ABSTRACT
In this study, we investigated the role and mechanism of imperatorin (IMP) in chronic inflammation and airway remodeling. The levels of TNF-α, IL-1β, IL-6, IL-8, VEGF, α-SMA, and ROS were detected by ELISA, immunohistochemistry (IHC), immunofluorescence, and Western blot. In addition, we evaluated the effect of IMP on MAPK, PI3K/Akt, NF-κB, and Nrf2/HO-1 signaling pathways. IMP treatment obviously attenuated the production of inflammatory cytokines and inflammatory cells in bronchoalveolar lavage fluid of OVA-induced airway remodeling model. Meanwhile, it significantly inhibited inflammatory cell infiltration, goblet cell hyperplasia, collagen deposition, VEGF production, α-SMA, and ROS expression. Our study has shown that IMP could regulate the signaling pathways including MAPK, PI3K/Akt, NF-κB, and Nrf2/HO-1 to release the inflammatory responses. IMP might attenuate airway remodeling by the down-regulation of Nrf2/HO-1/ROS/PI3K/Akt, Nrf2/HO-1/ROS/MAPK, and Nrf2/HO-1/ROS/NF-κB signaling pathways.
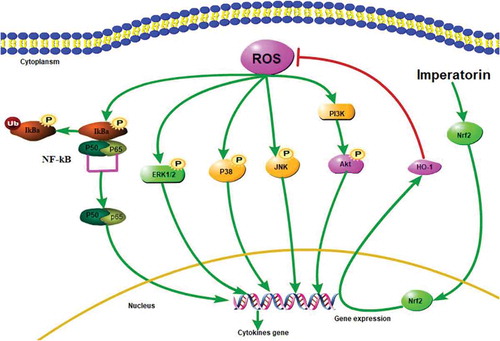
Graphical abstract
Schematic picture of the role of Imperatorin in alleviating ROS-mediated airway remodeling by targeting the Nrf2/HO-1 signaling pathway.
Bronchial asthma is one of the most common respiratory diseases, which is characterized by airway hyperresponsiveness, inflammation, and remodeling [Citation1]. Airway remodeling always happens at the early stage of asthma alone with inflammation [Citation2] and is closely related to the severity of asthma. However, the current focus of asthma treatment is to control the inflammatory response, which cannot effectively reverse the degree of airway remodeling. Till now, only a small number of laboratory indicators have been identified to reflect the degree of airway remodeling in asthma; thus, it is of great significance to study the pathogenesis of airway remodeling in asthma.
Pathophysiological changes in airway remodeling reconstruction include hyperplasia of mucus gland and goblet cell, subcutaneous fibrosis, increased smooth muscle mass, and vascular changes [Citation3]. These symptoms can lead to stubborn airway hyperresponsiveness and irreversible airway obstruction, further leading to lung dysfunction [Citation4]. Airway smooth muscle cells (ASMCs) can fight against allergens, infections and other environmental factors, and thus plays an important regulatory role in airway remodeling. ASMCs can produce a variety of extracellular matrix (ECM) proteins (including matrix metalloproteinases (MMPs) and collagen), anti-inflammatory cytokines and vascular endothelial growth factor (VEGF). The hypertrophy and hyperplasia of ASMCs have been observed in asthmatics [Citation5]. Therefore, new approaches targeting the proliferation of ASMCs in lung tissue may alleviate the process of airway remodeling in asthma.
Nuclear factor-erythroid 2-related factor 2 (Nrf2) is a cellular sensor of oxidative stress, which is associated with transcriptional activation of antioxidant-response element genes (ARE) [Citation6]. The heme oxygenase-1 (HO-1) has an anti-inflammatory feature related to cytoprotective responses and can be activated by multifarious phytochemicals involved in immune responses [Citation7]. At present, it has been confirmed that the disruption of the Nrf2 gene leads to allergen-driven airway remodeling in mice [Citation8]. Pulmonary alveolar cells, e.g. type I and II pneumocyte and alveolar macrophage, are known to be immunopositive to Nrf2 and HO-1 in immunohistochemistry of lung section [Citation9,Citation10].
Nuclear transcription factor kappa-B (NF-κB) is a transcriptional factor that mediates the transcription of many genes, including genes encoding pro-inflammatory chemokines and cytokines [Citation11]. In resting cells, the NF-κB pathway is inactive and binds to inhibitory IκB in the cytoplasm [Citation12]. Under the proper stimulations, for example, when stimulated by TNF-α, IκB is phosphorylated by IκB kinases (IKKs) and then dissociates from NF-κB. The activated NF-κB, which is translocated to the nucleus, plays a pivotal role in the progression of chronic allergic inflammation followed by airway remodeling [Citation13]. The pro-inflammatory cytokines can stimulate adaptive immunity and airway remodeling through activating NF-κB [Citation14]. Concretely, it has been shown that TNF-α can activate the PI3K/Akt pathway, which in turn has been shown to be able to upregulate the NF-κB signaling pathway through IKKα/β in HeLa cells [Citation15]. Likewise, the mitogen-activated protein kinase (MAPK) pathway is also responsible for the augmentation of NF-κB activity in inflammatory cytokine-mediated airway inflammation such as asthma [Citation16].
