ABSTRACT
The penta-EF-hand (PEF) protein family includes ALG-2 (gene name, PDCD6) and its paralogs as well as classical calpain family members. ALG-2 is a prototypic PEF protein that is widely distributed in eukaryotes and interacts with a variety of proteins in a Ca2+-dependent manner. Mammalian ALG-2 and its interacting partners have various modulatory roles including roles in cell death, signal transduction, membrane repair, ER-to-Golgi vesicular transport, and RNA processing. Some ALG-2-interacting proteins are key factors that function in the endosomal sorting complex required for transport (ESCRT) system. On the other hand, mammalian calpain-7 (CAPN7) lacks the PEF domain but contains two microtubule-interacting and trafficking (MIT) domains in tandem. CAPN7 interacts with a subset of ESCRT-III proteins through the MIT domains and regulates EGF receptor downregulation. Structures and functions of ALG-2 and those of its interacting partners as well as relationships with the calpain family are reviewed in this article.
GRAPHICAL ABSTRACT
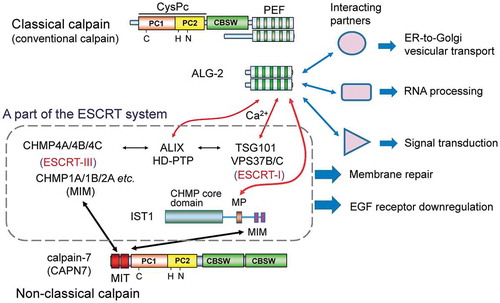
The penta-EF-hand (PEF) protein ALG-2 interacts with various proteins in versatile cell systems including a common binding partner with calpain-7 in the ESCRT system.
Calcium is a major essential mineral for animals and taken into the body from foods and milk. The total amount of calcium in the adult human body is as high as 1000–1200 grams. Over 99% of calcium is present in the form of hydroxyapatite in bones and teeth, which provides strength of the hard tissues. The remaining calcium is present as cations in blood and body fluids inside and outside cells, and these cations play important roles in mediating vascular contraction, vasodilatation, muscle function, intracellular signaling, nerve transmission and hormonal secretion [Citation1]. Calcium ions (Ca2+) are buffered with small organic compounds and with a variety of low-affinity Ca2+-binding proteins. Concentrations of Ca2+ in milk greatly differ among mammal species, but they are closely correlated with concentrations of casein, the major acidic milk protein [Citation2]. While the concentration of Ca2+ in extracellular fluid is 1–2 mM, it is maintained at an extremely low level (as low as 100 nM or less) in the cytosol. Thus, there is a more than 10,000-fold difference between concentrations of Ca2+ outside and inside the cells. This feature is in marked contrast to Mg2+, the concentrations which are similar at the millimolar (mM) order on both sides of the cell membrane [Citation3].
Biological effects of Ca2+ are mediated through various types of Ca2+-binding proteins, which have structural motifs such as an EF-hand (helix-loop-helix), a C2 domain, an endonexin fold (annexin domain) and acidic clusters [Citation4]. Binding of Ca2+ to these motifs stabilizes protein structures, induces conformational changes to activate enzymatic activities, triggers interaction with target factors (proteins and phospholipids), and keeps the free Ca2+ concentrations at fixed levels by buffering actions. The EF-hand proteins are the most extensively studied Ca2+-binding proteins [Citation5]. Calmodulin (CaM) is characterized by four EF-hands with sterically separated N- and C-terminal lobes, which contain two paired EF-hands, respectively. CaM is the best known signal transducer that plays important roles in eukaryotic cells and has been well reviewed in the literature [Citation6–Citation8]. Calpain was originally discovered as a Ca2+-dependent cysteine protease present in animal tissues, and it was found to possess a CaM-like Ca2+-binding domain in each large subunit and small subunit based on the primary structure [Citation9,Citation10]. However, 3D-structure analysis of the Ca2+-binding region revealed a novel domain structure with five EF-hand modules [Citation11,Citation12], which was later named penta-EF-hand (PEF) [Citation13]. The PEF domain is also found in non-calpain proteins including ALG-2 [Citation14], which is widely distributed in eukaryotes and regarded as a prototypic penta-EF-hand protein [Citation15]. On the other hand, structurally related homologs of calpain that lack the PEF domain but retain the protease domain (designated non-classical calpains or atypical calpains) have been identified in a wide range of eukaryotes [Citation16–Citation19]. Amino acid sequences related to the cysteine protease domain of calpain have also been reported in bacteria [Citation17,Citation18]. In this review, the author focuses on physiological relationships between ALG-2 and the non-classical calpain designated calpain-7 (CAPN7) from the viewpoint of interacting partners.
Partial overlap of the PEF family and the calpain family
Ubiquitously expressed conventional calpains designated μ-calpain and m-calpain (~110 kDa) are heterodimers of the large subunit (~80 kDa, CAPN1 or CAPN2) and the common regulatory small subunit (~30 kDa, CAPNS1). The required Ca2+ concentrations for protease activation in in vitro assays are at micromolar (μM) and millimolar (mM) levels for μ-calpain and m-calpain, respectively [Citation20]. Calcium sensitivity increases by binding to phospholipids, N-terminal processing of subunits and phosphorylation [Citation21–Citation23]. Skeletal muscle-specific p94/calpain-3 (CAPN3) requires Na+ instead of Ca2+ for its rapid and exhaustive auto-degradation [Citation24], suggesting a structural role of the PEF domain in CAPN3. Calpains have a cysteine protease core domain (CysPc; divided into the two subdomains PC1 and PC2), a calpain type β-sandwich domain (CBSW) (previously called domain III or C2-like domain) and a PEF domain (, calpain family) [Citation17,Citation19]. In the human genome, fifteen genes encode the calpain protease domain-containing sequences designated CAPN1-CAPN16, whereas CAPN4, encoding the non-catalytic small subunit, has been renamed and replaced with CAPNS1. While nine calpain paralogs (CAPN1, 2, 3, 8, 9, 11, 12, 13, and 14) contain PEF domains and are called classical or typical calpains, six calpain paralogs lack PEF domains and are called non-classical or atypical calpains [Citation19]. CAPN7 contains additional domains in place of the PEF domain: two microtubule-interacting and trafficking (MIT) domains in tandem and an additional CBSW domain. CAPN16 (also called androglobin; expressed in mammalian testes) has a CAPN7-like protease domain without readily recognizable catalytic His/Asn residues in the corresponding PC2-like subdomain (PC2ʹ) [Citation25].
Figure 1. Relationship between the penta-EF-hand (PEF) protein family and the calpain family in mammals.
