ABSTRACT
Two kinds of Kunitz-type protease inhibitors, AKPI1 and AKPI2, were purified from Apios americana tubers by four steps of column chromatographies and their cDNA cloning was performed. AKPI1 cDNA consist of 809 nucleotides, and the matured protein had 190 amino acids with 20,594 Da. AKPI2 cDNA consist of 794 nucleotides, and the matured protein had 177 amino acids with 19,336 Da. P1 site of AKPI2 was Leu88, suggested the target enzyme was chymotrypsin. On the other hand, Gly85-Ile86-Ser87 was positioned around P1 site of AKTI1. Sequence analysis suggested that two forms (single-chain and two-chain form) of AKPI2 protein were present in the tubers. Recombinant AKPI2 expressed by E.coli system showed inhibitory activity toward serine proteases and heat stability. The Ki values toward chymotrypsin and trypsin were 4 × 10−7 M and 6 × 10−6 M, respectively.
Abbreviations: AAL: Apios americana lectin; AATI: Apios americana Bowman-Birk type trypsin inhibitor; ACE: angiotensin-converting enzyme; IPTG: isopropyl-β-D-thio-galactopyranoside; Ki: inhibition constant; KPIs: Kunitz-type protease inhibitors; L-BAPA: Benzoyl-L-arginine p-nitroanilide monohydrochloride; L-BTPA: Benzoyl-L-tyrosine p-nitroanilide; PFLNA: Pyr-Phe-Leu-p-nitroanilide; RP-HPLC: reverse-phase high-performance liquid chromatography; RT-PCR: reverse transcription-polymerase chain reaction; SDS-PAGE: sodium dodecyl sulfate-polyacrylamide gel electrophoresis; SLIC: sequence and ligation independent cloning; STANA: N-Succinyl-Ala-Ala-Ala-p-nitroanilide; SHR: spontaneously hypertensive rats; TFA: trifluoroacetic acid; UTR: untranslated region.
Graphical abstract
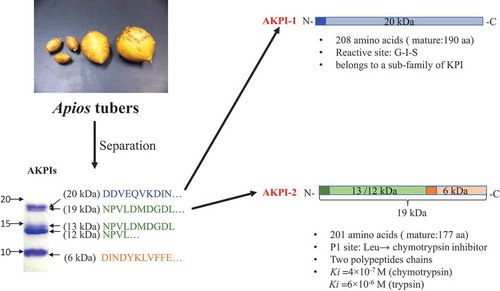
Kunitz-type trypsin inhibitors AKPI1 and AKPI2 were purified and the cDNA cloning and functional expression were performed.
Apios americana Medik is a kind of legume plants native to North America, that can produce tubers at stolons [Citation1], and the tubers were called as “American groundnut” or “potato bean” commonly. The tubers were used as a staple food source for native American Indians [Citation2], and the dried powdered tubers were used for bread in Japan due to the nutritional benefits [Citation3]. Apios tubers had been thought as healthy food material because of high contents of protein, carbohydrates, iron, dietary fiber and low levels of reducing sugars [Citation4,Citation5]. Recently some components with biological activities in Apios have reported such as isoflavones that have a potential role in anti-oxidative activity [Citation6] and angiotensin-converting enzyme (ACE) inhibitor that can reduce the blood pressure of spontaneously hypertensive rats (SHR) [Citation7,Citation8]. Besides, the extracts from the Apios flowers and leaves have benefits on an anti-oxidation mechanism [Citation9,Citation10].
Our research group has focused on bioactive proteins existed in Apios tubers and purified a protease inhibitor AATI [Citation11,Citation12], and a lectin AAL [Citation11,Citation12] so far. Protease inhibitors play an important role in plants such as against diseases, insects, and herbivores [Citation13]. In addition, the proteinase inhibitors can reduce the incidence of some chronic diseases like gastric ulcer and emphysema, and cancers such as colon, prostate, breast, and skin [Citation14]. AATI was classified as Bowman-Birk type inhibitors [Citation14,Citation15] and consist of 59 amino acid residues with the molecular mass of 6,437 Da. AATI not only showed inhibitory activity against trypsin and chymotrypsin [Citation11] but also have an inhibitory effect on the proliferation of cancer cells including U937, K563, J774.1 and HeLa cells [Citation12].
In the course of purification of AATI, we found another type of protease inhibitors in Apios tubers. N-terminal amino acid sequences of the inhibitors suggested that these inhibitors were kinds of Kunitz-type protease inhibitors. However, little is known about Kunitz-type protease inhibitors existed in tubers of legume plants. Here, we proved that two new Kunitz-type protease inhibitors (AKPI1 and AKPI2) existed in Apios tubers through purification and cDNA cloning. We showed two forms (single-chain and two-chain form) of AKPI2 protein was present in the tubers. Furthermore, we tried to expression of recombinant AKPI2 protein in E. coli cells and investigated the inhibitory activities.
Materials and methods
Materials
Apios americana tubers were harvested from the Experimental Farm of the College of Agriculture, Ibaraki University and stored at −20°C or −80°C. DEAE-Cellufine A-500 and Butyl-Cellufine were purchased from JNC (Tokyo). Sephadex G-50 was from GE Healthcare Japan and YMC-pack PROTEIN-RP and YMC-pack pro C18 were purchased from YMC. Trypsin, chymotrypsin, papain, subtilisin, and elastase were from Sigma-Aldrich Japan. Benzoyl-L-arginine p-nitroanilide monohydrochloride (L-BAPA), Benzoyl-L-tyrosine p-nitroanilide (L-BTPA), Pyr-Phe-Leu-p-nitroanilide (PFLNA) and N-Succinyl-Ala-Ala-Ala-p-nitroanilide (STANA) were from Peptide institute (Japan). Oligonucleotide primers were purchased from Fasmac. All other chemicals were of the analytical grade for biochemical use.