Reactive oxygen species (ROS) is constantly produced and removed in the body and are important to drive regulatory signaling pathways [Citation17]. However, under oxidative stress, excess ROS can disable intracellular proteins, leading to fatal cell damage. Moreover, ROS is a vital mediator of pulmonary vascular cell proliferation. Furthermore, the excessive production of ROS in asthma may induce enzymatic and non-enzymatic alterations, which give rise to an antioxidant/oxidant imbalance in airways [Citation18]. The imbalance produces a state of oxidative stress, resulting in the persistence of pulmonary fibrosis [Citation19]. In view of ROS-mediated signaling pathways, ROS appears to target the Nrf2 and NF-κB response pathways in chronic inflammatory disorders. Previous work has revealed that ROS can mediate the TNF-α-induced inflammation through the Akt-mediated activation of NF-κB [Citation20]. Moreover, the Nrf2/ARE/HO-1 pathway could be initiated by the ROS-dependent mechanism [Citation21]. Taken together, although the exact correlation in ROS-related molecular regulation and signaling is still unclear, ROS, PI3K/Akt, MAPK, NF-κB, and Nrf2/HO-1 signaling pathway seems to be implicated in the pulmonary inflammation-derived remodeling.
Imperatorin (IMP) [9-(3-methylbut-2-enyloxy)-7H-furo[3,2-g]chromen-7-one] is the main component of the dried root or rhizome of Radix Angelicae Dahuricae. It has several pharmacological effects, including anti-inflammation and tumor inhibition [Citation22], anti-HIV [Citation23], and anti-convulsant action [Citation24]. Moreover, IMP has been reported to exert antiallergic effects in Th2-mediated allergic asthma via induction of IL-10-producing regulatory T cells by modulating the function of dendritic cells [Citation25]. Aprevious study has demonstrated that IMP can reduce the inflammation response by inhibiting TNF-α-mediated activation of the ROS/PI3K/Akt/NF-κB pathway [Citation26]. It also can suppress inflammation via the MAPK signaling pathway [Citation27].
The signal pathways of MAPK and PI3K/Akt and the transcription factors such as NF-κB and Nrf2 are known to be the predominant cascades that regulate HO-1 expression [Citation28,Citation29]. The previous studies have shown that Nrf2 facilitates the induction of genes encoding antioxidant proteins and phase 2 detoxifying under oxidative or electrophilic stress [Citation30]. Thus, identification of these signal pathways and transcription factors may provide potential therapeutic targets for the prevention and treatment of allergic airway remodeling [Citation31]. Nevertheless, the regulatory role of IMP on the above-mentioned signaling networks in allergic airway remodeling and its underlying mechanism has not yet been studied. In this paper, we aim to investigate the effects and mechanisms of IMP against allergic airway remodeling in a mouse asthmatic model.
Material and methods
Animals
Specific 7-week-old pathogen-free (SPF) inbred female BALB/c mice (n = 40) were purchased from the House Section of Yanbian University Health Science Center (Yanji, China). The mice were kept under standard laboratory conditions for 7 days prior to experiments and provided with water and standard chow ad libitum. The experiments were performed based on the guidelines approved by the Institutional Animal Care and Use Committee of Yanbian University.
Asthma model
BALB/c mice were randomly divided into five groups, namely control, ovalbumin (OVA) model, OVA + IMP-L (15 mg/kg), OVA + IMP-M (30 mg/kg), and OVA + IMP-H (60 mg/kg) groups, with eight mice in each group. The asthmatic mice model was sensitized on days 0, 7, and 14 by intraperitoneal injection of 10 μg OVA (chicken egg albumin from Sigma, St. Louis, MO, USA) plus 1.0 mg of aluminum hydroxide adjuvant (Imject® Alum; Pierce, Rockford, IL, USA; supplementary Figure 1). These sensitized mice were then exposed to aerosolized 5% OVA in sterile saline for 8 weeks beginning on day 16 for 30 min, three times a week (supplementary Figure 1). The mice were placed in plastic chambers (18 × 14 × 8 cm) connected to an ultrasonic nebulizer (NE-U12; Omron, Tokyo, Japan) to obtain a whole-body inhalation system (supplementary Figure 1). IMP (15, 30 and 60 mg/kg) (purity>98%; Maya reagent, Zhejiang, China) was administered every day for 8 weeks at 30 min before nebulization (supplementary Figure 1). Control mice were sensitized and challenged with saline using the same way. The mice were sacrificed 24 h after the last challenge. The method of euthanasia is an intraperitoneal injection of pentobarbital (100mg/kg) so that mice can enter the anesthesia state quickly and reduce the fear and pain. The mice were sacrificed when the mice showed aggravating symptoms of eye congestion, scratched ears, and difficulty breathing. Animal death was confirmed when there was no spontaneous breathing for 2–3 min and no blinking reflex. The Bronchoalveolar lavage fluid (BALF) and lung tissues were collected for analysis.