Classical (typical) calpain sequences contain the PEF domain and the calpain type β-sandwich domain (CBSW) in addition to the cysteine protease core domain (CysPc), which is further divided into two subdomains named PC1 (containing a catalytic Cys residue) and PC2 (containing catalytic His and Asn residues). Conventional calpains (μ-calpain and m-calpain) are comprised of each catalytic large subunit (designated CAPN1 for μ-calpain or CAPN2 for m-calpain) and a common regulatory small subunit (CAPNS1). Non-classical (atypical) calpain sequences lack the PEF domain but contain additional domains or motifs [calcium-binding C2 domain, microtubule-interacting and trafficking (MIT) domain, Zinc finger (ZnF), SOL-homology domain (SOH), and circularly permutated globin domain (cpGB) split by the calmodulin-binding IQ motif]. Calpain-3 (CAPN3), specifically expressed in skeletal muscles, has distinct sequences: N-terminal sequence (NS), insertion sequence 1 (IS1), and insertion sequence 2 (IS2). Calpain-7 (CAPN7) is an ortholog of fungal PalB. PEF proteins are classified into two groups based on similarity of the first EF-hand (EF1) sequences [Citation15].
![Figure 1. Relationship between the penta-EF-hand (PEF) protein family and the calpain family in mammals.Classical (typical) calpain sequences contain the PEF domain and the calpain type β-sandwich domain (CBSW) in addition to the cysteine protease core domain (CysPc), which is further divided into two subdomains named PC1 (containing a catalytic Cys residue) and PC2 (containing catalytic His and Asn residues). Conventional calpains (μ-calpain and m-calpain) are comprised of each catalytic large subunit (designated CAPN1 for μ-calpain or CAPN2 for m-calpain) and a common regulatory small subunit (CAPNS1). Non-classical (atypical) calpain sequences lack the PEF domain but contain additional domains or motifs [calcium-binding C2 domain, microtubule-interacting and trafficking (MIT) domain, Zinc finger (ZnF), SOL-homology domain (SOH), and circularly permutated globin domain (cpGB) split by the calmodulin-binding IQ motif]. Calpain-3 (CAPN3), specifically expressed in skeletal muscles, has distinct sequences: N-terminal sequence (NS), insertion sequence 1 (IS1), and insertion sequence 2 (IS2). Calpain-7 (CAPN7) is an ortholog of fungal PalB. PEF proteins are classified into two groups based on similarity of the first EF-hand (EF1) sequences [Citation15].](/cms/asset/264e773b-f049-471a-8279-17f5315c45ff/tbbb_a_1700099_f0001_oc.jpg)
In addition to the two calpain small subunit genes (CAPNS1 and intron-less CAPNS2), four genes for PEF proteins (22 ~ 30 kDa) that lack catalytic domains are known (, PEF protein family). While ALG-2 (gene name, PDCD6) has the shortest non-PEF N-terminal sequence (23 amino acid residues rich in Pro/Gly/Ala), the closest paralog named peflin (gene name, PEF1) has the longest non-PEF sequence with 113 amino acids containing nine repeats of a nonapeptide (A/PPGGPYGGP) sequence [Citation15,Citation26]. Sorcin and grancalcin also contain Gly/Pro-rich sequences at the N-terminal regions [Citation15].
Evolutionary features of ALG-2 and CAPN7
Sequence comparison of the PEF proteins has revealed that the calpain PEF domains are evolutionarily closer to the sorcin/grancalcin subfamily than to the ALG-2/peflin subfamily [Citation15]. As shown in , ALG-2 and its orthologs are the most widely distributed PEF proteins in eukaryotes ranging from protists to mammals [Citation27]. PEF domain-containing calpains and other PEF proteins are found in higher animals, but their presence depends on classes in other eukaryotes. Interestingly, the fly does not have genes for CAPN7 (ortholog of fungal PalB and yeast Rim13) nor those for calpain small subunits but possesses a PEF-containing classical calpain. Protists and plants have other types of non-classical calpain homologs [Citation17,Citation18].
Table 1. Distribution of penta-EF-hand (PEF) proteins, classical calpains and calpain-7 orthologs in eukaryotes.
ALG-2-interacting proteins and binding motifs
Although ALG-2 was originally identified as a pro-apoptotic factor (apoptosis-linked gene 2) [Citation14], roles of ALG-2 in cell death remain unclear. ALG-2 interacts in a Ca2+-dependent manner with a variety of proteins that function in (i) the endosomal sorting complex required for transport (ESCRT) system, (ii) regulation of endoplasmic reticulum (ER)-to-Golgi vesicular transport, (iii) RNA processing, (iv) protein phosphorylation, and (v) other cellular processes () (See Ref [Citation28]. therein and Refs. [Citation29–Citation33] for new reports.). Binding of these proteins with ALG-2 may indirectly promote the cell death pathway at multiple steps. Importantly, the reported ALG-2-interacting partners are mutually physically associated or in close contact at specific subcellular localizations, e.g., ESCRT system components (ALIX, HD-PTP, TSG101, VPS37B/C, IST1) [Citation34–Citation38], ER exit sites (Sec31A, annexin A11, and TFG) [Citation30,Citation39], and the ER-to-Golgi pathway (MISSL and MAP1B) [Citation31,Citation32].
Figure 2. Schematic structures of ALG-2-interacting proteins reported in the literature.
The human or murine ALG-2-interacting proteins are classified into five groups for convenience sake based on functional properties: (a) ESCRT system, (b) ER-to-Golgi vesicular transport, (c) RNA processing, (d) protein kinases, and (e) miscellaneous. Underlined proteins have been studied in the author’s group. Red boxes and thick violet bars indicate Pro-rich regions (PRRs) and determined ALG-2-binding regions, respectively. PTP, phosphotyrosine phosphatase; UEV, ubiquitin E2 variant; CC, coiled-coil; SB, steadiness box; LC1, light chain 1; CID, C-terminal domain (CTD)-interacting domain; ZnF, zinc finger; RRM, RNA recognition motif; Ig-like C2, immunoglobulin-like constant domain type 2; ANK, ankyrin; TM, transmembrane; C2, Protein Kinase C C2-domain-like Ca2+-binding domain; vWFA, von Willebrand factor A.