Purification of protease inhibitors from Apios tubers
The crude protein from Apios tubers were extracted according to the methods of Zhang et al. [Citation11] and Kouzuma et al. [Citation16] Apios tubers were cleaned and ground by Physcotron homogenizer (Nition, Chiba, Japan) with 3 times volumes (w/v) of 50 mM Tris-HCl buffer (pH 7.5) for 30 min. After filtration by four-layer gauze, the precipitate was re-extracted with 3 times volumes (w/v) of 50 mM Tris-HCl buffer (pH 7.5) for 30 min at 4℃ again. The protein solution was centrifuged at 10,000 rpm for 30 min, and the supernatant was saturated with ammonium sulfate. After centrifugation, the crude protein precipitate was dialyzed against 50 mM Tris-HCl buffer (pH7.5), and applied onto a DEAE-Cellufine A-500 column (2.6 × 38 cm) equilibrated with the same buffer, and adsorbed proteins were eluted with a linear gradient of 0–0.3 M NaCl in the same buffer. Fractions that exhibited trypsin inhibitory activity (D2 fraction) were pooled and saturated with ammonium sulfate. After centrifuged, the precipitate was dialyzed against 50 mM phosphate buffer (pH 7.0) containing 0.15 M NaCl and 1 M ammonium sulfate. After centrifuged, the supernatant was applied onto a Butyl-Cellufine column (1.6 × 38 cm) equilibrated with the same buffer. Absorbed proteins were eluted with a linear gradient of 1–0 M ammonium sulfate in the same buffer. Fractions that exhibited trypsin inhibitory activity (B1 fraction) were pooled and saturated with ammonium sulfate. After centrifuged, the precipitate was dialyzed against 50 mM phosphate buffer (pH 7.0) and centrifuged. The supernatant was applied onto a Sephadex G-50 column (2.6 × 65 cm) equilibrated with the same buffer. The fractions that exhibited trypsin inhibitory activity (S1 fraction) were freeze-dried after dialysis against water and preserved at −20℃. The concentration of protein was measured by the Protein Assay Reagent (Pierce Chemical, Rockford, IL) with bovine serum albumin as a standard.
Protease inhibitors fraction (freeze-dried S1 fraction) was applied to reverse-phase high-performance liquid chromatography (RP-HPLC) on the YMC-pack PROTEIN-RP column (4.6 × 250 mm), equilibrated with 0.1% trifluoroacetic acid (TFA) at a flow rate of 1.0 mL/min. Proteins were eluted with a linear gradient of 80% acetonitrile containing 0.1%TFA for 45 min, followed by isocratic elution at 40% acetonitrile containing 0.1%TFA from 10 min to 18 min and 60% acetonitrile containing 0.1%TFA from 20 min to 25 min. The absorbance was monitored at 220 nm. All peaks were collected and dried by the centrifugal concentrator.
Protease inhibition assays
Trypsin and chymotrypsin inhibitory activity were measured as described by Zhang et al. [Citation11] Trypsin or chymotrypsin was dissolved in 50 mM Tris-HCl buffer (pH 7.5) at a concentration of 0.2 mg/mL. One hundred microlitres of the trypsin solution or chymotrypsin solution was incubated with 100 μL of inhibitor solution at room temperature for 10 min, and then 1 mL of 0.5 mM L-BAPA (trypsin substrate solution) or 0.5 mM L-BTPA (chymotrypsin substrate solution) was added. After incubated at 37 ℃ for 10 min, 150 μL of 30% acetic acid was added to stop the enzyme reaction, and the absorbance at 410 nm was measured. One unit of inhibitory activity was defined as the amount of inhibitor that inhibits 1 μg of the active enzyme. The papain inhibitory activity was followed by the method of Filippova [Citation17], using PFLNA as a substrate. The subtilisin inhibitory activity was followed by the method of Orawan [Citation18], using casein as a substrate. The inhibitory activity against Elastase was followed by the method of Stein [Citation19], using STANA as a substrate. Inhibition constants (Ki) toward trypsin and chymotrypsin were calculated using the Bieth plot [Citation20].
Molecular mass determination
The relative molecular mass of the inhibitor protein was calculated from the relative mobility in Tricine-SDS-PAGE in the presence or absence of β-mercaptoethanol [Citation21]. The Dual color standards (BIO-RAD) and low molecular weight marker (GE Healthcare Japan) were used for comparison. The logarithm of the molecular weight of the marker and its relative migration distance is plotted into a graph to make a standard curve and the molecular mass of inhibitor proteins were calculated based on the standard curve. Experiments were carried out in triplicate.
Enzymatic digestion of protease inhibitors
The purified inhibitor protein separated by RP-HPLC was used for enzymatic digestion. Lysyl-endopeptidase and 4 M urea were mixed with the inhibitor solution and incubated at 37 ℃ for 16 h. The digested peptides were separated by RP-HPLC on a YMC-Pack pro C18 column (4.6 × 250 mm) equilibrated with 0.1% TFA at a flow rate of 0.6 mL/min and eluted with a linear gradient from 0.1% TFA to 80% acetonitrile containing 0.1%TFA. The peptides were collected and dried by the centrifugal concentrator.
Amino acid sequencing of proteins and peptides
The proteins were electrophoretically transferred to PVDF membranes with Midi PVDF Transfer Packs (BIO-RAD) and Transfer System from BIO-RAD at the voltage of 7 V for 5 min. The membrane was stained for 5 min with staining solution (0.2% (v/w) Coomassie Brilliant Blue G250, 40% (w/w) methanol and 10% (w/w) acetic acid) and destained by destaining solution (10% (w/w) methanol and 10% (w/w) acetic acid) [Citation22]. N-terminal amino acid sequences of proteins and digested peptides were determined by a protein sequencer (Procise 494-HT, Applied Biosystems, Foster City).
