducible factor 1α signaling promotes repair of the alveolar epithelium after acute lung injury. Am J Pathol. 2017;187(8):1772–1786.
- He JY, Cui HJ, Tang LJ, et al. Inhibition of pre-B cell colony-enhancing factor attenuates inflammation induced by hyperoxia in EA. hy926 cells.. Int J Mol Med. 2017;40(3):859–866.
- Gao H, Zhang Q, Chen J, et al. Porcine IL‐6, IL‐1β, and TNF‐α regulate the expression of pro‐inflammatory‐related genes and tissue factor in human umbilical vein endothelial cells. Xenotransplantation. 2018;25(5):e12408.
- Fang Q, You M, Xu W, et al. pre-B cell colony enhancing factor negatively regulates Na+ and fluid transport in lung epithelial cells. Am J Transl Res. 2018;10(7):2047.
- Sun L CS-C, Gao H-N, Ren L-P, et al. Visfatin induces the apoptosis of endothelial progenitor cells via the induction of pro-inflammatory mediators through the NF-κB pathway. Int J Mol Me. 2017;40(3):637–646.
- Coleman FT, Blahna MT, Kamata H, et al. Capacity of pneumococci to activate macrophage nuclear factor κB: influence on necroptosis and pneumonia severity. J Infect Dis. 2017;216(4):425–435.
- Jackson IL, Zodda A, Gurung G, et al. BIO 300, a nanosuspension of genistein, mitigates pneumonitis/fibrosis following high‐dose radiation exposure in the C57L/J murine model. Br J Pharmacol. 2017;174(24):4738–4750.
- Liu F-C, Wang -C-C, Lu J-W, et al. Chondroprotective effects of genistein against osteoarthritis induced joint inflammation. Nutrients. 2019;11(5):1180.
- Mukund V BSK, Alam A, Nagaraju GP. Molecular docking analysis of nuclear factor-κB and genistein interaction in the context of breast cancer. Bioinformation. 2019;15(1):11–17.
- Zheng Y, Liu H, Liang Y. Genistein exerts potent antitumour effects alongside anaesthetic, propofol, by suppressing cell proliferation and nuclear factor-κb-mediated signalling and through upregulating microrna-218 expression in an intracranial rat brain tumour model. J Pharm Pharmacol. 2017;69 (11):1565–1577.
- Xie W, Lu Q, Wang K, et al. miR‐34b‐5p inhibition attenuates lung inflammation and apoptosis in an LPS‐induced acute lung injury mouse model by targeting progranulin. J Cell Physiol. 2018;233(9):6615–6631.
- Chen L, Deng H, Cui H, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204.
- Maldonado M, Salgado-Aguayo A, Herrera I, et al. Upregulation and nuclear location of MMP28 in alveolar epithelium of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2018 Jul;59(1):77–86. PubMed PMID: 29373068; eng.
- Zeng M, Sang W, Chen S, et al. 4-PBA inhibits LPS-induced inflammation through regulating ER stress and autophagy in acute lung injury models. Toxicol Lett. 2017;271:26–37.
- Cheng P, Wang T, Li W, et al. Baicalin alleviates lipopolysaccharide-induced liver inflammation in chicken by suppressing TLR4-Mediated NF-kappaB pathway. Front Pharmacol. 2017;8:547. PubMed PMID: 28868036; PubMed Central PMCID: PMCPMC5563358. eng.
- Shu B, Feng Y, Gui Y, et al. Blockade of CD38 diminishes lipopolysaccharide-induced macrophage classical activation and acute kidney injury involving NF-kappaB signaling suppression. Cell Signal. 2018 Jan;42:249–258. PubMed PMID: 29080804; eng.
- Zhang X, Wang Y, Xiao C, et al. Resveratrol inhibits LPS-induced mice mastitis through attenuating the MAPK and NF-kappaB signaling pathway. Microb Pathog. 2017 Jun;107:462–467. PubMed PMID: 28389348; eng.
- Zhang Q, Zhu S, Cheng X, et al. Euphorbia factor L2 alleviates lipopolysaccharide-induced acute lung injury and inflammation in mice through the suppression of NF-kappaB activation. Biochem Pharmacol. 2018 Sep;155:444–454. PubMed PMID: 30055150; eng.
