ABSTRACT
Kudoa septempunctata
, a myxosporean parasite infecting the trunk muscles of olive flounder (Paralichthys olivaceus), is reported to cause food poisoning in humans. The molecular mechanisms underlying the toxicity of K. septempunctata spores remain largely unknown. In the present study, we examine the molecular basis of such toxicity using DNA microarray analysis of K. septempunctata-inoculated human colon adenocarcinoma cells (Caco-2). We observed that the transepithelial resistance of the K. septempunctata-inoculated Caco-2 cell monolayers decreased markedly. DNA microarray analysis revealed that the mRNA expression profiles of control and inoculated cells clearly differed. Inflammatory and bacteria-related pathways, such as interleukin-8 (IL-8) production and MAPK/NF-kappa B pathway, were enriched. The concentrations of IL-8 and serotonin (5-HT) were higher in inoculated cells than in controls. K. septempunctata invasion damages the human intestinal epithelium, causing increased production of IL-8 and 5-HT, which likely results in the vomiting associated with K. septempunctata invasion.
Abbreviations
AP-1: activator protein 1; DAVID: Database for Annotation, Visualization and Integrated Discovery; ENS: enteric nervous system; FARMS: Factor Analysis for Robust Microarray Summarization; FDR: false discovery rate; GO: Gene Ontology; 5-HT: 5-hydroxytryptamine; IL-8: Interleukin-8; KEGG: Kyoto Encyclopedia of Genes and Genomes; K. septempunctata: Kudoa septempunctata; NF-kappa B: nuclear factor-kappa B; TJ: tight junction; TER: transepithelial electrical resistance
Graphical abstract
K. septempunctata
invasion damages the human intestinal epithelium, causing increased production of IL-8 and 5-HT.
Kudoa septempunctata (K. septempunctata), a myxosporean parasite infecting the trunk muscles of olive flounder (Paralichthys olivaceus), is reported to cause food poisoning in humans [Citation1,Citation2]. Patients experience acute diarrhea and vomiting after eating the raw flesh of infected olive flounder. The toxicity of Kudoa myxosporeans has been investigated using experimental animals such as the house musk shrew and suckling mice2, 3) and in Caco-2 cell monolayers. Caco-2 is a human colon carcinoma cell line used to investigate intestinal epithelial transport [Citation3,Citation4]. The toxicity of K. septempunctata was verified by measuring tight junction (TJ) barrier integrity in intestinal Caco-2 cells [Citation5].
After feeding on olive founder meat containing K. septempunctata spores, the lag phase is only 1–20 h. Symptoms are transient but severe, including diarrhea and emesis, as demonstrated in the house musk shrew [Citation2]. Ohnishi et al. [Citation3] described the mechanism of K. septempunctata invasion of human epithelial cells using Caco-2 cells. The sporoplasms of K. septempunctata very quickly intrude directly into the cytoplasm, reaching the basolateral side within 1 h. This rapid invasion causes a rapid decrease in transepithelial electrical resistance (TER) and is responsible for the short lag phase before the appearance of clinical symptoms.
In the present study, we perform DNA microarray analysis of Caco-2 cells inoculated with K. septempunctata spores. We report that K. septempunctata spores induce a variety of changes in intestinal function that may contribute to the observed toxic effects.