Nuclear and cytosolic protein extractions
The samples of lung tissue were washed and lysed in two volumes of lysis buffer A (50 mM Tris-HCl, pH 7.5, 1 M EDTA, 10% glycerol, 0.5 mM dithiothreitol, 5 mM MgCl2, 1 mM phenylmethanesulfonylfluoride (PMSF) and protease inhibitor cocktails) for 5 min at 4°C. The suspension was centrifuged at 1,000 xg for 15 min at 4°C. The supernatant fraction was incubated on ice for 10 min and centrifuged at 100,000 x g for 1 h at 4°C to obtain cytosolic protein extracts. The cytosolic protein extracts were used for western blot analysis. The pelleted nuclei were resuspended in buffer B (1.3 M sucrose, 1.0 mM MgCl2, 10 mM potassium phosphate buffer, pH 6.8) and centrifuged at 1,000 xg for 15 min. The pellets were suspended in buffer B with a final sucrose concentration of 2.2 M and centrifuged at 100,000 xg for 1 h. The resulting nuclear pellets were washed once with a solution containing 0.25 M sucrose, 0.5 mM MgCl2, and 20 mM Tris-HCl (pH 7.2), and centrifuged at 1,000 xg for 10 min. The nuclear pellets were solubilized with a solution of 50 mM Tris-HCl (pH 7.2), 0.3 M sucrose, 150 mM NaCl, 2 mM EDTA, 20% glycerol, 2% Triton X-100, 2 mM PMSF, and protease inhibitor cocktails, and the resulting mixture was kept on ice for 1 h with gentle stirring, after which it was centrifuged at 12,000 x g for 30 min. The resulting soluble nuclear protein supernatant samples were used for western blot analysis.
BALF collection and histological analysis of lung tissue
The mice were fixed on the surgical plate and sacrificed after pentobarbital anesthesia. The neck skin was longitudinally cut and the trachea was fully exposed. A small incision was made in the trachea to insert the hose. One milliliter of 4°C normal saline was injected slowly and the BALF was collected slowly. The recovery rate of the BALF was more than 85%. The right lung was then removed and stored at −80°C, and the left lung was fixed in 4% formaldehyde.
Paraffin-embedded lung tissue sections were stained with hematoxylin-eosin (H&E), Masson trichrome, and periodic acid-Schiff (PAS) for light microscopic examinations. Goblet cell hyperplasia was evaluated by PAS. To determine the production of mucus, the percentage of PAS staining-positive cells in the airway epithelium was quantified. The Masson trichrome’s staining was used to observe the collagen formation in the bronchial airway. Under 200× magnification, three fields were randomly selected from each section and photographed.
ELISA
The ELISA kits (R&D Systems, Minneapolis, MN, USA) were used to measure the concentration of IL-1β, IL-6, IL-8, and TNF-α in BALF according to the manufacturer’s instructions. The lower limits of detection for the cytokines were as follows (pg/mL): TNF-α, 5.2; IL-1β, 3.3; IL-6, 4.1; IL-8, 0.2.
Immunohistochemistry
Paraffin-processed lung tissue sections were incubated with antigen retrieval solution in a microwave at high power for 15 min. After blocking, the sections were incubated sequentially with primary antibodies: anti-α-SMA (#19,245, 1:1000, Cell Signaling, Beverly, MA, USA), anti-VEGF (sc4570, 1:1000), anti-Nrf2 (sc722, 1:1000), anti-HO-1 (sc1796, 1:1000), anti-NF-κB p65 (sc-109; 1:1000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). After that, the samples were rinsed and then incubated with biotin-labeled secondary antibody (#5151, 1:2000, Cell Signaling). Finally, the sections were developed with DAB, counterstained with hematoxylin, dehydrated, cleared in xylene, and fixed. Quantitative expression analysis of α-SMA, VEGF, HO-1, Nrf2, and NF-κB were performed by calculating the average optical density of the positive staining around the airway.
Immunofluorescence staining
After deparaffinization, the lung sections were incubated with the anti-α-SMA antibody (1:500, #19,245, Cell Signaling Tech, Danvers, MA, USA) at 4°C overnight. After rinsing with PBS, the sections were incubated with the anti-rabbit secondary antibodies (#5151, 1:3000, Cell Signaling) at room temperature for 2 h. The sections were observed under a fluorescence microscope (Nikon Eclipse Ti-SR, Nikon, Tokyo, Japan). Five fields were randomly chosen under 200× magnification and photographed.