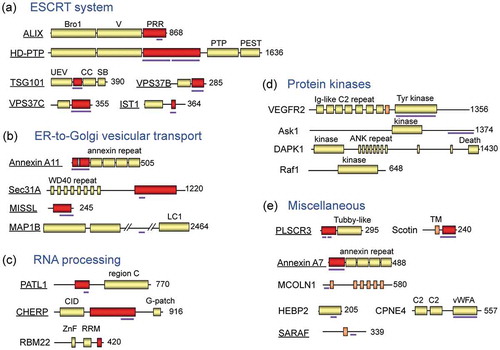
In vitro binding assays of various deletion and amino acid-substituted mutants enabled narrowing down of the major binding regions for ALG-2: ALIX [Citation40,Citation41], TSG101 [Citation35], Scotin [Citation42], PLSCR3 [Citation43], MCOLN1 (mucolipin-1) [Citation44], Sec31A [Citation45], PATL1 [Citation46], IST1 [Citation38], CHERP [Citation47], MAP1B [Citation32], and SARAF [Citation33,Citation48]. Not all but the majority of ALG-2-interacting proteins possess Pro-rich regions (PRRs) (). Comparison of the multiple predicted ALG-2-binding sequences has revealed at least three types of ALG-2-binding motifs (ABMs) that are rich in prolines ()). X-ray crystal structure analyses of human recombinant ALG-2 proteins in complex with an ALIX peptide [Citation49] and with a Sec31A peptide [Citation48] further defined the motifs and clarified the nature of interactions. Unlike CaM, ALG-2 does not drastically change its conformation to bind its target [Citation49]. Interestingly, ALIX and Sec31A bind ALG-2 at different hydrophobic pockets (,c)). Although binding sites in some proteins have not been clarified yet, incomplete resemblance to the type 1 ABM (ABM-1) sequence (annexin A7, annexin A11, TSG101, VPS37B, VPS37C, MISSL and Scotin) or to the ABM-2 sequence (PLSCR3 and TFG) suggests that the binding strength of a suboptimal motif is augmented by surrounding sequences [Citation48] or by oligomerization/polymerization [Citation30]. ALIX and IST1 contain Met-Pro (MP) repeat sequences, designated ABM-3. While the ABM-3 sequence in IST1 is essential for interaction with ALG-2 [Citation38], this short motif sequence found in ALIX may be subsidiary to the main ABM-1 sequence. The ALG-2 binding sites described above are all located in the predicted intrinsically disordered regions, which have advantages in protein–protein interactions [Citation50,Citation51]. However, ALG-2 also binds the structurally stable region of HEBP2 [Citation29,Citation52], indicating the presence of diversity in the binding modes.
Figure 3. PEF-binding motifs and 3D structures.
(a) Three types of Pro-rich ALG-2-binding motifs (ABMs). Residues conserved among the identified ALG-2-interacting proteins in each type of ABM are indicated in red, and residues compatible with the type 2 motif at the Ω position are indicated in violet. [PΦ], Pro or hydrophobic; [FW], Phe or Trp; Ω, large side chain; x, variable. (b) Overall 3D structure of the complex between ALG-2 (homodimer) and ALIX peptides (indicated by magenta arrows) is shown by a cartoon in rainbow colors (from blue in the N-terminal region to red in the C-terminal region) using the 3D presentation software PyMOL and Protein Data Bank (PDB) code 2ZNE. (c) Overall 3D structure of the complex between ALG-2 and Sec31A peptides. PDB code 3WXA. A side view (left panel) and a 90°-rotated bottom view (right panel). (d) Schematic representation of a three-binding-site model of calpain inhibition by calpastatin. Among the three conserved regions of the four repeated domains of calpastatin, region B binds the protease domain and inhibits the proteolytic activity of calpain. Regions A and C bind the PEF domains of the large subunit (L-PEF) and the small subunit (S-PEF), respectively. (e) Amino acid sequences of regions A and C of human calpastatin. Conserved (identical or similar) residues are highlighted in light green for region A and in cyan for region C. Conserved residues between the two regions are marked with asterisks, where high conservation is indicated by bold face. (f) Overall 3D structure of the complex between rat m-calpain and calpastatin domain 1 (PDB code 3DF0). The PEF domains and the calpastatin peptide are shown by cartoon models in rainbow colors and in magenta, respectively. Other calpain domains are shown by surface representation in pale colors.
![Figure 3. PEF-binding motifs and 3D structures.(a) Three types of Pro-rich ALG-2-binding motifs (ABMs). Residues conserved among the identified ALG-2-interacting proteins in each type of ABM are indicated in red, and residues compatible with the type 2 motif at the Ω position are indicated in violet. [PΦ], Pro or hydrophobic; [FW], Phe or Trp; Ω, large side chain; x, variable. (b) Overall 3D structure of the complex between ALG-2 (homodimer) and ALIX peptides (indicated by magenta arrows) is shown by a cartoon in rainbow colors (from blue in the N-terminal region to red in the C-terminal region) using the 3D presentation software PyMOL and Protein Data Bank (PDB) code 2ZNE. (c) Overall 3D structure of the complex between ALG-2 and Sec31A peptides. PDB code 3WXA. A side view (left panel) and a 90°-rotated bottom view (right panel). (d) Schematic representation of a three-binding-site model of calpain inhibition by calpastatin. Among the three conserved regions of the four repeated domains of calpastatin, region B binds the protease domain and inhibits the proteolytic activity of calpain. Regions A and C bind the PEF domains of the large subunit (L-PEF) and the small subunit (S-PEF), respectively. (e) Amino acid sequences of regions A and C of human calpastatin. Conserved (identical or similar) residues are highlighted in light green for region A and in cyan for region C. Conserved residues between the two regions are marked with asterisks, where high conservation is indicated by bold face. (f) Overall 3D structure of the complex between rat m-calpain and calpastatin domain 1 (PDB code 3DF0). The PEF domains and the calpastatin peptide are shown by cartoon models in rainbow colors and in magenta, respectively. Other calpain domains are shown by surface representation in pale colors.](/cms/asset/0b2c982a-cb45-4253-97e1-f054755880d7/tbbb_a_1700099_f0003_oc.jpg)
Calpastatin, an endogenous calpain inhibitor protein, has four repeated inhibitory domains [Citation53–Citation55]. Each inhibitory domain is comprised of three conserved regions, among which region B is the inhibitory center [Citation56,Citation57], and both regions A and C augment inhibitory potency ()) [Citation58,Citation59]. Regions A and C are similar in amino acid sequences ()) but specifically bind PEF domains of the classical calpain large subunit (L-PEF) and the small subunit (S-PEF), respectively, in a Ca2+-dependent manner [Citation57,Citation60]. The peptides of regions A and C form acidic amphiphilic helices ()) [Citation61–Citation63]. They bind each calpain PEF domain in the hydrophobic cavity similarly found as Pocket 3 in ALG-2. However, the modes of interactions analyzed in the 3D structures of complexes of respective peptides with ALG-2, sorcin, and calpain are different and seem to have diverged during evolution of the PEF proteins [Citation64].