Materials and methods
K. septempunctata samples
One or two juvenile olive flounder naturally infected with K. septempunctata were transported on ice to the Laboratory of Fish Diseases at the University of Tokyo from the National Research Institute of Aquaculture (Kamiura Branch, Oita Prefecture, Japan), where infected fish lots were stocked in a land-based tank. Since the infection with Kudoa cannot be achieved by artificial method (injection or exposure to Kudoa spores), it must rely on natural exposure to infective waters. Thus, the fish were consistently (continuously) born (from the hatch) and raised in a laboratory tank using a running natural seawater. When we collected the spores, the fish were already dead. The fish were determined to have died during the refrigerated transportation (2–3 days) from the Research Institute to the University of Tokyo. The fish were placed in a container with 15-L of seawater containing 2-phenoxyethanol at 0.5-mL/L for 2 min. At this condition, the fish were completely anesthetized. Somatic muscle was minced, suspended in PBS, and then passed through 425-μm and 212-μm steel mesh (Tokyo Screen Co. Ltd.) and 100-μm nylon mesh (Cell Strainer, BD Falcon, NY, USA). The spores were combined after they were obtained from 1 or 2 juvenile olive flounders. After the spore suspensions were centrifuged at 430 × g for 15 min, the supernatant was discarded, and the pellet was re-suspended in 1.0–3.0 mL PBS. The spore suspension layered onto a 25%/50% Percoll gradient solution (GE healthcare, Tokyo, Japan) in a 15-mL plastic tube. After centrifugation at 2330 × g for 30 min, the pellet was collected and re-suspended in PBS. The purified spore suspension was again centrifuged at 430 × g for 15 min, and the pellet was collected and used as the source of the parasite. The above procedures were repeated several times so that research materials were provided on each occasion. We conducted the procedures in this study according to the Institutional Animal Care and Use Committee (the University of Tokyo and the National Research Institute of Aquaculture).
Measurement of TJ barrier integrity of Caco-2 cells
The human adenocarcinoma cell line Caco-2 (European Collection of Cell Cultures [ECACC] 86,010,202) was cultured in Eagle’s minimal essential medium (Nissui Pharmaceutical Co., Tokyo, Japan) supplemented with 2 mM glutamine (MP Biomedical, Santa Ana, CA, USA), 10% fetal bovine serum (GIBCO/Life Technologies, Carlsbad, CA, USA) and 1% non-essential amino acids (GIBCO) at 37°C in 5% CO2. The Corning Biocoat Intestinal Epithelium Differentiation Environment Kit (Corning, Catalog No. 355,057) was used for differentiation. Caco-2 cells were suspended in Seeding Basal Medium with MITO, seeded at 4 × 105 cells/well in the Biocoat cell culture insert, and then incubated at 37°C in 5% CO2. After 24 h, the culture medium was replaced with the assay kit’s Enterocyte Differentiation Medium with MITO and incubated for 48 h. The Caco-2 monolayer was made up to four days from seeding in this kit. The cell monolayer integrity was investigated by measuring the TER with an epithelial volt-ohm-milliammeter (World Precision Instruments, Sarasota, FL, USA). Inserts showing a TER of more than 1,000 Ωcm2 were used for the assay. Fresh K. septempunctata spores were suspended in Enterocyte Differentiation Medium with MITO and the serum expander of the Caco-2 assay system. Fresh spore suspensions containing medium (1 × 106 spores/well) (K. septempunctata group: K group) or not extant medium (Control group: C group) were added to the apical side, and the TER was measured hourly.
DNA microarray analysis
The DNA microarray and data analysis procedures were based on the Kamei et al [Citation6]. Total RNA was extracted from approximately 50 mg of Caco-2 cell monolayers ()) using ISOGEN (Nippon gene, Tokyo, Japan). After combining the extracts from six wells, the RNA was isolated and purified using the RNeasy Mini Kit and RNase-Free DNase Set (Qiagen K.K., Tokyo, Japan). The quantity and quality were measured using a spectrophotometer and Agilent 2100 Bioanalyzer and an Agilent RNA 6000 Nano Kit (Agilent Technologies, Santa Clara, CA), respectively. cDNA was synthesized from 100 ng of purified total RNA using a One-Cycle cDNA Synthesis Kit (Thermo Fisher Scientific). A biotinylated anti-sense RNA probe was synthesized from cDNA using a GeneChip Expression 3ʹ IVT Express Kit (Affymetrix, Santa Clara, CA, USA). The cRNA quality was evaluated by agarose gel electrophoresis. The cRNA was hybridized to a GeneChip Human Genome U133 Plus 2.0 Array (Thermo Fisher Scientific) for 16 h. The chip was washed and stained with GeneChip Fluidics Station 450 (Thermo Fisher Scientific). Fluorescence signals were scanned using a GeneChip Scanner 3000 7G (Thermo Fisher Scientific). The Affymetrix GeneChip Command Console software program (Thermo Fisher Scientific) was used to convert the array images into intensity values for each probe (CEL files).