Western blot
Total protein was extracted from lung tissues and its concentration was determined by BCA kit (Beyotime, Hunan, China). Cytosolic or nuclear extractions from harvested lung tissues were performed as described previously [Citation32]. Proteins samples of equal quantity were separated by SDS-PAGE and then transferred to nitrocellulose membranes. The membranes were blocked with 5% skimmed milk, and incubated with anti-IκBα, anti-phosphorylated (p)-IκBα (sc-847, 1:1000), anti-NF-κB p65 (sc-109; 1:1000), anti-Akt, anti-P-Akt (sc-7985-R, 1:500), anti-ERK, anti-P-ERK (sc-7976; 1:1000), anti-p38, anti-P-p38 (sc-535; 1:1000), anti-JNK, anti-P-JNK (sc-135642; 1:1000), anti-Nrf2 (sc722, 1:1000), anti-HO-1 (sc1796, 1:1000), anti-VEGF (sc4570, 1:1000), anti-β-actin (sc-130656, 1:1,000, Santa Cruz Biotechnology, Inc.) and anti-α-SMA (#19245, 1:1000), anti-GAPDH (#2118, 1:1000), and anti-MMP-9 (#13667, 1:1000, Cell Signaling) respectively, at 4°C overnight. After that, the samples were rinsed and incubated with goat anti-rabbit secondary antibody which is conjugated with horseradish peroxidase (#5151, 1:2000, Cell Signaling) at room temperature for 2h. Immunodetection was developed with ECL detection reagent (Beyotime, Hunan, China). Photographs were taken and the optical densities of the bands were scanned and quantified with the Gel Doc 2000 (Bio-Rad, Hercules, CA, USA).
Cell line and treatment
Normal human ASMCs were purchased from ScienCell Research Laboratories (Carlsbad, CA, USA). The cells were cultured at 37°C with 5% CO2 in DMEM (Invitrogen-Gibco, Paisley, Scotland) supplemented with 20 U/L penicillin, 20μg/mL streptomycin, and 10% fetal bovine serum (HyClone, Logan, UT, USA). According to different treatments, cells were divided into five groups of the control group (0.5% DMSO, 1 μM), OVA group (1 μM) + IMP-L (1 μM), OVA + IMP-M (5 μM), and OVA + IMP-H (10 μM) groups. Cells were then treated with these agents for 48 h at 37°C, respectively.
The measurement of intracellular ROS
Intracellular ROS was measured using the 2′,7′-dichlorofluorescin diacetate (DCFH-DA, Sigma, USA). After treatment for 48 h, the cells were harvested and incubated with DCFH-DA at 37°C for 20 min and washed twice with cold PBS. After incubation, the cells were washed twice with PBS and analyzed within 30 min using FACScan (Becton Dickinson, San Jose, CA, United States) with excitation at 488 nm. The specific fluorescence signals corresponding to DCFH-DA were collected with a 525-nm bandpass filter. A total of 10,000 cells were counted in each determination. For each culture, a minimum of five random fields was captured.
Statistical analysis
The data are shown as the means ± standard error (SD). All tests were performed using Prism 6.00 (GraphPad Software, San Diego, CA, USA). The results were analyzed by one-way analysis of variance for repeated measures, followed by Tukey’s post hoc test to determine differences in multiple comparisons. p < 0.05 was considered statistically significant.
Results
Effect of IMP on chronic airway inflammation, goblet cell hyperplasia, and collagen deposition
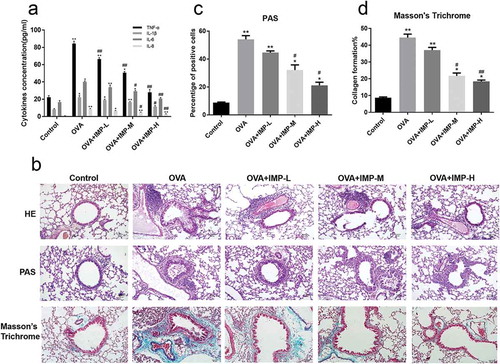
Asthmatic airway remodeling is featured by inflammation, collagen deposition, and goblet cell hyperplasia [Citation33]. In this study, we examined whether IMP could alleviate these symptoms. As shown in ), the levels of IL-1β, IL-6, IL-8, TNF-α in the BALF were tested. All of them were significantly increased in the OVA group than the Control group. Compared with the OVA group, these proinflammatory cytokines in the OVA + IMP-L, OVA + IMP-M, and OVA + IMP-H groups were significantly decreased in a dose-dependent manner. Nevertheless, all of them in IMP treatment groups were still significantly higher than those in the Control group. HE staining showed that compared to the Control group, a great number of inflammatory cells were infiltrated around the airway in the OVA group ()). The IMP groups showed clearly less inflammatory cell infiltration than the OVA group. The mucus productions of goblet cells were evaluated by PAS staining. As shown in ,c), the percentage of PAS-positive cells in the OVA group and IMP groups were significantly higher than the Control group, whereas OVA + IMP-M and OVA + IMP-H groups significantly reduced the goblet cells in the airway epithelium and thus the mucus hypersecretion than the OVA group (P < 0.05). Meanwhile, the collagen deposition was evaluated by Masson’s trichrome staining. As shown in ,d), collagen deposition was significantly increased in the OVA group and IMP groups than the Control group. Compared with the OVA group, the OVA + IMP-M and OVA + IMP-H groups showed significantly less collagen deposition (P < 0.05). These results indicate that IMP inhibits the production of proinflammatory factors, the inflammatory cell infiltration, collagen deposition, and goblet cell hyperplasia, thus alleviating airway remodeling.