ALG-2 functions as a Ca2+-dependent adaptor protein
PEF proteins form homodimers or heterodimers by pairing the fifth EF-hands (EF5s) as revealed by X-ray crystal structure analyses [Citation11,Citation12,Citation65–Citation69]. The presence of one or two ligand-binding sites per one monomeric PEF molecule suggests a di- or multi-valent mode of interactions for dimeric PEF proteins. The Ca2+-dependent adaptor function of ALG-2 was first demonstrated for ALG-2 to bridge ALIX and TSG101 [Citation70]. Results of in vitro multi-complex formation experiments using either mammalian cell expression constructs or recombinant proteins of ALG-2, ALIX, and ESCRT-I complex (TSG101, VPS28, MVB12A, and one of the VPS37 isoforms A/B/C/D) further indicated that VPS37 isoforms with no or different ALG-2-binding capacities differentially modulate the ternary complex formation of ALG-2, ALIX, and ESCRT-I [Citation37]. Since ALIX contains a PSAP motif and weakly interacts with TSG101 [Citation71], a role of ALG-2 appears to be stabilization of the complex between ALIX and ESCRT-I [Citation28,Citation72]. In the ESCRT system, ESCRT-III plays a key role in membrane deformation by spiral polymerization of CHMP (CHarged Multivesicular body Protein) subunit proteins on the membrane [Citation73]. In mammals, twelve CHMP paralogs are known (CHMP1A/1B/2A/2B/3/4A/4B/4C/5/6/7 and IST1), among which CHMP4s (yeast Snf7 orthologs) are major subunits of ESCRT-III and interact with the Bro1 domain of ALIX [Citation34,Citation73]. Thus, ALIX is recognized as an ESCRT-III-recruiting adaptor and bridges ESCRT-I and -III, which is essential for membrane deformation of HIV-1 budding, abscission of cells at the final stage of cytokinesis, and membrane repair [Citation73,Citation74]. For multivesicular body (MVB) biogenesis, ALIX and the ALIX paralog HD-PTP differentially activate ESCRT-III depending on cargoes [Citation75]. A complex of Hrs and STAM1/2 recruits ESCRT-I in MVB biogenesis and is sometimes called ESCRT-0 [Citation73]. However, upstream factors that recruit ESCRT-I are different among the ESCRT systems employed in cellular functions: i.e., Gag in retrovirus budding, CEP55 in cytokinesis, and Arrestin-Domain Containing Protein 1 (ARRDC1) in extracellular release of microvesicles [Citation73,Citation74,Citation76–Citation78]. ALG-2 functions as an upstream factor of ESCRT-III or an initiator in membrane repair upon a plasma membrane lesion, where ESCRT-0 (Hrs-STAM1/2), ESCRT-I except for TSG101, and ESCRT-II are not recruited [Citation79]. Injury of the plasma membrane causes Ca2+ flux into the cytoplasm and recruitment of the Ca2+-dependent phospholipid-binding protein annexin A7, which forms a complex with ALG-2 [Citation80,Citation81], to facilitate proper recruitment of ALIX to the damaged membrane [Citation82]. The ESCRT system is also involved in lysosomal membrane repair and precedes engulfment of unrepairable lysosomes by the autophagic membrane (lysophagy) [Citation83,Citation84]. Requirement of ALG-2 remains to be established in this case.
The Ca2+-dependent adaptor function of ALG-2 has also been demonstrated in proteins involved in regulation of the ER-to-Golgi vesicular transport system [Citation85]: Sec31A-annexin A11 complex formation [Citation39], MISSL-MAP1B complex formation [Citation31,Citation32], and TFG polymerization [Citation30]. Peflin acts as a negative regulator in this transport system [Citation86]. However, positive or negative effects of PEF proteins may depend on cargoes that are to be analyzed. An ALG-2/peflin heterodimeric complex plays a role as a co-adaptor to bridge ubiquitin ligase CUL3 and its substrate adaptor protein KLHL12 for mono-ubiquitination of Sec31A to form larger coat protein complex II (COPII) coats and promote collagen secretion from the ER exit sites [Citation87]. CaM, which has two independently folded Ca2+ binding N- and C-lobes that interact differentially with target proteins, has also recently been reported to act as a Ca2+-dependent adaptor [Citation88]. It remains unknown whether the presence of two separated binding sites in one ALG-2 molecule for different types of binding motifs (Pocket 1/2 and Pocket 3 for ABM-1 and ABM-2, respectively) enables monomeric ALG-2 to serve as a link to target proteins.
Activation of calpain-7 in the ESCRT system
An oligomeric complex of VPS4 (occasionally called ESCRT-IV), a member of meiotic clade AAA type ATPases (ATPases associated with diverse cellular activities), disassembles ESCRT-III complexes at the last stage of membrane remodeling and abscission [Citation73,Citation89]. The microtubule-interacting and trafficking (MIT) domain of VPS4 binds the C-terminal regions of ESCRT-III proteins by recognizing MIT-interacting motifs (MIMs) [Citation73,Citation89]. Human CAPN7, a non-classical calpain, lacks the PEF domain but possesses a tandem repeat of the MIT domains located at the N-terminus (). CAPN7 physically interacts with a subset of CHMP proteins (ESCRT-III subunit paralogs) through the MIT domains such as CHMP1A, CHMP1B, CHMP4B and IST1 [Citation90,Citation91]. Orthologs of CAPN7 in yeast (Rim13) and Aspergillus (PalB) have been shown to be activated on the ESCRT-III platform and to play essential roles in alkaline adaptation by limited proteolysis of transcription factors [Citation92]. These eukaryotic microbial CAPN7 orthologs have different preferences for interactions with ESCRT-III proteins, i.e., Rim13 to Snf7 (CHMP4) and PalB to Vps24 (CHMP3) [Citation93,Citation94]. Rim13 does not contain any discernable MIT domain, and PalB has only one MIT domain in contrast to two MIT domains in mammalian CAPN7. The variation or lack of the MIT domains might have allowed the protease activation mechanism to evolve differently in the detailed process.
Although physiological substrates have not been identified yet, human CAPN7 has been shown to possess both catalytic activities for autolysis of the monomeric green fluorescent protein (mGFP)-fused protease and those for processing of artificially designed non-physiological substrates [Citation91,Citation95]. These proteolytic activities were abrogated by substitution of the active site Cys-290 with Ser (C290S) and were enhanced with different degrees of efficiency by overexpression of ESCRT proteins in HEK293 cells [Citation95]. IST1 activated CAPN7 in an in vitro proteolytic assay [Citation91] but showed little effects in transfected cells probably due to a sufficient basal level of the protein [Citation95]. CAPN7 may form a ternary complex with IST1 and with CHMP1B [Citation96]. Fluorescence microscopic analysis of autolysis-defective mGFP-CAPN7C290S revealed time-dependent transient accumulation of CAPN7 at epidermal growth factor (EGF) receptor (EGFR)-positive endosomes after stimulation of HeLa cells with EGF [Citation97]. Knockdown of IST1 by the RNA interference method decreased the rate of subcellular localization of CAPN7 in the EGFR-positive endosomes. Knockdown of CAPN7 caused a decrease in the rate of EGF-stimulated EGFR degradation in HeLa cells. Similarly, mouse embryonic fibroblast (MEF) cells derived from CAPN7 knockout (Capn7−/-) mice showed a reduced rate of EGFR degradation compared with that of wild-type MEF cells [Citation97]. The rate of EGFR degradation was recovered by exogenous expression of wild-type CAPN7 but not by expression of the CAPN7C290S mutant. These experimental data clearly indicate that CAPN7 plays roles in degradation of endocytosed EGFR. However, the physiological substrate of CAPN7 has not been identified yet. CAPN7 may accelerate multivesicular body (MVB) sorting by cleaving unknown factors that are involved in the pathway from endocytosis to lysosomal degradation ().