DNA microarray data analysis
The probe signals were normalized using the Distribution Free Weighted method, the Affymetrix Microarray Suite version 5.0, the Factor Analysis for Robust Microarray Summarization (FARMS), or the Robust Multi-array Averaging values obtained by comparison of expression levels between experimental groups. To identify probe sets that were differentially expressed between the groups, the rank products method [Citation7] was applied to the quantified data. Probe sets with a false discovery rate (FDR) of 0.05 were considered to be differentially expressed. The annotation file for the Human Genome U133 Plus 2.0 Array was downloaded from the Affymetrix website.
The selected probe sets were analyzed using the Database for Annotation, Visualization and Integrated Discovery (DAVID 6.8; http://david.abcc.ncifcrf.gov/) [Citation8] for their significant representation in certain Gene Ontology (GO) terms. The Benjamini and Hochberg FDR were used to correct for multiple tests. A GO term with a FDR‐corrected p‐value < 0.05 was considered to be significantly enriched. The QuickGO (https://www.ebi.ac.uk/QuickGO/) was used to determine the hierarchical structure of the enriched GO terms [Citation9].
Measurement of interleukin-8 and serotonin in Caco-2 medium
The concentrations of interleukin-8 (IL-8) and serotonin (5-hydroxytryptamine, 5-HT) in cell culture media were measured using an ELISA kit (Abcam) according to the manufacturer’s instructions. After the TER measurement, both apical and basolateral side medium were collected and subjected to ELISA.
Results
Tight junction barrier integrity of Caco-2 cells
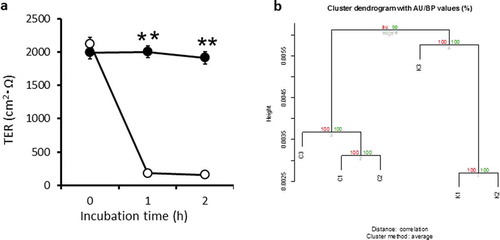
The TER in the Control group remained around 2,000 Ω cm2 during the experimental period (2 h). However, the TER in the wells inoculated with fresh spores of K. septempunctata sharply decreased by 80% from 1 h ()). These results suggested that barrier function of Caco-2 cell monolayer was damaged by K. septempunctata inoculation, which were consistent with our previous data [Citation5]. We extracted total RNA from Caco-2 cell monolayers both C and K groups after the TER measurement assay of ).
DNA microarray analysis
For all four types of normalization methods, each group formed clearly different clusters, which was particularly pronounced for FARMS. These data suggest that the transcriptomic profiles differ markedly between the C and K groups. Normalization of gene expression levels using the FARMS method resulted in the best segregation of clusters in the K group ()); consequently, we used this method for the remainder of the study. The probe sets were then selected according to their FDR values (0.05), determined in between-group comparisons using the rank product method. Setting the FDR significance level as ≤ 0.05, we identified 214 upregulated and 253 downregulated genes.
Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathway analysis
To identify GO terms, we performed a gene-annotation enrichment analysis using the online software program DAVID. The GO terms in the deepest hierarchy level that were significantly up-and down-regulated genes regulated by K. septempunctata invasionare shown in . The names of genes in are shown in Supplemental Table 1. The extracted GO terms were related to apoptosis and its positive regulation, cellular responses (responses to molecules of bacterial origin, mechanical stimuli, hormones, cytokines, hydrogen peroxide, cAMP and peptides), the cytoskeleton (skeletal muscle cell differentiation), transcription and cell proliferation (negative regulation of transcription from RNA polymerase II promoter, negative regulation of cell proliferation, positive regulation of transcription from RNA polymerase II promoter, regulation of the cell cycle) and MAPK (inactivation of MAPK activity). These data suggest that all of these factors were altered in the K group.