Effect of IMP on α-SMA and MMP-9 expression
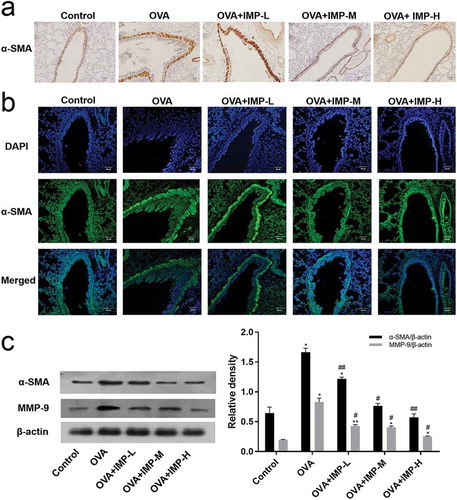
The expression of α-SMA and MMP-9 was analyzed. The immunohistochemical staining showed that the expression of α-SMA around the airways was increased in the OVA group compared with the control group ()). IMP treatment obviously decreased the level of α-SMA. Immunofluorescence staining of α-SMA showed similar results ()). Western blot results for the α-SMA and MMP-9 are shown in ), which showed that the expression of α-SMA and MMP-9 was significantly decreased in IMP groups, compared with the OVA group. These results demonstrated that IMP reduced the expression of α-SMA and MMP-9 induced by OVA inoculation.
Effect of IMP on the production of ROS
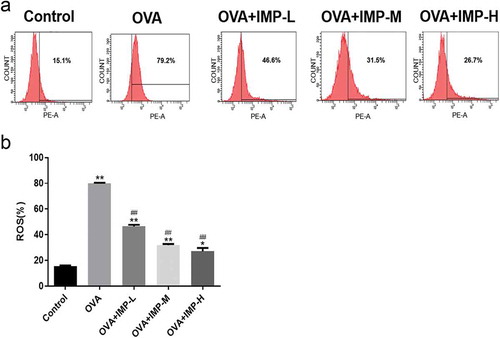
Flow cytometry analysis showed that the production of ROS in the OVA group and IMP groups were significantly increased, compared with the Control group (P < 0.05, ,b)). The intracellular levels of ROS in IMP-L, M, and H groups were significantly decreased to 46.6%, 31.5% or 26.7%, respectively, compared with the OVA group (P < 0.01). The results indicated that IMP might alleviate allergic inflammation and airway remodeling by reducing the production of ROS.
Effect of IMP on the expression of VEGF
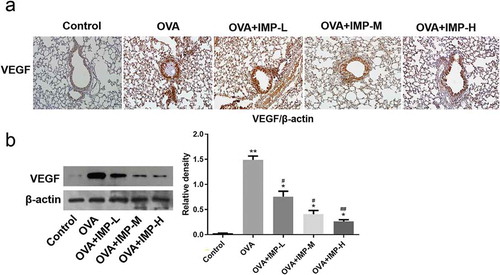
Immunohistochemistry and western blot were used to detect the effect of IMP on VEGF expression. The IHC staining for VEGF in the airways is shown in ). The VEGF level around the airways was increased in the OVA group and IMP groups compared with the Control ()). IMP treatment obviously decreased the VEGF expression compared to the OVA group. Consistently, as revealed by Western blot, the VEGF level in the OVA group and IMP groups was significantly higher than that in the control group ()). Meanwhile, IMP groups had significantly lower levels of VEGF than the OVA group (P < 0.05). These results demonstrated that IMP inhibited VEGF expression, thus regulating airway remodeling.
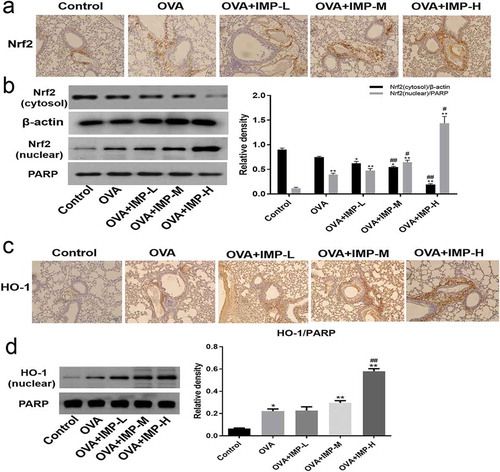
Effect of IMP on the expression of Nrf2 and HO-1
The immunohistochemical staining for Nrf2 and HO-1 in the airways is shown in ,c). The staining of Nrf2 and HO-1 around the airways was increased in the OVA group and IMP groups, compared with the Control group. Western blot results showed that the Nrf2 (nuclear) level in the OVA group and the IMP groups was significantly higher than that in the Control group (P < 0.01). Meanwhile, IMP groups had significantly higher levels of Nrf2 (nuclear) than the OVA group. Nevertheless, the expression of Nrf2 (cytosol) is contrary to the expression of Nrf2 (nuclear). The Nrf2 (cytosol) level in the OVA group and the IMP groups was significantly lower than that in the Control group. And IMP groups had significantly lower levels of Nrf2 (cytosol) than the OVA group ()). As revealed by Western blot, the HO-1 level in the OVA group and the IMP groups was significantly higher than that in the Control group. And IMP groups had significantly higher levels of HO-1 than the OVA group ()). The results imply that IMP might alleviate allergic inflammation and airway remodeling by promoting activation of the Nrf2/HO-1 pathway.