Figure 4. Schematic diagram of calpain-7 actions on ESCRT-mediated EGF receptor downregulation in the endosome-to-lysosome pathway.
Calpain-7 (CAPN7) is recruited to endosomes after stimulation of cells with epidermal growth factor (EGF) and regulates downregulation of the ubiquitinated and endocytosed EGF receptor (EGFR). Calpain-7 interacts via the tandemly repeated microtubule-interacting and trafficking (MIT) domains with a subset of ESCRT-III subunits (CHMP proteins) and related proteins that contain MIT-interacting motifs (MIMs). ALG-2 interacts with IST1, a CHMP-like protein, in a Ca2+-dependent manner at the Met-Pro repeat (MP) region. Endogenous substrates of calpain-7 have not been identified yet. Fungal and yeast orthologs of calpain-7 cleave ALIX-homolog-interacting transcription factors in association with ESCRT-III proteins. VPS4 (isoforms A and B) and spastin, meiotic clade AAA ATPases containing MIT domains, disassemble ESCRT-III polymers and microtubules, respectively [Citation101,Citation102]. CHMP, charged multivesicular body protein; MTBD, microtubule-binding domain; MVB, multivesicular body; Ub, ubiquitin.
![Figure 4. Schematic diagram of calpain-7 actions on ESCRT-mediated EGF receptor downregulation in the endosome-to-lysosome pathway.Calpain-7 (CAPN7) is recruited to endosomes after stimulation of cells with epidermal growth factor (EGF) and regulates downregulation of the ubiquitinated and endocytosed EGF receptor (EGFR). Calpain-7 interacts via the tandemly repeated microtubule-interacting and trafficking (MIT) domains with a subset of ESCRT-III subunits (CHMP proteins) and related proteins that contain MIT-interacting motifs (MIMs). ALG-2 interacts with IST1, a CHMP-like protein, in a Ca2+-dependent manner at the Met-Pro repeat (MP) region. Endogenous substrates of calpain-7 have not been identified yet. Fungal and yeast orthologs of calpain-7 cleave ALIX-homolog-interacting transcription factors in association with ESCRT-III proteins. VPS4 (isoforms A and B) and spastin, meiotic clade AAA ATPases containing MIT domains, disassemble ESCRT-III polymers and microtubules, respectively [Citation101,Citation102]. CHMP, charged multivesicular body protein; MTBD, microtubule-binding domain; MVB, multivesicular body; Ub, ubiquitin.](/cms/asset/c935265e-de3b-434f-bc1c-af6163fbabfa/tbbb_a_1700099_f0004_oc.jpg)
Perspective
ALIX is a scaffold protein that binds and recruits multiple proteins by using different domains: the Bro1 domain (CHMP4), the V domain (LYPXnL motif-containing proteins including HIV-1 Gag p6, PAR1, and Syntenin), and the Pro-rich region (TSG101, ALG-2, endophilin, and CIN85) (See Refs. [Citation28,Citation76,Citation98] and references therein.). Yeast and fungal substrates of CAPN7 orthologs (yeast Rim13, Rim101; fungal PalB, PacC) bind the V domain of ALIX homologs (yeast Rim20; fungal PalA) that recognize the YPXL/I motif [Citation99]. During evolution of eukaryotes, a prototype of CAPN7 might have acquired a primitive MIT domain to localize the protease by interaction with ESCRT-III proteins and to increase efficiency of encountering substrates that are recruited by ESCRT-III-interacting adaptor proteins (ALIX homologs). Knockdown of ALG-2 retards EGFR degradation, suggesting inhibition of ALIX activation by ALG-2 [Citation100]. Interestingly, IST1 has a Met-Pro repeat sequence that serves as a binding site for ALG-2 [Citation38]. Moreover, ALG-2 interacts with ESCRT-I proteins (TSG101, VPS37B and VPS37C) [Citation35,Citation37,Citation70]. Thus, ALG-2 seems to play diverse modulatory roles in the MVB sorting pathway including the ESCRT system and CAPN7. It is intriguing to speculate that ALG-2 interacts with CAPN7 substrates directly or indirectly through either the V domain of ALIX (or HD-PTP) or IST1 in a Ca2+-dependent fashion and recruits them to the ESCRT platform on the endosomal membrane. Moreover, classical calpains might have evolved by acquiring PEF domains to change the Ca2+-dependent activation locality from the ESCRT platform to the phospholipid membranes. The author hopes that these hypothetical ideas will be verified in the future by finding endogenous substrates of CAPN7 and elucidating its proteolytic activation mechanism.
Acknowledgments
The author would like to express his sincere gratitude to the late Professor Takashi Murachi (Kyoto University School of Medicine), Professor Masakazu Hatanaka (Institute for Virus Research, Kyoto University) and colleagues for continuous support for his study on the calpain-calpastatin system. The author thanks all current and past members of the Laboratory of Molecular and Cellular Regulation (Graduate School of Bioagricultural Sciences, Nagoya University) and collaborators throughout the world for further deepening the study on calpains and the penta-EF-hand proteins. Finally, the author would like to thank the late Professor Hideo Chiba, Professor Ryuzo Sasaki, Professor Masaaki Hirose and colleagues in the School of Agriculture (Kyoto University) for giving him invaluable advice with encouragement to become an independent scientist in biochemistry and molecular biology.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Ross AC, Taylor CL, Yaktine AL, et al., editors. Dietary reference intakes for calcium and vitamin D. Washington (DC): The National Academies Collection: Reports funded by National Institutes of Health; 2011. DOI:10.17226/13050.
- Neville MC. Calcium secretion into milk. J Mammary Gland Biol Neoplasia. 2005;10:119–128.
- Carafoli E, Krebs J. Why calcium? How calcium became the best communicator. J Biol Chem. 2016;291:20849–20857.
- Yanez M, Gil-Longo J, Campos-Toimil M. Calcium binding proteins. Adv Exp Med Biol. 2012;740:461–482.