- Lin G, Li C, Huang C, et al. Co‐expression of NF‐κB‐p65 and phosphorylated NF‐κB‐p105 is associated with poor prognosis in surgically resectable non‐small cell lung cancer. J Cell Mol Med. 2018;22(3):1923–1930.
- Ruiz-Perera LM, Schneider L, Windmoller BA, et al. NF-kappaB p65 directs sex-specific neuroprotection in human neurons. Sci Rep. 2018 Oct 30;8(1):16012. PubMed PMID: 30375448; PubMed Central PMCID: PMCPMC6207661. eng. doi: 10.1038/s41598-018-34394-8.
- Huang Y, Chen J, Jiang T, et al. Gallic acid inhibits the release of ADAMTS4 in nucleus pulposus cells by inhibiting p65 phosphorylation and acetylation of the NF-κB signaling pathway. Oncotarget. 2017;8(29):47665.
- Zhang X, Cook KL, Warri A, et al. Lifetime genistein intake increases the response of mammary tumors to tamoxifen in rats. Clin Cancer Res. 2017;23(3):814–824.
- Hegab II, Abd-Ellatif RN, Sadek MT. The gastroprotective effect of n-acetylcysteine and genistein in indomethacin-induced gastric injury in rats. Can J Physiol Pharmacol. 2018;96(11):1161–1170.
- Daghigh F, Alihemmati A, Karimi P, et al. Genistein preserves the lungs of ovariectomized diabetic rats: addition to apoptotic and inflammatory markers in the lung. Iran J Basic Med Sci. 2017;20(12):1312.
- Cui H, Li S, Xu C, et al. Emodin alleviates severe acute pancreatitis-associated acute lung injury by decreasing pre-B-cell colony-enhancing factor expression and promoting polymorphonuclear neutrophil apoptosis. Mol Med Rep. 2017;16(4):5121–5128.
- Wang A, Wei J, Lu C, et al. Genistein suppresses psoriasis-related inflammation through a STAT3-NF-kappaB-dependent mechanism in keratinocytes. Int Immunopharmacol. 2019 Apr;69:270–278. PubMed PMID: 30743203; eng.
- Liu FC, Wang CC, Lu JW, et al. Chondroprotective effects of genistein against osteoarthritis induced joint inflammation. Nutrients. 2019 May 27;11(5). PubMed PMID: 31137797; PubMed Central PMCID: PMCPMC6566664. eng.
- Yellayi S, Zakroczymski MA, Selvaraj V, et al. The phytoestrogen genistein suppresses cell-mediated immunity in mice. J Endocrinol. 2003 Feb;176(2):267–274. PubMed PMID: 12553875; eng.
- Mukund V, Mukund D, Sharma V, et al. Genistein: its role in metabolic diseases and cancer. Crit Rev Oncol Hematol. 2017 Nov;119:13–22. PubMed PMID: 29065980; eng.
- Sun Z, Lei H, Zhang Z. Pre-B cell colony enhancing factor (PBEF), a cytokine with multiple physiological functions. Cytokine Growth Factor Rev. 2013 Oct;24(5):433–442. PubMed PMID: 23787158; PubMed Central PMCID: PMCPMC3791181. eng. .
- Jia SH, Li Y, Parodo J, et al. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest. 2004 May;113(9):1318–1327. PubMed PMID: 15124023; PubMed Central PMCID: PMCPMC398427. eng.
- Jayne JG, Bensman TJ, Schaal JB, et al. Rhesus θ-defensin-1 attenuates endotoxin-induced acute lung injury by inhibiting proinflammatory cytokines and neutrophil recruitment. Am J Respir Cell Mol Biol. 2018;58(3):310–319.
- Chen Y, Liang Y, Hu T, et al. Endogenous Nampt upregulation is associated with diabetic nephropathy inflammatory-fibrosis through the NF-kappaB p65 and Sirt1 pathway; NMN alleviates diabetic nephropathy inflammatory-fibrosis by inhibiting endogenous Nampt. Exp Ther Med. 2017 Nov;14(5):4181–4193. PubMed PMID: 29104634; PubMed Central PMCID: PMCPMC5658765. eng.