cDNA cloning of protease inhibitors
Total RNA was extracted from Apios americana tubers with ISOGEN with a spin column (Nippon Gene). Reverse transcription reaction was performed using the High Fidelity RT-PCR Kit (Takara) and oligo (dT) primer. The cDNA fragment of AKPI1 were amplified with the degenerated oligonucleotide primers (RT-AKPI1-F and RT-AKPI1-R, Supplemental Table S1 and ) and performed under the following PCR conditions: 94℃ for 3 min (1 cycle), 94℃ for 1 min, 50℃ for 1 min and 72℃ for 1 min (35 cycles), and 72℃ for 10 min (1 cycle). The PCR products were purified using Wizard® SV Gel and PCR Clean-Up System (Promega) and cloned into TOPO Vector using TOPO TA Cloning Kit (Invitrogen) for sequencing. The nucleotide sequence was determined using BigDye Terminator v3.1 Cycle sequencing Ready Reaction Kit (Applied Biosystems), Big Dye X-terminator Kit (Applied Biosystems) and automated DNA sequencer ABI Prism 3100 (Applied Biosystems). The 5ʹ-terminal-region of AKPI1 cDNA was determined by 5ʹ-RACE. The single-stranded cDNA was obtained using 5ʹ-Full RACE core set (Takara) and 5-AKPI1-RT primer. SLIC (Sequence and ligation independent cloning) primer (5ʹ-GTAGGAATTCGGGTTGTAGGGAGGTCGACATTGCC-3ʹ) was ligated with the single-stranded cDNA. Primer AP1 and 5-AKPI1-R1 were used for first PCR, and AP2 and 5-AKPI1-R2 were used for second PCR (Supplemental Table S1 and ). The 3ʹ-terminal-region of AKPI1 cDNA was determined by 3ʹ-RACE with the primer oligo (dT) for single-stranded cDNA synthesis by reverse transcription, and the primers, 3-AKPI1-F, and dT for PCR (Supplemental Table S1 and ). Full-length of AKPI1 cDNA was obtained using KOD-plus (TOYOBO), 5ʹ-UTR forward primer, Full-AKPI1-F and 3ʹ-UTR forward primer, Full-AKPI1-R. The cDNA cloning of AKPI2 were obtained by the same procedures with AKPI1. The primers used were shown in Supplemental Table S1 and .
The homologous proteins were searched by the Basic local alignment of sequences tool (BLAST) from the National Center for Biotechnology Information (NCBI) (https://blast.ncbi.nlm.nih.gov).
Expression of recombinant AKPI2 in E.coli cells
Recombinant AKPI2 protein was expressed following the methods of Shimizu et al. [Citation23] AKPI2 gene coding mature region was amplified using primers, EX-AKPI2-F and EX-AKPI2-R (Supplemental Table S1) with the restriction site of NdeI or BamHI and full-length AKPI2 cDNA as a template for PCR. The amplified DNA were ligated with TOPO Vector for subcloning. After confirmation of the nucleotide sequence, the obtained TOPO-rAKPI2 plasmid and expression vector pET-22b (Merck Japan) were digested with NdeI and BamHI (Nippon Gene), and the insert DNA and the expression vector were ligated by DNA ligation kit ver.2.1 (Takara). The obtained pET-rAKPI2 were transformed into BL21 CodonPlus (DE3) RIL strain (Stratagene) for expression. E. coli cells harboring plasmid pET-rAKPI2 were incubated in 1 L of LB medium containing 50 μg/mL ampicillin, and the recombinant protein was induced with 1 mM IPTG at 37℃ for 4 h. After induction, E.coli cells were harvested and disrupted by sonication. After centrifugation, the obtained inclusion bodies were dissolved in solubilization buffer (8 M urea, 30 mM Tris-HCl (pH 7.5), 30 mM NaCl, 1 mM dithiothreitol) and dialyzed against refolding buffer A (4 M urea, 30 mM Tris-HCl (pH 7.5), 30 mM NaCl, 1 mM dithiothreitol) for 8 h, refolding buffer B (30 mM Tris-HCl (pH 7.5), 30 mM NaCl, 1 mM dithiothreitol) for 24 h, and 10 mM phosphate buffer (pH 7.2) for 24 h. The refolded recombinant proteins were purified by Sephadex G-75 column (1.6 × 75 cm), equilibrated with 10 mM phosphate buffer (pH 7.2). The fractions that exhibited chymotrypsin inhibitory activity were recovered, dialyzed against water, and freeze-dried for inhibitory activity analysis.
Heat stability of recombinant protease inhibitor
The purified recombinant protease inhibitor was dissolved in 30 mM Tris-HCl (pH 7.5) and incubated at different temperatures (37–100°C) for different times (30, 60, 120 min). After heat-treated, the proteins solutions was returned to room temperature and the inhibitory activity against trypsin and chymotrypsin were investigated. Experiments were carried out in triplicate.
Results
Purification of protease inhibitors from Apios tubers
The crude extracts from Apios tubers were applied onto a DEAE-Cellufine A-500 column, and adsorbed proteins were eluted with a linear gradient of 0–0.3 M NaCl in the equilibrating buffer. As shown in ), high trypsin inhibitory activities were shown in flow-through fractions (D1) and adsorbed fractions (D2). Since D1 fraction contained Bowman-Birk trypsin inhibitor protein (AATI) [Citation11] as previously reported, we recovered D2 fraction for purification of novel protease inhibitors. D2 fraction was applied onto a Butyl-Cellufine column, and adsorbed proteins were eluted with a linear gradient of 1–0 M (NH4)2SO4 in equilibrating buffer. As shown in ), the fractions showed high trypsin inhibitory activity (B1) was collected and applied onto a Sephadex G-50 column ()). The second peak showed high trypsin inhibitory activity (S1) was collected and subjected to SDS-PAGE ()). S1 fraction gave five protein bands and the molecular mass of them were estimated to be 20 kDa, 19 kDa, 13 kDa, 12 kDa and 6 kDa in the presence or absence of β-mercaptoethanol. Protein sequencing analysis indicated that the three N-terminal amino acids sequences (12, 13 and 19 kD) of the five bands were identical (). The BLAST search results suggested that two kinds of Kunitz-type protease inhibitors were existed in Apios tubers. Therefore, the 20 kDa and 19 kDa (13/12 kDa and 6 kDa) proteins from Apios tubers were named as AKPI1 and AKPI2, respectively. The presence of smaller molecular mass protein with the same N-terminal amino acid sequence suggested 19 kDa protein was processed and generated 13 or 12 kDa protein fragment and 6 kDa protein fragment in the tubers. Furthermore, 13 or 12 kDa protein fragment and 6 kDa protein fragment were combined each other without disulfide bond (or with noncovalent bond) between both chains in the tubers because their protein bands were detected in the absence of β-mercaptoethanol ()).
Table 1. N-terminal amino acid sequences of the protease inhibitors obtained by Sephadex G-50 from Apios americana tubers.
Figure 1. Purification of protease inhibitors from Apios americana tubers.