- Kawasaki H, Kretsinger RH. Structural and functional diversity of EF-hand proteins: evolutionary perspectives. Protein Sci. 2017;26:1898–1920.
- Cheung WY. Calmodulin plays a pivotal role in cellular regulation. Science. 1980;207:19–27.
- Hoeflich KP, Ikura M. Calmodulin in action: diversity in target recognition and activation mechanisms. Cell. 2002;108:739–742.
- Sharma RK, Parameswaran S. Calmodulin-binding proteins: a journey of 40 years. Cell Calcium. 2018;75:89–100.
- Ohno S, Emori Y, Imajoh S, et al. Evolutionary origin of a calcium-dependent protease by fusion of genes for a thiol protease and a calcium-binding protein? Nature. 1984;312:566–570.
- Sakihama T, Kakidani H, Zenita K, et al. A putative Ca2+-binding protein: structure of the light subunit of porcine calpain elucidated by molecular cloning and protein sequence analysis. Proc Natl Acad Sci USA. 1985;82:6075–6079.
- Blanchard H, Grochulski P, Li Y, et al. Structure of a calpain Ca2+-binding domain reveals a novel EF-hand and Ca2+-induced conformational changes. Nat Struct Biol. 1997;4:532–538.
- Lin GD, Chattopadhyay D, Maki M, et al. Crystal structure of calcium bound domain VI of calpain at 1.9 Å resolution and its role in enzyme assembly, regulation, and inhibitor binding. Nat Struct Biol. 1997;4:539–547.
- Maki M, Narayana SV, Hitomi K. A growing family of the Ca2+-binding proteins with five EF-hand motifs. Biochem J. 1997;328:718–720.
- Vito P, Lacanà E, D’Adamio L. Interfering with apoptosis: ca2+-binding protein ALG-2 and Alzheimer’s disease gene ALG-3. Science. 1996;271:521–525.
- Maki M, Kitaura Y, Satoh H, et al. Structures, functions and molecular evolution of the penta-EF-hand Ca2+-binding proteins. Biochim Biophys Acta. 2002;1600:51–60.
- Goll DE, Thompson VF, Li H, et al. The calpain system. Physiol Rev. 2003;83:731–801.
- Croall DE, Ersfeld K. The calpains: modular designs and functional diversity. Genome Biol. 2007;8:218.
- Sorimachi H, Hata S, Ono Y. Impact of genetic insights into calpain biology. J Biochem. 2011;150:23–37.
- Ono Y, Sorimachi H. Calpains: an elaborate proteolytic system. Biochim Biophys Acta. 2012;1824:224–236.
- Suzuki K, Hata S, Kawabata Y, et al. Structure, activation, and biology of calpain. Diabetes. 2004;53:S12–S18.
- Suzuki K, Saido TC, Hirai S. Modulation of cellular signals by calpain. Ann NY Acad Sci. 1992;674:218–227.
- Saido TC, Nagao S, Shiramine M, et al. Distinct kinetics of subunit autolysis in mammalian m-calpain activation. FEBS Lett. 1994;346:263–267.
- Glading A, Bodnar RJ, Reynolds IJ, et al. Epidermal growth factor activates m-calpain (calpain II), at least in part, by extracellular signal-regulated kinase-mediated phosphorylation. Mol Cell Biol. 2004;24:2499–2512.
- Ono Y, Ojima K, Shinkai-Ouchi F, et al. An eccentric calpain, CAPN3/p94/calpain-3. Biochimie. 2016;122:169–187.
- Hoogewijs D, Ebner B, Germani F, et al. Androglobin: a chimeric globin in metazoans that is preferentially expressed in mammalian testes. Mol Biol Evol. 2012;29:1105–1114.
- Kitaura Y, Watanabe M, Satoh H, et al. Peflin, a novel member of the five-EF-hand-protein family, is similar to the apoptosis-linked gene 2 (ALG-2) protein but possesses nonapeptide repeats in the N-terminal hydrophobic region. Biochem Biophys Res Commun. 1999;263:68–75.
- Maki M, Maemoto Y, Osako Y, et al. Evolutionary and physical linkage between calpains and penta-EF-hand Ca2+-binding proteins. Febs J. 2012;279:1414–1421.
- Maki M, Takahara T, Shibata H. Multifaceted roles of ALG-2 in Ca2+-regulated membrane trafficking. Int J Mol Sci. 2016;17:pii: E1401.
- Ma J, Zhang X, Feng Y, et al. Structural and functional study of apoptosis-linked gene-2·Heme-binding protein 2 interactions in HIV-1 production. J Biol Chem. 2016;291:26670–26685.
- Kanadome T, Shibata H, Kuwata K, et al. The calcium-binding protein ALG-2 promotes endoplasmic reticulum exit site localization and polymerization of Trk-fused gene (TFG) protein. Febs J. 2017;284:56–76.
- Takahara T, Inoue K, Arai Y, et al. The calcium-binding protein ALG-2 regulates protein secretion and trafficking via interactions with MISSL and MAP1B proteins. J Biol Chem. 2017;292:17057–17072.
- Takahara T, Arai Y, Kono Y, et al. A microtubule-associated protein MAP1B binds to and regulates localization of a calcium-binding protein ALG-2. Biochem Biophys Res Commun. 2018;497:492–498.
- Zhang W, Matsuo R, Takahara T, et al. High sensitive quantitative binding assays using a nanoluciferase-fused probe for analysis of ALG-2-interacting proteins. Methods Mol Biol. 2019;1929:501–516.
- Katoh K, Shibata H, Suzuki H, et al. The ALG-2-interacting protein Alix associates with CHMP4b, a human homologue of yeast Snf7 that is involved in multivesicular body sorting. J Biol Chem. 2003;278:39104–39113.
- Katoh K, Suzuki H, Terasawa Y, et al. The penta-EF-hand protein ALG-2 interacts directly with the ESCRT-I component TSG101, and Ca2+-dependently co-localizes to aberrant endosomes with dominant-negative AAA ATPase SKD1/Vps4B. Biochem J. 2005;391:677–685.
- Ichioka F, Takaya E, Suzuki H, et al. HD-PTP and Alix share some membrane-traffic related proteins that interact with their Bro1 domains or proline-rich regions. Arch Biochem Biophys. 2007;457:142–149.
- Okumura M, Katsuyama AM, Shibata H, et al. VPS37 isoforms differentially modulate the ternary complex formation of ALIX, ALG-2, and ESCRT-I. Biosci Biotechnol Biochem. 2013;77:1715–1721.
- Okumura M, Takahashi T, Shibata H, et al. Mammalian ESCRT-III-related protein IST1 has a distinctive Met-Pro repeat sequence that is essential for interaction with ALG-2 in the presence of Ca2+. Biosci Biotechnol Biochem. 2013;77:1049–1054.