Table 1. GO terms in the deepest level of hierarchy and genes significantly up/down regulated by K. septempunctata infection (FDR<0.05, Benjamini<0.05). Gene title list addressed in S1 Table.
There is no TJ related GO term at the deepest hierarchy level. Claudin and occludin are very important molecules involved in tight junctions, but they did not significantly change. The FERM domain containing 4A, which regulates epithelial cell polarity, was the only gene change labeled with the GO term “tight junction” (). The genes labeled with the GO term “cell adhesion” are also shown in . The cell adhesion-related genes were both down- and upregulated. The cysteine-rich, angiogenic inducer, 61, which is related to collagen degradation, was upregulated.
Table 2. A list of biological process Gene Ontology (GO) terms of “cell adhesion” and “tight junction” and their component genes by K. septempunctata infection (FDR<0.05, Benjamini<0.05).
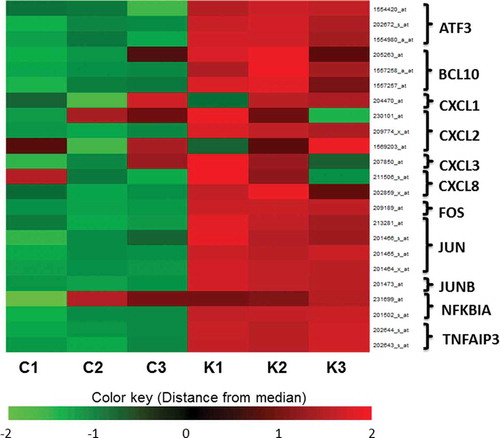
B-cell CLL/lymphoma 10 (BCL10), chemokine (C-X-C motif) ligand 2 (CXCL2), chemokine (C-X-C motif) ligand 8 (CXCL8, IL-8) and tumor necrosis factor, alpha-induced protein 3 (TNFAIP3) are general inflammatory-related genes, categorized as “response to molecules of bacterial origin.” All of these genes were upregulated in the K group.
To identify significantly enriched pathways, we also assessed the pathways described in the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways (p < 0.05). KEGG pathway analysis showed that the expression of genes in the “TNF signalingpathway,” “MAPK signaling pathway” and “NF-kappa B signaling pathway” was altered in the K group (). All of these pathways are related to inflammation. The gene expression patterns and related pathways of inflammation caused by K. septempunctata invasion are shown in and . The activator protein 1 (AP-1) is a mixture of heterodimeric complexes of proteins derived from the Fos and Jun families including FOS, are also elevated in the K group ( and ). As a result, the expression of the leukocyte recruitment chemokines CXCL1, 2, and 3 and the TNF signaling molecules nuclear factor of kappa light polypeptide gene enhancer in B-cell inhibitor, alpha (NFKBIA) and TNFAIP3 are upregulated. The bacterial and viral infection pathways, such as HTLV-I infection, and epithelial cell signaling in Helicobacter pylori infection were also in the annotated KEGG pathways. It was suggested that the gene expression of K. septempunctata-infected Caco-2 monolayers were changed in a similar way to Helicobacter pylori infection. AP-1 was also annotated and noted as activated in “TNF signaling,” “NF-kappa B signaling,” and “Epithelial cell signaling in Helicobacter pylori infection” pathways. These data suggest that AP-1 may be a key molecule against inflammation during K. septempunctata invasion.
Table 3. The enriched KEGG pathway of K. septempunctata infection.
Figure 3. Mapping of the extracted gene products in pathways stimulated by K. septempunctata invasion.