(a) DEAE Cellufine A-500 column chromatography, (b) Butyl-Cellufine column chromatography, (c) Sephadex G-50 column chromatography and SDS-PAGE of S1 fractions (M, Marker; Lane 1, S1 fraction in the absence of β-mercaptoethanol, Lane 2, S1 fraction in the presence of β-mercaptoethanol), and (d) Elution profile of RP-HPLC on a YMC-Pack Protein-RP and SDS-PAGE of fractions 1 ~ 4.
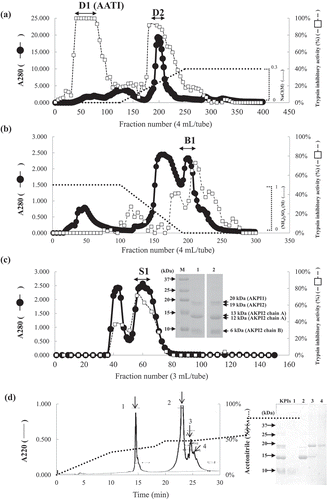
Since the separation of AKPI1 and AKPI2 proteins using a conventional liquid chromatography was very difficult, the proteins were separate using RP-HPLC on a YMC-Pack Protein-RP column. The typical separation pattern is shown in ). Peak 1 and 2 included 6 and 12 kDa proteins, respectively, and peaks 3 and 4 included mainly 19 and 20 kDa proteins, respectively. After rechromatography, protease inhibitory activities of the purified protease inhibitors and the fragments were investigated. However, their yields were very low and they lost the protease inhibitory activity due to the influence of acetonitrile or TFA. The purification until the Sephadex G-75 step is summarized in . In total, 50 mg of AKPI1 and AKPI2 (fraction S1) were obtained from 50 g Apios tubers.
Table 2. Summary of the purification of Kunitz-type protease inhibitors from Apios americana tubers.
cDNA Cloning of AKPIs
In order to obtain the information of amino acid sequences of AKPI1 for cDNA cloning, AKPI1 was digested by lysyl-endopeptidase and the digested peptides were separated by RP-HPLC on a YMC-Pack pro C18 column (4.6 × 250 mm). Amino acid sequences of three peptides were determined and the specific-degenerated PCR primers of AKPI1 were designed based on the amino acid sequences of the peptides and the N-terminal ()). From degenerated PCR, 5ʹ-RACE and 3ʹ-RACE, 348, 242 and 448 bp AKPI1 cDNA fragments were obtained, respectively. To combine these fragments, the full-length AKPI1 cDNA was obtained by RT-PCR using KOD-plus PCR enzyme and primers, Full-AKPI1-F and R, designed from 5ʹ-and 3ʹ-UTR. The AKPI1 cDNA consist of 809 nucleotides, 624-bp open reading frame which coded a polypeptide of 208 amino acids, a 46-bp 5ʹ-UTR, and a 139-bp 3ʹ-UTR (DDBJ accession no. LC504755). N-terminal amino acid sequence of AKPI1 protein in tubers suggested that the mature protein had a polypeptide of 190 amino acids with a molecular mass of 20,594 Da (). The AKPI1 was relatively similar with Kunitz-type inhibitors in legumes such as Gm2, Glycine max Kunitz Typsin Inhibitor (ACA23207.1), Ca, Cicer arietinum Kunitz Typsin Inhibitor (NP_001266050.1), and Ps, Pisum sativum Kunitz Protease Inhibitor (O82711.1) from BLAST search (). These KPIs shared 65–75% amino acid residues with AKPI1 and had five cysteine residues located at the position of 62, 107, 125, 158 and 165 unlike general KPI proteins that have four cysteine residues in the molecule. The putative reactive site of a serine protease inhibitor was speculated to be Gly85-Ile86-Ser87 (, and ).
Figure 2. Nucleotide sequences and their deduced amino acid sequences of AKPI1 cDNA (a) and AKPI2 cDNA(b).
The predicted amino acid sequence is shown above the nucleotide sequence. The amino acid sequences determined by a protein sequencer are marked by a double underline. Arrowheads indicate the processing sites of mature AKPI1 and AKPI2. The N-terminal sequence of Chain B of AKPI2 is shown in box with gray shading. The putative poly (A) additional signals (AATAAA) are indicated by boxes. The primers used in RT-PCR, 5ʹ-RACE, and 3ʹ-RACE are indicated by arrows.
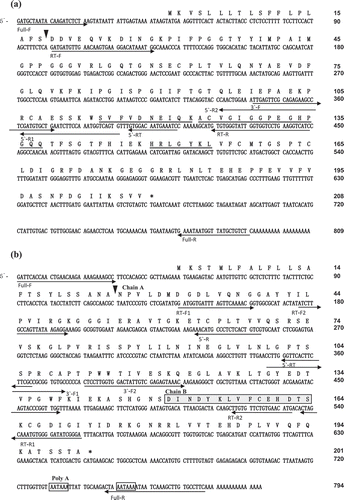
Figure 3. Amino acid sequence comparisons of AKPI1 with AKPI2.
The alignment was done using CLUSTALW and ESpript 3.0. Similar residues are highlighted with boxes. Strictly conserved residues are highlighted with boxes with gray shading.
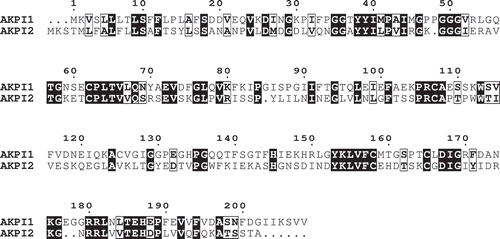
Figure 4. Amino acid sequence comparisons of AKPI1 and AKPI2 with various KPIs from plants.
Arrowheads indicate the processing sites of mature proteins. Cysteine residues are indicated by boxes. The deduced P1 amino acid residues (or reactive sites) are indicated by boxes with gray shading. Gm2, Glycine max Kunitz-type trypsin inhibitor (ACA23207.1); Ca, Cicer arietinum Kunitz-type trypsin inhibitor (NP_001266050.1) [Citation24]; Ps, Pisum sativum Kunitz-type protease inhibitor (O82711.1); Gm1, Glycine max Kunitz-type trypsin inhibitor (P01070.2); Ac, Acacia confusa Kunitz-type Protease Inhibitor (AAA32618.1) Ec, Enterolobium contortisiliquum Kunitz-type trypsin inhibitor (P86451.1); St, Solanum tuberosum Kunitz-type protease inhibitor (P58515.1).