Effect of IMP on the nuclear translocation of NF-κB, phosphorylation of Akt, ERK, p38, and JNK
To further investigate the mechanisms underlying the effects of IMP on airway remodeling, the nuclear translocation of NF-κB and the phosphorylation of Akt, ERK, p38, and JNK were investigated. The immunohistochemical staining for NF-κB p65 is shown in ). NF-κB p65 in the nuclear around the airways was increased in the OVA group compared with the Control group. IMP obviously decreased the staining of NF-κB p65 in the nuclear. In ), the NF-κB (nuclear) and P-IκBα levels in the OVA group and the IMP groups were significantly higher than those in the Control group. Moreover, IMP groups had significantly lower levels of NF-κB (nuclear) and P-IκBα than the OVA group. Nevertheless, the expression of NF-κB (cytosol) was contrary to the expression of NF-κB (nuclear). As revealed by Western blot, the NF-κB (cytosol) level in the OVA group and the IMP groups were significantly lower than that in the control group. In addition, IMP groups had significantly higher levels of NF-κB (cytosol) than the OVA group.
Figure 6. Effect of IMP on the nuclear translocation of NF-κB, phosphorylation of Akt, ERK, p38, and JNK.
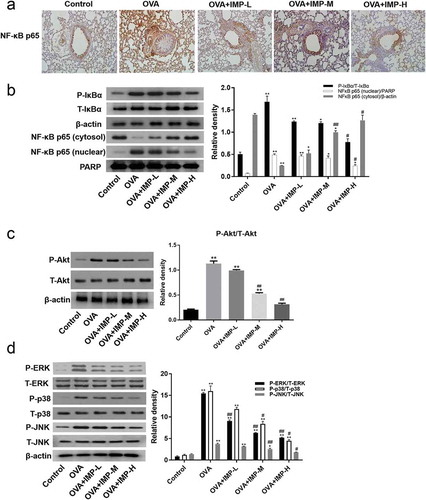
We also found that the expression of P-Akt, P-ERK, P-p38, and P-JNK in the OVA group and the IMP groups was significantly higher than that in the Control group. IMP groups had significantly lower levels of P-Akt, P-ERK, P-p38, and P-JNK than the OVA group. As revealed by Western blot, the levels of Akt, ERK, p38, and JNK had no difference in all groups (,d)). These results show that IMP likely alleviates airway remodeling by inhibiting activation of NF-κB, MAPK, and Akt pathway.
Discussion
At present, natural products have been known as valuable sources for drug discovery and many natural products have been discovered as new drugs. IMP is considered as a Chinese medicinal ingredient of coumarins. It has several pharmacological effects including anti-inflammation and tumor inhibition [Citation22], anti-HIV [Citation23], and anticonvulsant [Citation24]. Although the introduction of natural medicines has been used as the most common treatment, the administration of a single, effective compound increases bioavailability and reduces side effects. In this study, we investigated the anti-inflammatory effect of IMP in a chronic allergic asthma model.
It is well known that dexamethasone (DEX) is used as the first-line agent for asthma treatment, but it has few therapeutic effects on airway remodeling [Citation34]. Therefore, it is extremely urgent to discover new therapeutic drugs for asthmatic patients that are effective and safe. Airway remodeling that leads to airway obstruction may be an important cause of refractory asthma in patients [Citation35]. In this paper, we made a chronic model of allergic asthma to explore the effect of IMP on airway remodeling. Ya-Nan Liu et al. had illuminated that collagen deposition, goblet cell hyperplasia and hyperplasia and hypertrophy of airway smooth muscle, and these processes should be the crux of the problem in asthmatic airway remodeling [Citation33]. Collagen deposition and mucus hypersecretion could lead to airway obstruction, contributing to the morbidity and mortality of asthma [Citation36,Citation37]. The results showed that IMP obviously decreased collagen deposition and goblet cell hyperplasia, indicating that IMP may have an inhibitory action in asthmatic airway remodeling.
The α-SMA is commonly deemed as a contractile element in airway smooth muscle cell (ASMCs) which is related to the progression of airway remodeling. Improve the levels of α-SMA can promote ASMC migration and proliferation, which are critical for airway remodeling [Citation38]. The results of immunohistochemical staining and western blot showed that the level of α-SMA in airways was decreased by IMP in the chronic model of allergic asthma. And it has been confirmed that DEX treated model has no obvious effect in reducing the level of α-SMA [Citation33], indicating that IMP is superior to DEX about the inhibition of ASMC hyperplasia and hypertrophy [Citation39].