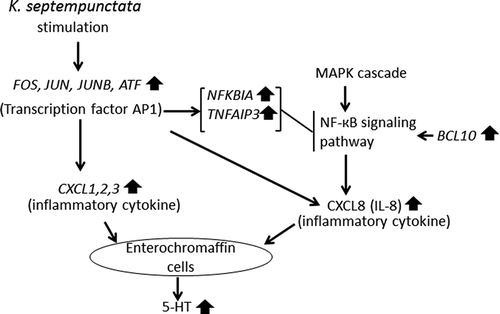
The transcription nuclear factor-kappa B (NF-kappa B) signaling pathway is located downstream of the MAPK signaling pathway. Both of these pathways were upregulated in the K group. Both NF-kappa B and AP-1 contain transcriptional regulator binding sites for most inflammatory mediators [Citation10]. Human hosts express multiple transcription factors, such as AP-1 and NF-kappa B, that mediate inflammation. We observed that CXCL8 (IL-8) was upregulated ( and ). AP-1 and NF-kappa B are known to regulate IL-8 [Citation11].
Cytokine and serotonin concentrations in Caco-2 cell medium
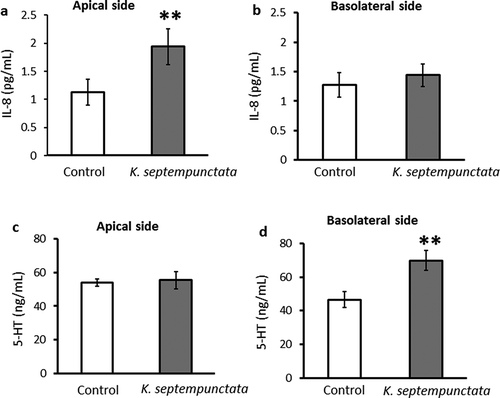
We measured IL-8, CXCL2 and 5-HT concentrations in the apical and basolateral medium using an ELISA kit. The concentration of IL-8 on the apical side of the medium increased significantly in the K group as compared to the Control group ()). On the basolateral side, the IL-8 concentration in the K group did not differ from that of controls ()). The CXCL2 concentration was too low to detect in Caco-2 medium. The 5-HT concentration in the medium of the basolateral side was significantly higher in the K group than in the C group ()). On the apical side, the 5-HT concentration did not differ between the K group and controls ()).
Discussion
We observed that the TER of Caco-2 cells decreased within one hour of K. septempunctata inoculation. This rapid response was shown in a previous study, indicating that the lag phase of K. septempunctata is short. Calatayud et al [Citation12] reported that the IL-8 direction in Caco-2 cells was affected by increasing the stimulation time. Most of the treatments with inorganic arsenic or lipopolysaccharide increased IL-8 release into the apical medium after 2 and 4 h of exposure. In contrast, the IL-8 levels on the basolateral side changed later (between 4 and 24 h). We observed that IL-8 levels increased only on the apical side () because the incubation was only 2 h. Ahn et al. reported that K. septempunctata did not cause inflammation in Caco-2 cells [Citation13]. They concluded that K. septempunctata spores did not affect the expression of inflammatory mediators in Caco-2 cells. However, their experiments were limited because they measured only four mediators, which were nitric oxide synthase, cyclooxygenase, nitric oxide, and prostaglandin E2. These genes were also not changed in our DNA microarray data. In this study, we found other inflammatory mediators and pathways by an exhaustive gene expression analysis using DNA microarray. Takeuchi et al., reported that K. septempunctata are genetically classified into 3 genotypes ST1, ST2, and ST3 [Citation14]. K. septempunctata detected from aquaculture-raised and natural fish from Japan were ST1 and ST2 genotypes but those from fish inspected in quarantine from Korea to Japan were the ST3 genotype. Ahnn used ST3 genotype, which was obtained from Jeju-do in Korea. Although they didn’t measure the TER, we observed that the TER of the K. septempunctata-inoculated Caco-2 cell monolayers markedly decreased in this study, which is consistent with other studies of Japanese K. septempunctata-inoculated Caco-2 cells [Citation3,Citation15]. We used K. septempunctata which was obtained from Oita Prefecture in Japan. It remains a possibility that there is a difference in pathogenicity between Korean and Japanese K. septempunctata, which we will examine in the future.