![Figure 4. Amino acid sequence comparisons of AKPI1 and AKPI2 with various KPIs from plants.Arrowheads indicate the processing sites of mature proteins. Cysteine residues are indicated by boxes. The deduced P1 amino acid residues (or reactive sites) are indicated by boxes with gray shading. Gm2, Glycine max Kunitz-type trypsin inhibitor (ACA23207.1); Ca, Cicer arietinum Kunitz-type trypsin inhibitor (NP_001266050.1) [Citation24]; Ps, Pisum sativum Kunitz-type protease inhibitor (O82711.1); Gm1, Glycine max Kunitz-type trypsin inhibitor (P01070.2); Ac, Acacia confusa Kunitz-type Protease Inhibitor (AAA32618.1) Ec, Enterolobium contortisiliquum Kunitz-type trypsin inhibitor (P86451.1); St, Solanum tuberosum Kunitz-type protease inhibitor (P58515.1).](/cms/asset/7edb93c4-6586-44d3-9a40-b1ad601ba99d/tbbb_a_1698281_f0004_b.gif)
The specific-degenerated PCR primers of AKPI2 were designed based on the N-terminal amino acid sequences of AKPI2 (19 kDa) and 6 kDa protein. Degenerated PCR, 5ʹ-RACE and 3ʹ-RACE gave 315, 254 and 450 bp PCR products coding AKPI2 protein. To combine these fragments, the full-length AKPI2 cDNA was obtained by RT-PCR using KOD-plus PCR enzyme and primers, Full-AKPI2-F and R, designed from 5ʹ-and 3ʹ-UTR. The AKPI2 cDNA consist of 794 nucleotides and had an open reading frame of 603 nucleotides encoding a polypeptide of 201 amino acid residues, a 49 bp 5ʹ-UTR and a142 bp 3ʹ-UTR (DDBJ accession no. LC504756). The AKPI2 had a signal sequence consist of 24 amino acids and a mature AKPI2 protein consist of 177 amino acid residues with a molecular mass of 19,336 Da. N-terminal amino acid sequence of 6 kDa AKPI2 fragment suggested that the AKPI2 was cleaved between Ser149-Asp150 and generated 12/13 kDa fragment (chain A) consist of at least 125 amino acid residues and 6 kDa fragment (chain B) consist of at least 52 amino acid residues ()). Amino acid sequence comparison between AKPI1 and AKPI2 indicated that only 30% amino acid residues are identical to each other (). Furthermore, AKPI2 was similar to Kunitz type inhibitors from Glycine max (Gm1, P01070.2), Acacia confusa (Ac, AAA32618.1), Enterolobium contortisiliquum (Ec, P86451.1), and these KPIs shared 43–37% amino acid residues with AKPI2. The sequence alignment suggested that the P1 site of AKPI2 was Leu88. Four cysteine residues were located at the position of 64, 108, 159 and 166 that can compose two disulfide bridges (, and ).
Expression of recombinant AKPI2
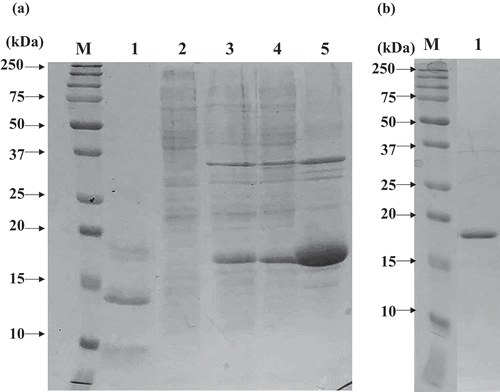
Purified AKPI2 is mainly existed in the form of two polypeptides, chain A (12/13 kDa) and chain B (6 kDa). In order to study whether the complete form of AKPI2 (19 kDa) have protease inhibitory activity, the recombinant AKPI2 protein was produced in E. coli. E. coli cells harboring plasmid pET-rAKPI2 were incubated in LB medium and the recombinant protein was induced by the addition of 1 mM IPTG. After induction, E.coli cells were harvested and disrupted by sonication. SDS–PAGE showed that the recombinant AKPI2 protein (rAKPI2) was mainly expressed in the insoluble fraction (inclusion body) after sonication ()). rAKPI2 recovered in the form of the inclusion body was refolded by the dialysis method and purified by Sephadex G-75 column chromatography (data not shown). The yield of purified AKPI2 ()) was 16 mg/one liter of medium. The purified rAKPI2 were used for inhibition assay.
Figure 5. Expression of recombinant AKPI2 in E.coli.
(a) Expression of recombinant AKPI2 protein in E. coli cells. M, Marker Lane 1, S1 fraction obtained from gel-filtration chromatograpy; Lane 2, before induction by IPTG; Lane 3, after induction by IPTG; Lane 4, soluble fraction of E.coli after sonication; Lane 5, insoluble fraction of E.coli after sonication. (b) Purified rAKPI2. M, Marker; Lane 1, rAKPI2 after Sephadex G-75 column chromatography.
Inhibitory properties and stability of rAKPI2
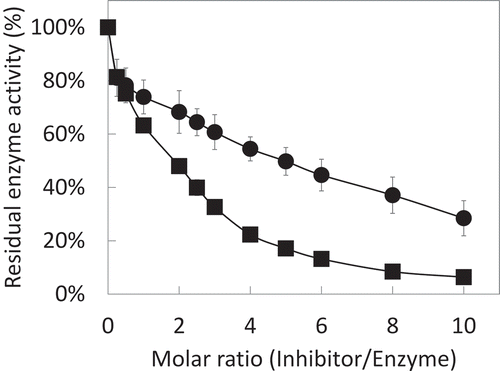
Inhibitory activities of rAKPI2 toward trypsin or chymotrypsin were investigated using L-BAPA or L-BTPA as a substrate (). The rAKPI2 showed stronger inhibition toward chymotrypsin than trypsin. However, the stoichiometry between rAKPI2 and chymotrypsin was estimated as 5:1 and rAKPI2 was not able to completely inhibit trypsin even the molar ratio is 10:1. The inhibition constants (Ki) of rAKPI2 toward trypsin and chymotrypsin were calculated to be 6 × 10−6 M and 4 × 10−7 M based on the Bieth plot.
Figure 6. Inhibitory activity of rAKPI2 toward trypsin and chymotrypsin.