It is well known that MMP-9 is related to the collagen deposition in airway walls, which can cause narrowed airways [Citation40,Citation41]. The results here showed that IMP obviously decreased the expression of MMP-9, compared with the control group. It implied that IMP should attenuate the ECM deposition and fibrosis. Thus, we indicated that IMP might have an anti-fibrotic effect on the ECM in chronic asthma.
ROS is the critical mediator of pulmonary vascular cell proliferation. The results showed that IMP obviously reduces the production of ROS in BALF. It has been shown that ROS could increase VEGF expression, and VEGF enhances the pulmonary arterial walls and vascular remodeling [Citation42,Citation43]. As an endothelial cell-specific mitogenic peptide, VEGF can modulate angiogenesis and vasculogenesis, which results in airway obstruction [Citation44]. VEGF is also known to induce airway and vascular remodeling and enhances Th2-mediated sensitization and inflammation in the lung [Citation45]. The previous data have already shown that activation of Nrf2 blocks VEGF induction of VEGFR2-PI3K/Akt in vascular endothelial cells [Citation46]. In this paper, IMP remarkably decreased the expression of VEGF. Therefore, it demonstrated that IMP might downregulate the VEGF level related to oxidative stress.
Oxidative stress, the physiological damage that appears due to the effects of ROS, has been confirmed to influence smooth muscle contraction and increase mucus secretion [Citation47]. Furthermore, the excessive production of ROS in asthma may induce enzymatic and non-enzymatic alterations, which give rise to an antioxidant/oxidant imbalance in airways [Citation18]. The imbalance produces a state of oxidative stress, resulted in the persistence of pulmonary fibrosis [Citation19]. Pro-inflammatory cytokines, such as TNF-α, IL-6, IL-8, and IL-1β have significant roles in allergic inflammation [Citation48]. The data showed that IMP decreased the expression of these pro-inflammatory cytokines. IL-6, IL-8, and IL-1β promote allergic diseases, such as bronchial asthma, contact hypersensitivity, and atopic dermatitis [Citation49,Citation50]. One of the main sources of TNF-α is mast cell in human dermis, and it is essential in the progression of chronic allergic inflammation [Citation51]. The pro-inflammatory cytokines can stimulate adaptive immunity through activating NF-κB [Citation14]. It is widely viewed that NF‑κB is the main regulator of inflammatory cytokine [Citation52]. Many studies have demonstrated that ROS can activate an inflammatory response by the NF-κB signaling pathway [Citation53]. As is well known that PI3K and MAPK play a critical role in signal transduction related to cell proliferation [Citation33]. Burgess et al. even discovered that MAPK and Akt activation were obviously enhanced in ASMCs from the asthmatic model, and U0126 (the MEK/ERK inhibitor) and LY294002 (the PI3K inhibitor) remarkably inhibited cell proliferation in ASMCs from asthmatic model [Citation54]. ROS, MAPK, PI3K/Akt, and NF-κB are closely related to airway inflammation and pulmonary cell proliferation. However, the exact mechanism is unknown. Previous studies have proved that ERK phosphorylation relies on ROS in Siglec-8-mediated eosinophil cell death [Citation55] and that JNK phosphorylation is a vital molecular target of ROS in TNF-α-induced cell death [Citation56]. Additionally, pretreatments of anti-oxidant enzymes can inhibit the thalidomide-induced p38 phosphorylation in erythropoiesis [Citation57]. Moreover, the inhibition of ROS by the chemicals can reduce MAPK phosphorylation in human ASMCs [Citation58], which implies that ROS plays a critical role in mediating MAPK activation [Citation59]. The high levels of ROS also can activate PI3K/Akt signaling pathways [Citation60]. Taken together, ROS plays critical roles in MAPK, PI3K/Akt, and NF-κB signaling pathways. In this paper, the results showed that IMP remarkably increased the expression of NF‑κB p65 in the cytoplasm and decreased the expression of P-IκBα and NF-κB p65 (nuclear). Besides, IMP inhibits the expression of P-ERK, P-p38, P-JNK, and P-Akt in a dose-dependent manner. Some researchers have demonstrated that the allergic airway inflammation and remodeling were closely related to the activation of NF-κB [Citation61,Citation62]. In summary, this information showed that IMP inhibits airway inflammation and remodeling through blocking ROS/PI3K/Akt, ROS/MAPK, and ROS/NF-κB signaling pathways.
Previous studies have shown that HO-1 plays a key role in regulating ROS [Citation63]. The Nrf2/Kelch-like ECH-associated protein 1 (KEAP1) system can regulate the induction of HO-1 [Citation6]. HO-1 is an anti-oxidative response and can regulate the expression of antioxidant enzymes [Citation64]. ROS makes Nrf2 (cytosol) depart from the Nrf2/KEAP1 complex, and Nrf2 translocates to the nucleus. This results in increasing the expression of HO-1 [Citation65]. In this paper, IMP increased the expression of Nrf2 (nuclear) and HO-1, compared with the OVA group. And IMP dose-dependently inhibited expression of P-ERK, P-p38, P-JNK, and P-Akt, compared with the OVA group. In summary, these results showed that IMP inhibits airway inflammation and remodeling through modulating Nrf2/HO-1/ROS/PI3K/Akt and Nrf2/HO-1/ROS/MAPK signaling pathways.