- Shibata H, Kanadome T, Sugiura H, et al. A new role for annexin A11 in the early secretory pathway via stabilizing Sec31A protein at the endoplasmic reticulum exit sites (ERES). J Biol Chem. 2015;290:4981–4993.
- Trioulier Y, Torch S, Blot B, et al. Alix, a protein regulating endosomal trafficking, is involved in neuronal death. J Biol Chem. 2004;279:2046–2052.
- Shibata H, Yamada K, Mizuno T, et al. The penta-EF-hand protein ALG-2 interacts with a region containing PxY repeats in Alix/AIP1, which is required for the subcellular punctate distribution of the amino-terminal truncation form of Alix/AIP1. J Biochem. 2004;135:117–128.
- Draeby I, Woods YL, la Cour JM, et al. The calcium binding protein ALG-2 binds and stabilizes Scotin, a p53-inducible gene product localized at the endoplasmic reticulum membrane. Arch Biochem Biophys. 2007;467:87–94.
- Shibata H, Suzuki H, Kakiuchi T, et al. Identification of Alix-type and Non-Alix-type ALG-2-binding sites in human phospholipid scramblase 3: differential binding to an alternatively spliced isoform and amino acid-substituted mutants. J Biol Chem. 2008;283:9623–9632.
- Vergarajauregui S, Martina JA, Puertollano R. Identification of the penta-EF-hand protein ALG-2 as a Ca2+-dependent interactor of mucolipin-1. J Biol Chem. 2009;284:36357–36366.
- Shibata H, Inuzuka T, Yoshida H, et al. The ALG-2 binding site in Sec31A influences the retention kinetics of Sec31A at the endoplasmic reticulum exit sites as revealed by live-cell time-lapse imaging. Biosci Biotechnol Biochem. 2010;74:1819–1826.
- Osugi K, Suzuki H, Nomura T, et al. Identification of the P-body component PATL1 as a novel ALG-2-interacting protein by in silico and far-Western screening of proline-rich proteins. J Biochem. 2012;151:657–666.
- Sasaki-Osugi K, Imoto C, Takahara T, et al. Nuclear ALG-2 protein interacts with Ca2+ homeostasis endoplasmic reticulum protein (CHERP) Ca2+-dependently and participates in regulation of alternative splicing of inositol trisphosphate receptor type 1 (IP3R1) pre-mRNA. J Biol Chem. 2013;288:33361–33375.
- Takahashi T, Kojima K, Zhang W, et al. Structural analysis of the complex between penta-EF-hand ALG-2 protein and Sec31A peptide reveals a novel target recognition mechanism of ALG-2. Int J Mol Sci. 2015;16:3677–3699.
- Suzuki H, Kawasaki M, Inuzuka T, et al. Structural basis for Ca2+-dependent formation of ALG-2/Alix peptide complex: ca2+/EF3-driven arginine switch mechanism. Structure. 2008;16:1562–1573.
- Pancsa R, Fuxreiter M. Interactions via intrinsically disordered regions: what kind of motifs? IUBMB Life. 2012;64:513–520.
- Berlow RB, Dyson HJ, Wright PE. Functional advantages of dynamic protein disorder. FEBS Lett. 2015;589:2433–2440.
- Mikasa T, Kugo M, Nishimura S, et al. Thermodynamic characterization of the Ca2+-dependent interaction between SOUL and ALG-2. Int J Mol Sci. 2018;19:pii: E3802.
- Emori Y, Kawasaki H, Imajoh S, et al. Endogenous inhibitor for calcium-dependent cysteine protease contains four internal repeats that could be responsible for its multiple reactive sites. Proc Natl Acad Sci USA. 1987;84:3590–3594.
- Maki M, Takano E, Mori H, et al. All four internally repetitive domains of pig calpastatin possess inhibitory activities against calpains I and II. FEBS Lett. 1987;223:174–180.
- Takano E, Maki M, Mori H, et al. Pig heart calpastatin: identification of repetitive domain structures and anomalous behavior in polyacrylamide gel electrophoresis. Biochemistry. 1988;27:1964–1972.
- Maki M, Bagci H, Hamaguchi K, et al. Inhibition of calpain by a synthetic oligopeptide corresponding to an exon of the human calpastatin gene. J Biol Chem. 1989 Nov 15;264:18866–18869.
- Ma H, Yang HQ, Takano E, et al. Requirement of different subdomains of calpastatin for calpain inhibition and for binding to calmodulin-like domains. J Biochem. 1993;113:591–599.
- Yang HQ, Ma H, Takano E, et al. Analysis of calcium-dependent interaction between amino-terminal conserved region of calpastatin functional domain and calmodulin-like domain of mu-calpain large subunit. J Biol Chem. 1994;269:18977–18984.
- Ma H, Yang HQ, Takano E, et al. Amino-terminal conserved region in proteinase inhibitor domain of calpastatin potentiates its calpain inhibitory activity by interacting with calmodulin-like domain of the proteinase. J Biol Chem. 1994;269:24430–24436.
- Takano E, Ma H, Yang HQ, et al. Preference of calcium-dependent interactions between calmodulin-like domains of calpain and calpastatin subdomains. FEBS Lett. 1995;362:93–97.
- Todd B, Moore D, Deivanayagam CC, et al. A structural model for the inhibition of calpain by calpastatin: crystal structures of the native domain VI of calpain and its complexes with calpastatin peptide and a small molecule inhibitor. J Mol Biol. 2003;328:131–146.
- Moldoveanu T, Gehring K, Green DR. Concerted multi-pronged attack by calpastatin to occlude the catalytic cleft of heterodimeric calpains. Nature. 2008;456:404–408.
- Hanna RA, Campbell RL, Davies PL. Calcium-bound structure of calpain and its mechanism of inhibition by calpastatin. Nature. 2008;456:409–412.
- Kawasaki H, Mizutome H, Kretsinger RH. Interaction sites of PEF proteins for recognition of their targets. Int J Biol Macromol. 2019;133:1035–1041.
- Hosfield CM, Elce JS, Davies PL, et al. Crystal structure of calpain reveals the structural basis for Ca2+-dependent protease activity and a novel mode of enzyme activation. Embo J. 1999;18:6880–6889.
- Strobl S, Fernandez-Catalan C, Braun M, et al. The crystal structure of calcium-free human m-calpain suggests an electrostatic switch mechanism for activation by calcium. Proc Natl Acad Sci USA. 2000;97:588–592.
- Jia J, Han Q, Borregaard N, et al. Crystal structure of human grancalcin, a member of the penta-EF-hand protein family. J Mol Biol. 2000;300:1271–1281.