The inhibitory activity of rAKPI2 were measured against trypsin (●) and chymotrypsin (■). Data points are the means of three determinations.
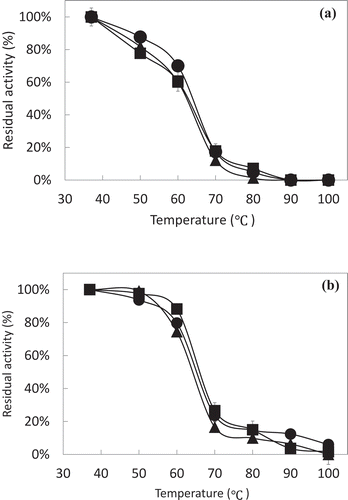
The heat-stability of rAKPI2 was investigated at different temperatures. The trypsin inhibitory activity was gradually reduced from 37 ℃ to 60 ℃ and reduced rapidly at 70℃ ()).On the other hand, the chymotrypsin inhibitory activity was stable from 37℃ to 60℃ and 80% of inhibitory activity was lost at 70℃ ()). The heat treatment time have no influence on the decrease of inhibitory activity. Although inhibitory activities of rAKTI2 toward papain, subtilisin, and elastase were measured, no inhibition of papain and subtilisin was detected, but rAKPI2 had weak inhibitory activity toward elastase (Supplemental Table S2).
Discussion
The Kunitz-type protease inhibitors from Apios tubers were purified by the DEAE-Cellufine A-500 column, Butyl-Cellufine column, Sephadex G-50 column, and RP-HPLC. N-terminal amino acid sequences of purified proteins showed that two kinds of Kunitz-type protease inhibitors (AKPI1: 20 kDa and AKPI2: 19 kDa) existed in Apios tubers. Besides the presence of 19 kDa AKPI2 protein, two-chain form of AKPI2 protein fragments [13/12 kDa (Chain A), and 6 kDa (Chain B)] were observed. Although AKPI1, AKPI2, and its fragments were able to be separated by RP-HPLC, the yields were very low and their inhibitory activities could not be detected. Therefore to characterize AKPI1 and AKPI2 proteins, we tried to cDNA cloning and functional expression of the recombinant protein.
For serine protease inhibitors, P1 residue of reactive site is mainly responsible for the specificity of inhibitors to specific proteases [Citation25]. The amino acid residues near P1 site of Kunitz-type protease inhibitor can form a protruding reaction loop, that can bind to the groove of protease, like a key and lock [Citation26]. If P1 residue is Lys or Arg, the inhibitor has strong inhibitory activity toward trypsin and trypsin-like enzymes. If P1 residue is Tyr, Phe, Trp, Leu or Met, the inhibitor has strong inhibitory activity toward chymotrypsin and chymotrypsin-like enzymes. In the case of Ala or Ser, it showed inhibitory activity toward elastase-like enzymes [Citation15]. The P1 residue of AKPI2 is speculated as Leu88 based on the sequence alignment with Kunitz trypsin inhibitor from Glycine max () Gm1) (P01070.2) [Citation27], suggesting that AKPI2 is a kind of chymotrypsin inhibitor. This speculation was consistent with the results of inhibitory assay of rAKPI2 that rAKPI2 prefer to inhibit chymotrypsin than trypsin (). However, the stoichiometry between AKPI2 and chymotrypsin is 5:1, suggesting that AKPI2 cannot form the strong EI complex with chymotrypsin. Inhibitory activity and the specificity of some KPIs sometimes were affected by the amino acid residues of P3-P2 sites [Citation28,Citation29]. Therefore, these amino acid residues of AKPI2 may influence of the inhibitory activity toward chymotrypsin. On the other hand, Gly85-Ile86-Ser87 motif was positioned around the reactive site of AKPI1. This motif has been reported in the Kunitz-type protease inhibitors from Cicer arietinum L. (CaKPI) (AAT45474) [Citation30], Glycine max (GmKTI2) (ACA23207.1) [Citation31] and Pisum sativum (PsTI) (O82711.1) [Citation32,Citation33] so far (). Among them, CaKPI has strong inhibitory activity against insect (H. armigera) gut proteinases and weak activity against trypsin [Citation30]. Another Kunitz-type protease inhibitor from Canavalia lineata (ClSI) (p81726) can inhibit subtilisin and its reactive site is known as Gly-Arg-Gly [Citation34,Citation35]. Thus, there are a few examples that the relationship between P1 amino acid residues and specificity of target serine proteases are obscure. In addition, the Kunitz-type protease inhibitors with Gly-Ile-Ser motif had five cysteine residues different from classical Kunitz-type inhibitors that have four cysteine residues. The presence of extra single cysteine residue tends to form dimer easily. As CaKPI and PsTI were erroneously recognized as α-L-fucosidase due to sequence homology [Citation30,Citation32], there is the little information about this type of Kunitz inhibitors. Based on the above features, those inhibitors may be categorized a new sub-family of Kunitz-type protease inhibitors and AKPI1 may belong to this sub-family. Future study of AKPI1 can help extend the knowledge of those sub-family of KPIs with abnormal P1 site.
Although most of Kunitz-type protease inhibitors were single polypeptide chains, some inhibitors were constituted from two polypeptide chains [Citation14,Citation31]. The Kunitz-type trypsin inhibitor purified from Cassia grandis seeds (CgTI, 20.0 kDa) is constituted two polypeptides chains (12.0 kDa and 7.0 kDa) [Citation36]. The Kunitz-type trypsin inhibitor from Enterolobium timbouva (EtTI) is composed of two polypeptides chains, which presents fungicidal effects against Candida species [Citation37]. These two polypeptide chains were generally connected by disulfide bridge. For instance, the Kunitz-type proteinase inhibitor from Solanum tuberosum L. (potato tubers) (PSPI-21, P58515.1, 21kDa) (St in ), which can inhibit trypsin, chymotrypsin and human leukocyte elastase (HLE) are consisted of two polypeptide chains (16.5 kDa and 4.5 kDa) [Citation38]. The large chain contained three cysteine residues and the small chain contained one cysteine residue so that the two subunits can be held together by a disulfide bridge [Citation39] which can be broken by β-mercaptoethanol [Citation40]. Similarly, the large chain (16 kDa) of Kunitz-type trypsin inhibitor from Enterolobium contortisiliquum (ECTI, P86451.1) (Ec in ) also contained three cysteine residues and the small chain (6 kDa) contained one cysteine residue, which can inhibit trypsin, chymotrypsin in the stoichiometric ratio of 1:1, and also human plasma kallikrein, factor XIIa and plasmin [Citation41]. Identically, the Kunitz-type serine protease inhibitor from Acacia confusa named ACTI (AAA32618.1) (Ac in ) consisted of two subunits (16 kDa, 5 kDa) which contained three and one cysteine residues, respectively, [Citation42]. The ACTI can inhibit trypsin and chymotrypsin at the molar ratio of 1:1 and 1:2 [Citation43]. On the other hand, the short-chain (chain B) of AKPI2 contained two cysteine residues and the two polypeptide chains (chain A and B) were detected by SDS-PAGE in the absence of β-mercaptoethanol ()). This suggested that the two polypeptide chains were not held together by a disulfide bridge unlike PSPI, ECTI, and ACTI which two polypeptide chains were combined by one disulfide bond. Therefore, AKPI2 may belong to another type of Kunitz-type protease inhibitor that has not been reported.