We examined the relationship between gene expression and TER reduction. However, there is no TJ related GO term in the deepest hierarchy level, and claudin and occludin related genes did not change. We investigated the GO term “cell adhesion” (). The cell adhesion-related genes were both down- and upregulated. The cadherin, collagen, and fibronectin related genes CDHR5, CEACAM1, COL12A1, FN1, and PTK2 were down regulated. The collagenase activating factor CYR61 was upregulated. The adhering matrix, actin, epithelial barrier function, and cell repair related genes CTGF, ITGA8, KLFA, RND1, and SERP1NE1 were upregulated. These data suggested that cell adhesion function was reduced, however, the repair also started at the same time. It is not inconsistent that K. septempunctata invasion symptoms are transient.
DNA microarray and ELISA results are summarized in . These results demonstrate that elevated IL-8 production, through the MAPK pathway, nuclear factor-kappa B (NF-kappaB), and AP-1, is an important signal of inflammation by K. septempunctata infection in the human intestine. Inflammation is a natural biological response to stimuli such as microbial infection. As described in detail previously [Citation16], many of the genes activated in the intestinal epithelial cells after bacterial infection are target genes of the transcription NF-kappaB. This dimeric transcription factor is composed of protein homo- or heterodimers, of which there are five family members in mammalian cells [Citation17]. The genes annotated in GO as: “response to molecule of bacterial origin” BCL10, CXCL2, CXCL8, and TNFAIP3 were all upregulated. BCL10 is required for activation of the NF-kappaB signaling pathway. According to our data, “NF-kappa B signaling pathway” and “Epithelial cell signaling in Helicobacter pylori infection” both indicated upregulation of IL-8, which is downstream of the NF-kappaB signaling pathway. NF-kappaB activates the transcription of cytokines such as IL-8 [Citation18]. IL-8 induces an immediate immune response in the intestine, including chemotaxis of neutrophils to the inflammation site [Citation19,Citation20]. We observed that the IL-8 concentration on the apical side of the medium was greater in the K group than in the Control group, indicating that IL-8 also induces an immediate immune response to K. septempunctata.
QuickGO and KEGG pathway analysis showed that AP-1 production was upregulated after K. septempunctata infection. AP-1 is a transcription factor that regulates gene expression in response to a variety of stimuli, including cytokines, growth factors, stress and bacterial and viral infections [Citation21]. The AP-1structure is heterodimeric, composed of proteins belonging to the c-Fos, c-Jun, ATF, and JDP families. AP-1 is commonly activated during bacterial and viral infections [Citation22,Citation23]. The AP-1 elements FOS, JUN and JUNB were upregulated in this study (). These data suggest that involvement of the early intestinal cell responders to K. septempunctata invasion is followed by upregulation of the transcription factor AP-1. K. septempunctata infection upregulated CXCL1, 2, and 3. CXCL1, 2, and 3 are inflammatory chemokines. These chemokines and IL-8 can cause degranulation of enterochromaffin (EC) cells [Citation24].