- Jia J, Tarabykina S, Hansen C, et al. Structure of apoptosis-linked protein ALG-2: insights into Ca2+-induced changes in penta-EF-hand proteins. Structure. 2001;9:267–275.
- Xie X, Dwyer MD, Swenson L, et al. Crystal structure of calcium-free human sorcin: a member of the penta-EF-hand protein family. Protein Sci. 2001;10:2419–2425.
- Okumura M, Ichioka F, Kobayashi R, et al. Penta-EF-hand protein ALG-2 functions as a Ca2+-dependent adaptor that bridges Alix and TSG101. Biochem Biophys Res Commun. 2009;386:237–241.
- von Schwedler UK, Stuchell M, Müller B, et al. The protein network of HIV budding. Cell. 2003;114:701–713.
- Maki M, Suzuki H, Shibata H. Structure and function of ALG-2, a penta-EF-hand calcium-dependent adaptor protein. Sci China Life Sci. 2011;54:770–779.
- McCullough J, Frost A, Sundquist WI. Structures, functions, and dynamics of ESCRT-III/Vps4 membrane remodeling and fission complexes. Annu Rev Cell Dev Biol. 2018;34:85–109.
- Christ L, Raiborg C, Wenzel EM, et al. Cellular functions and molecular mechanisms of the ESCRT membrane-scission machinery. Trends Biochem Sci. 2017;42:42–56.
- Woodman P. ESCRT-III on endosomes: new functions, new activation pathway. Biochem J. 2016;473:e5–8.
- Weiss ER, Göttlinger H. The role of cellular factors in promoting HIV budding. J Mol Biol. 2011;410:525–533.
- Agromayor M, Martin-Serrano J. Knowing when to cut and run: mechanisms that control cytokinetic abscission. Trends Cell Biol. 2013;23:433–441.
- Nabhan JF, Hu R, Oh RS, et al. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci USA. 2012;109:4146–4151.
- Scheffer LL, Sreetama SC, Sharma N, et al. Mechanism of Ca2+-triggered ESCRT assembly and regulation of cell membrane repair. Nat Commun. 2014;5:5646.
- Satoh H, Shibata H, Nakano Y, et al. ALG-2 interacts with the amino-terminal domain of annexin XI in a Ca2+-dependent manner. Biochem Biophys Res Commun. 2002;291:1166–1172.
- Satoh H, Nakano Y, Shibata H, et al. The penta-EF-hand domain of ALG-2 interacts with amino-terminal domains of both annexin VII and annexin XI in a Ca2+-dependent manner. Biochim Biophys Acta. 2002;1600:61–67.
- Sønder SL, Boye TL, Tölle R, et al. Annexin A7 is required for ESCRT III-mediated plasma membrane repair. Sci Rep. 2019;9:6726.
- Skowyra ML, Schlesinger PH, Naismith TV, et al. Triggered recruitment of ESCRT machinery promotes endolysosomal repair. Science. 2018;360:eaar5078.
- Radulovic M, Schink KO, Wenzel EM, et al. ESCRT-mediated lysosome repair precedes lysophagy and promotes cell survival. Embo J. 2018;37:pii: e99753.
- Shibata H. Adaptor functions of the Ca2+-binding protein ALG-2 in protein transport from the endoplasmic reticulum. Biosci Biotechnol Biochem. 2019;83:20–32.
- Rayl M, Truitt M, Held A, et al. Penta-EF-hand protein peflin is a negative regulator of ER-To-Golgi transport. PLoS One. 2016 Jun 8;11:e0157227.
- McGourty CA, Akopian D, Walsh C, et al. Regulation of the CUL3 ubiquitin ligase by a calcium-dependent co-adaptor. Cell. 2016;167:525–538.
- Villalobo A, Ishida H, Vogel HJ, et al. Calmodulin as a protein linker and a regulator of adaptor/scaffold proteins. Biochim Biophys Acta Mol Cell Res. 2018;1865:507–521.
- Monroe N, Hill CP. Meiotic Clade AAA ATPases: protein polymer disassembly machines. J Mol Biol. 2016;428:1897–1911.
- Yorikawa C, Takaya E, Osako Y, et al. Human calpain 7/PalBH associates with a subset of ESCRT-III-related proteins in its N-terminal region and partly localizes to endocytic membrane compartments. J Biochem. 2008;143:731–745.
- Osako Y, Maemoto Y, Tanaka R, et al. Autolytic activity of human calpain 7 is enhanced by ESCRT-III-related protein IST1 through MIT-MIM interaction. Febs J. 2010;277:4412–4426.
- Maeda T. The signaling mechanism of ambient pH sensing and adaptation in yeast and fungi. Febs J. 2012;279:1407–1413.
- Rodríguez-Galán O, Galindo A, Hervás-Aguilar A, et al. Physiological involvement in pH signaling of Vps24-mediated recruitment of Aspergillus PalB cysteine protease to ESCRT-III. J Biol Chem. 2009;284:4404–4412.
- Subramanian S, Woolford CA, Desai JV, et al. cis- and trans-acting localization determinants of pH response regulator Rim13 in Saccharomyces cerevisiae. Eukaryot Cell. 2012;11:1201–1209.
- Maemoto Y, Kiso S, Shibata H, et al. Analysis of limited proteolytic activity of calpain-7 using non-physiological substrates in mammalian cells. Febs J. 2013;280:2594–2607.
- Maemoto Y, Osako Y, Goto E, et al. Calpain-7 binds to CHMP1B at its second alpha-helical region and forms a ternary complex with IST1. J Biochem. 2011;150:411–421.
- Maemoto Y, Ono Y, Kiso S, et al. Involvement of calpain-7 in epidermal growth factor receptor degradation via the endosomal sorting pathway. Febs J. 2014;281:3642–3655.
- Sadoul R, Laporte MH, Chassefeyre R, et al. The role of ESCRT during development and functioning of the nervous system. Semin Cell Dev Biol. 2018;74:40–49.
- Vincent O, Rainbow L, Tilburn J, et al. YPXL/I is a protein interaction motif recognized by Aspergillus PalA and its human homologue, AIP1/Alix. Mol Cell Biol. 2003;23:1647–1655.
- Sun S, Zhou X, Corvera J, et al. ALG-2 activates the MVB sorting function of ALIX through relieving its intramolecular interaction. Cell Discov. 2015;1:15018.
- Schöneberg J, Lee IH, Iwasa JH, et al. Reverse-topology membrane scission by the ESCRT proteins. Nat Rev Mol Cell Biol. 2017;18:5–17.
- Schöneberg J, Pavlin MR, Yan S, et al. ATP-dependent force generation and membrane scission by ESCRT-III and Vps4. Science. 2018;362:1423–1428.