Both the separated AKPIs fraction (S1 fraction) and recombinant rAKPI2 are prone to precipitate easily. Precipitation of rAKPI2 also occurs during the process of protease inhibitory activity determination. This phenomenon may affect the measurement and calculation of the inhibition constants (Ki) because of the decrease in concentration. The stoichiometry also may be affected since the stoichiometry between rAKPI2 and chymotrypsin was not 1:1. The formation of two chains of AKPI2 may enhance the solubility to avoid precipitation. Furthermore, the two-chain form of AKPI2 may effectively function as a strong inhibitor in the tuber. This hypothesis will be verified by future studies in which recombinant polypeptide chains A and B of AKPI2 are expressed and the inhibitory activity of complex of chains A and B are investigated.
Protease inhibitors are existed in plant widely, and they can help regulate and balance protease activities. They also play important roles in the exogenous defense [Citation44]. We have already reported that a Bowman-Birk type trypsin inhibitor (AATI) is existed in Apios tubers [Citation11]. In this study, we confirmed that Apios tubers contained AKPI1 that may inhibit other protease and Kunitz-type chymotrypsin inhibitor AKPI2. Those three protease inhibitors that can inhibit different target proteases protect Apios to resist different exogenous stresses. In addition, due to the special characteristics (formation of tubers) of Apios different from other leguminous plants, the AKPIs that occurred in Apios may be different from other KPIs. The application of protease inhibitors used as anti-disease, anti-pest and anti-cancer has been researched extensively [Citation44]. We had reported the AATI have an inhibitory effect on the proliferation of cancer cell lines [Citation12]. Besides, if novel bioactivities of AKPI1 and AKPI2 are clarified in the future work, utilization of Apios tubers for functional foods may spread widely. [Citation24]
Author contribution
Y. K. and M.Y. conceived and designed the study; Y. K., and L. J. performed the experiment; Y.K. and L.J. wrote the manuscript. All authors have read and approved the final manuscript.
FinalApios_Supplemental_Table.pdf
Download PDF (69.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Musgrave ME, Blackmon WJ. Respiration in a new tuber crop, Apios americana. J Am Soc Hortic Sci. 1995;120(4):656–660.
- Asch DL, Hart JP. Crop domestication in prehistoric Eastern North America. In: Goodman RM, editor. Encyclopedia of plant and crop science. New York: Marcel Dekker; 2004. p. 314–319.
- Kikuta C, Sugimoto Y, Konishi Y, et al. Physicochemical and structural properties of starch isolated from Apios americana Medikus. J Appl Glycosci. 2011;59(1):21–30.
- Carlisi J, Wollard D. History, culture, and nutrition of Apios americana. J Nutraceuticals, Funct Med Foods. 2004;4(3-4):85–92. Haworth Press Inc..
- Belamkar V, Farmer AD, Weeks NT, et al. Genomics-assisted characterization of a breeding collection of Apios americana, an edible tuberous legume. Sci Rep. 2016;6:34908.
- Belamkar V, Wenger A, Kalberer SR, et al. Evaluation of phenotypic variation in a collection of: an edible tuberous legume. Crop Sci. 2015;55(2):712–726.
- Iwai K, Matsue H. Ingestion of Apios americana Medikus tuber suppresses blood pressure and improves plasma lipids in spontaneously hypertensive rats. Nutr Res. 2007;27(4):218–224.
- Kuramoto S, Kaneyoshi G, Morinaga Y, et al. Angiotensin-converting enzyme-inhibitory peptides isolated from pepsin hydrolyzate of Apios americana tuber and their hypotensive effects in spontaneously hypertensive rats. Food Sci Technol Res. 2013;19(3):399–407.
- Chu Q, Chen M, Song D, et al. Apios americana Medik flowers polysaccharide (AFP-2) attenuates H2O2 induced neurotoxicity in PC12 cells. Int J Biol Macromol. 2019;123:1115–1124.
- Yan F, Yang Y, Yu L, et al. Effects of C-glycosides from Apios americana leaves against oxidative stress during hyperglycemia through regulating mitogen-activated protein kinases and nuclear factor erythroid 2-related factor 2. J Agric Food Chem. 2017;65(34):7457–7466.
- Zhang Y, Kouzuma Y, Miyaji T, et al. Purification, characterization, and cDNA cloning of a Bowman-Birk type trypsin inhibitor from Apios americana Medikus tubers. Biosci Biotechnol Biochem. 2008;72(1):171–178.
- Zhang Y, Zhou C, Tang S, et al. Effect of AATI, a Bowman-Birk type inhibitor from Apios americana, on proliferation of cancer cell lines. Food Chem. 2011;128(4):909–915.
- Srikanth S, Chen Z. Plant protease inhibitors in therapeutics-focus on cancer therapy. Front Pharmacol. 2016;7:470.
- Cristina Oliveira de Lima V, Piuvezam G, Leal Lima Maciel B, et al. Trypsin inhibitors: promising candidate satietogenic proteins as complementary treatment for obesity and metabolic disorders? J Enzyme Inhib Med Chem. 2019;34(1):405–419.