The pathophysiological basis of emesis induction by K. septempunctata is particularly poorly understood. EC cells infected with virus release 5-HT, which then acts as a pro-inflammatory molecule [Citation25]. As described in detail previously [Citation26], 5-HT has physiological effects on gut motility, secretion and visceral sensation [Citation27]. The human enteric nervous system (ENS) contains about 100 million neurons, including sensory, inter and motor neurons [Citation26,Citation27]. The luminal EC cells sense the luminal contents and release mediators such as 5-HT to activate ENS as well as extrinsic vagal afferents to the brain [Citation24]. The major depository of the body’s 5-HT is the mucosa of the gastrointestinal tract [Citation25], but the details of gut 5-HT remain to be clarified [Citation28]. Activation of the extrinsic vagal and spinal afferent fibers by 5-HT results in slowed gastric emptying, pancreatic secretion, satiation, pain, and discomfort, as well as nausea and vomiting [Citation24]. Within the gut, 5-HT also exerts nonconventional actions such as the promotion of inflammation. Inflammation is a complex biological immune response to tissue damage, either from pathogens or irritants, with the goal of removing the irritating stimulus. The hypothesis that 5-HT released from EC cells acts at a pro- and anti- inflammatory molecule is strongly supported by evidence [Citation29,Citation30]. Rotavirus is reported to stimulate human EC cells in the gut, causing 5-HT release, which activates vagal afferents and the brain stem vomiting center, a reaction cascade associated with vomiting [Citation31]. Of the multiple 5-HT receptors identified to date, the 5-HT3 receptor appears to be most important in the acute phase of nausea and vomiting [Citation32]. 5-HT3 is located on the terminal ends of the vagal afferents. This receptor lies in close proximity to enteroendocrine cells located in the gastrointestinal mucosa of the proximal small intestine. Among the various local mediators, 5-HT, located in enterochromaffin-like cells, is believed to play the most important role [Citation32]. After the release of 5-HT from the enterochromaffin cells 5-HT interacts with 5-HT3 receptors on vagal afferent terminals in the wall of the bowel. We observed an increase in 5-HT in response to K. septempunctata infection, as indicated by ELISA (). Serotonin secretion induced by K. septempunctata might play a key role in the associated emesis.
We observed previously unreported responses of human intestinal epithelial cells to invasion by K. septempunctata, including increased 5-HT production and increased IL-8 production via AP-1 upregulation and induction of the MAPK/NF-kappa B pathway. IL-8 and 5-HT likely cause the inflammation and vomiting associated with K. septempunctata invasion. While the mechanism whereby K. septempunctata invasion induces vomiting was not clarified, we showed that 5-HT production might be one causative factor. Because this study uses an intestinal epithelial cell model, our results must be confirmed in future studies using animals.
Author contribution
HY and SK designed the project. SY, FK, HY, and SK conducted experiments and analyzed data. SY, HY, and SK interpreted the data. SY, HY, and SK wrote the manuscript. All authors critically read and contributed to the manuscript.
Supplemental_Table_1.docx
Download MS Word (19.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemented data of this article can be accessed here.
Additional information
Funding
References
- Harada T, Kawai T, Jinnai M, et al. Detection of Kudoa septempunctata 18S ribosomal DNA in patient fecal samples from novel food-borne outbreaks caused by consumption of raw olive flounder (Paralichthys olivaceus). Clin Microbiol. 2012;50:2964–2968.
- Kawai T, Sekizuka T, Yahata Y, et al. Identification of Kudoa septempunctata as the causative agent of novel food poisoning outbreaks in Japan by consumption of Paralichthys olivaceus in raw fish. Clin Infect Dis. 2012;54:1046–1052.
- Ohnishi T, Kikuchi Y, Furusawa H, et al. Kudoa septempunctata invasion increases the permeability of human intestinal epithelial monolayer. Foodborne Pathog Dis. 2013;10:137–142.
- Suzuki J, Murata R, Yokoyama H, et al. Detection rate of diarrhoea-causing Kudoa hexapunctata in Pacific bluefin tuna Thunnus orientalis from Japanese waters. Int J Food Microbiol. 2015;194:1–6.
- Yokoyama H, Funaguma N, Kobayashi S. In vitro inactivation of Kudoa septempunctata spores infecting the muscle of olive flounder Paralichthys olivaceus. Foodborne Pathog Dis. 2016;13:21–27.
- Kamei A, Watanabe Y, Kondo K, et al. Influence of a short-term iron-deficient diet on hepatic gene expression profiles in rats. PLoS One. 2013;8:e65732.
- Breitling R, Armengaud P, Amtmann A, et al. Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett. 2004;573:83–92.
- Dennis G Jr, Sherman BT, Hosack DA, et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3.
- Barrell D, Dimmer E, Huntley RP, et al. The GOA database in 2009–an integrated gene ontology annotation resource. Bioinformatics. 2009;25:3045–3046.
- Wang A, Al-Kuhlani M, Johnston SC, et al. Transcription factor complex AP-1 mediates inflammation initiated by Chlamydia pneumoniae infection. Cell Microbiol. 2013;15:779–794.