- Haq SK, Atif SM, Khan RH. Protein proteinase inhibitor genes in combat against insects, pests, and pathogens: natural and engineered phytoprotection. Arch Biochem Biophys. 2004;431(1):145–159.
- Kouzuma Y, Irie S, Yamazaki R, et al. Purification and cDNA cloning of a lectin and a lectin-like protein from Apios americana Medikus tubers. Biosci Biotechnol Biochem. 2014;78(4):574–581.
- Filippova IY, Lysogorskaya EN, Oksenoit ES, et al. L-Pyroglutamyl-L-phenylalanyl-L-leucine-p-nitroanilide—a chromogenic substrate for thiol proteinase assay. Anal Biochem. 1984;143(2):293–297.
- Bunyatang O, Chirapongsatonkul N, Bangrak P, et al. Molecular cloning and characterization of a novel bi-functional alpha-amylase/subtilisin inhibitor from Hevea brasiliensis. Plant Physiol Biochem. 2016;101:76–87.
- Stein RL. Catalysis by human leukocyte elastase: substrate structural dependence of rate-limiting protolytic catalysis and operation of the charge relay system. J Am Chem Soc. 1983;105(15):5111–5116.
- Bieth J. Some kinetic consequences of the tight binding of protein-proteinase-inhibitors to proteolytic enzymes and their application to the determination of dissociation constants. Berlin, Heidelberg: Springer; 1974. ( (Proteinase Inhibitors)).
- Schägger H. Tricine–SDS-PAGE. Nat Protoc. 2006;1:16–22.
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76(9):4350–4354.
- Shimizu Y, Yamazaki H, Yoshida S, et al. Molecular cloning, functional expression, and characterization of isolectin genes of hemolytic lectin CEL-III from the marine invertebrate Cucumaria echinata. Biosci Biotechnol Biochem. 2012;76(2):276–282.
- Jiménez T, Martín I, Hernández-Nistal J, et al. The accumulation of a Kunitz trypsin inhibitor from chickpea (TPI-2) located in cell walls is increased in wounded leaves and elongating epicotyls. Physiol Plant. 2008;132(3):306–317.
- Schoofs L, Clynen E, Trypsin SM. Chymotrypsin inhibitors in insects and gut leeches. Curr Pharm Des. 2002;8(7):483–491.
- Zhou D, Lobo YA, Batista IF, et al. Crystal structures of a plant trypsin inhibitor from Enterolobium contortisiliquum (EcTI) and of its complex with bovine trypsin. PLoS One. 2013;8(4):e62252.
- Ozawa K, Michael L. The reactive site of trypsin inhibitors. J Biol Chem. 1966;241(17):3955–3961.
- Kouzuma Y, Yamasaki N, Kimura M. Th e tissue-type plasminogen activator inhibitor ETIa from Erythrina variegata: structural basis for the inhibitory activity by cloning, expression, and mutagenesis of the cDNA encoding ETIa. J Biochem. 1997;121(3):456–463.
- Kouzuma Y, Yamasaki N, Kimura M. Cloning, expression, and mutagenesis of trypsin inhibitor ETIb from Erythrina variegata seeds. Biosci Biotechnol Biochem. 1997;61(6):1041–1043.
- Srinivasan A, Giri AP, Harsulkar AM, et al. A Kunitz trypsin inhibitor from chickpea (Cicer arietinum L.) that exerts anti-metabolic effect on podborer (Helicoverpa armigera) larvae. Plant Mol Biol. 2005;57(3):359–374.
- Rashed NA, Macdonald MH, Matthews BF. Protease inhibitor expression in soybean roots exhibiting susceptible and resistant interactions with soybean cyst nematode. J Nematol. 2008;40(2):138.
- Christopher A, Virginia S, Alan D, et al. Molecular cloning and pattern of expression of an α-L-fucosidase gene from pea seedlings. J Biol Chem. 1995;270(42):24839–24843.
- Tarragó T, Martínez I, Torrent M, et al. The fuc1 gene product (20 kDa FUC1) of Pisum sativum has no α-L-fucosidase activity. Plant Mol Biol. 2003;51(6):877–884.
- Terada S, Fujimura S, Katayama H, et al. Purification and characterization of two kunitz family subtilisin inhibitors from seeds of Canavalia lineata. J Biochem. 1994;115(3):392–396.
- Terada S, Katayama H, Noda K, et al. Amino acid sequences of kunitz family subtilisin inhibitors from seeds of Canavalia lineata. J Biochem. 1994;115(3):397–404.
- Brandao-Costa RMP, Araujo VF, Porto ALF. CgTI, a novel thermostable Kunitz trypsin-inhibitor purified from Cassia grandis seeds: purification, characterization and termiticidal activity. Int J Biol Macromol. 2018;118(Pt B):2296–2306.
- de Oliveira CFR, Oliveira CT, Taveira GB, et al. Characterization of a Kunitz trypsin inhibitor from Enterolobium timbouva with activity against Candida species. Int J Biol Macromol. 2018;119:645–653.
- Valueva Tatyana A, Revina Tatyana A, Kladnitskaya Galina V, et al. Kunitz-type proteinase inhibitors from intact and Phytophthora- infected potato tubers. FEBS Lett. 1998;426(1):131–134.
- Valueva TA, Revina TA, Mosolov VV, et al. Primary structure of potato Kunitz-type serine proteinase inhibitor. Biol Chem. 2000;381(12):1215–1221.
- Pouvreau L, Gruppen H, Van Koningsveld GA, et al. The most abundant protease inhibitor in potato tuber (cv. Elkana) is a serine protease inhibitor from the Kunitz family. J Agric Food Chem. 2003;51(17):5001–5005.
- Batista IFC, Oliva MLV, Araujo MS, et al. Primary structure of a kunitz-type trypsin inhibitor from Enterolobium contortisiliquum seeds. Phytochemistry. 1996;41(4):1017–1022.
- Wu HC, Lin JY. The complete amino acid sequence of a kunitz family trypsin inhibitor from seeds of acacia confusa. J Biochem. 1993;113(2):258–263.
- Lin JY, Chu SC, Wu HC, et al. Trypsin inhibitor from the seeds of Acacia confusa. J Biochem. 1991;110(6):879–883.
- Habib H, Fazili KM. Plant protease inhibitors: a defense strategy in plants. Biotechnol Mol Biol Rev. 2007;2(3):68–85.