- Thiel G, Ulrich M, Mukaida N, et al. Resveratrol stimulation induces interleukin-8 gene transcription via NF-κB. Pharmacol Res. 2018;134:238–245.
- Calatayud M, Gimeno-Alcañiz JV, Devesa V, et al. Proinflammatory effect of trivalent arsenical species in a co-culture of Caco-2 cells and peripheral blood mononuclear cells. Arch Toxicol. 2015;89:555–564.
- Ahn M, Ko HJ, Kim J, et al. Evaluation of the inflammatory response to Kudoa septempunctata genotype ST3 isolated from olive flounder (Paralichthys olivaceus) in Caco-2 cells. Parasite. 2018;25:12.
- Takeuchi F, Ogasawara Y, Kato K, et al. Genetic variants of Kudoa septempunctata (Myxozoa: multivalvulida), a flounder parasite causing foodborne disease. J Fish Dis. 2016;39:667–672.
- Ohnishi T, Fujiwara M, Tomaru A, et al. Cryopreservation of Kudoa septempunctata sporoplasm using commercial freezing media. Parasitol Res. 2017;116:425–427.
- Kim JM, Cho SJ, Oh YK, et al. Nuclear factor-kappa B activation pathway in intestinal epithelial cells is a major regulator of chemokine gene expression and neutrophil migration induced by Bacteroides fragilis enterotoxin. Clin Exp Immunol. 2002;130:59–66.
- Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179.
- Khalaf H, Jass J, Olsson PE. Differential cytokine regulation by NF-kappaB and AP-1 in Jurkat T-cells. BMC Immunol. 2010;11:26.
- Singer M, Sansonetti PJ, Immunol J. IL-8 is a key chemokine regulating neutrophil recruitment in a new mouse model of Shigella-induced colitis. J Immunol 173. 2004. 4197–4206.
- Hyland KA, Kohrt L, Vulchanova L, et al. Mucosal innate immune response to intragastric infection by Salmonella enterica serovar Choleraesuis. Mol Immunol. 2006. 43(11): 1890–1899
- Pan H, Xie J, Ye F, et al. Modulation of Kaposi’s sarcoma-associated herpesvirus infection and replication by MEK/ERK, JNK, and p38 multiple mitogen-activated protein kinase pathways during primary infection. J Virol. 2005;79:15027–15037.
- Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3:859–868.
- Seo JH, Lim JW, Kim H, et al. Helicobacter pylori in a Korean isolate activates mitogen-activated protein kinases, AP-1, and NF-kappaB and induces chemokine expression in gastric epithelial AGS cells. Lab Invest. 2004;84:49–62.
- Rao M, Gershon MD. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol. 2016;13:517–528.
- Spohn SN, Mawe GM. Non-conventional features of peripheral serotonin signalling - the gut and beyond. Nat Rev Gastroenterol Hepatol. 2017;14:412–420.
- Mawe GM, Hoffman JM. Serotonin signalling in the gut–functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473–486.
- Linan-Rico A, Ochoa-Cortes F, Beyder A, et al. mechanosensory signaling in enterochromaffin cells and 5-HT release: potential implications for gut inflammation. Front Neurosci. 2016;19:564.
- Hansen MB. The enteric nervous system I: organisation and classification. Toxicol Pharmacol. 2003;92:105–113.
- Margolis KG, Stevanovic K, Li Z, et al. Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut. 2014;63:928–937.
- Kim JJ, Wang H, Terc JD, et al. Blocking peripheral serotonin synthesis by telotristat etiprate (LX1032/LX1606) reduces severity of both chemical- and infection-induced intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2015;309:G455–465.
- Hagbom M, Istrate C, Engblom D, et al. Rotavirus stimulates release of serotonin (5-HT) from human enterochromaffin cells and activates brain structures involved in nausea and vomiting. PLoS Pathog. 2011;7:e1002115.
- Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482–2494.