In conclusion, this paper demonstrates that the IMP has the effect of anti-inflammatory and anti-proliferative in the chronic model of allergic asthma. IMP significantly alleviated the chronic inflammation and airway remodeling. The mechanisms might be that IMP attenuates inflammation by the Nrf2/HO-1/ROS/PI3K/Akt, Nrf2/HO-1/ROS/MAPK, and Nrf2/HO-1/ROS/NF-κB signaling pathways. Overall, IMP alleviates ROS-mediated airway remodeling by targeting the Nrf2/HO-1 signaling pathway.
Authors’ Contributions
Zhemin Xian performed the animal and cell experiments, analyzed the data, and drafted the manuscript. Yun Ho Choi and Mingyu Zheng helped in the animal and cell experiments and analyzed the data. Jingzhi Jiang and Yuzhe Zhao conducted the molecular experiments. Chongyang Wang participated in the animal experiments. Junfeng Li, Yan Li, and Liangchang Li provided help in cell experiments. Hongmei Piao and Guanghai Yan conceived the idea, designed the study, and revised the manuscript.
Supplementary_Figure_1.pdf
Download PDF (151.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemented data of this article can be accessed here.
Additional information
Funding
References
- Fehrenbach H, Wagner C, Wegmann M. Airway remodeling in asthma: what really matters. Cell Tissue Res. 2017;367:551–569.
- Ma K, Fan Y, Dong X, et al. MTA1 promotes epithelial to mesenchymal transition and metastasis in non-small-cell lung cancer. Oncotarget. 2017;8:38825–38840.
- Wang KC, Le Cras TD, Larcombe AN, et al. Independent and combined effects of airway remodelling and allergy on airway responsiveness. Clin Sci (Lond). 2017;132:327–338.
- van der Velden JL, Hoffman SM, Alcorn JF, et al. Absence of c-Jun NH2-terminal kinase 1 protects against house dust mite-induced pulmonary remodeling but not airway hyperresponsiveness and inflammation. Am J Physiol Lung Cell Mol Physiol. 2014;306:L866–75.
- Capra V, Rovati GE. Rosuvastatin inhibits human airway smooth muscle cells mitogenic response to eicosanoid contractile agents. Pulm Pharmacol Ther. 2014;27:10–16.
- Kaspar JW, Niture SK, Jaiswal AK. Nrf2:iNrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47:1304–1309.
- Zhang QH, Zhou ZS, Lu GS, et al. Melatonin improves bladder symptoms and may ameliorate bladder damage via increasing HO-1 in rats. Inflammation. 2013;36:651–657.
- Wang Z, Zhang H, Sun X, et al. The protective role of vitamin D3 in a murine model of asthma via the suppression of TGF-beta/Smad signaling and activation of the Nrf2/HO-1 pathway. Mol Med Rep. 2016;14:2389–2396.
- Li W, Cai ZN, Mehmood S, et al. Polysaccharide FMP-1 from Morchella esculenta attenuates cellular oxidative damage in human alveolar epithelial A549 cells through PI3K/AKT/Nrf2/HO-1 pathway. Int J Biol Macromol. 2018;120:865–875.
- Jayawardena TU, Asanka Sanjeewa KK, Shanura Fernando IP, et al. Sargassum horneri (Turner) C. Agardh ethanol extract inhibits the fine dust inflammation response via activating Nrf2/HO-1 signaling in RAW 264.7 cells. BMC Complement Altern Med. 2018;18:249.
- Hayden MS, Ghosh S. NF-kappaB in immunobiology. Cell Res. 2011;21:223–244.
- Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64:27–34.
- Tian B, Patrikeev I, Ochoa L, et al. NF-kappaB mediates mesenchymal transition, remodeling, and pulmonary fibrosis in response to chronic inflammation by viral RNA patterns. Am J Respir Cell Mol Biol. 2017;56:506–520.
- Lee NY, Chung KS, Jin JS, et al. Effect of chicoric acid on mast cell-mediated allergic inflammation in vitro and in vivo. J Nat Prod. 2015;78:2956–2962.
- Seo SH, Jeong GS. Fisetin inhibits TNF-alpha-induced inflammatory action and hydrogen peroxide-induced oxidative damage in human keratinocyte HaCaT cells through PI3K/AKT/Nrf-2-mediated heme oxygenase-1 expression. Int Immunopharmacol. 2015;29:246–253.
- Chung KF. p38 mitogen-activated protein kinase pathways in asthma and COPD. Chest. 2011;139:1470–1479.
- Dickinson BC, Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol. 2011;7:504–511.
- Nadeem A, Masood A, Siddiqui N. Oxidant–antioxidant imbalance in asthma: scientific evidence, epidemiological data and possible therapeutic options. Ther Adv Respir Dis. 2008;2:215–235.
- Cui Y, Robertson J, Maharaj S, et al. Oxidative stress contributes to the induction and persistence of TGF-beta1 induced pulmonary fibrosis. Int J Biochem Cell Biol. 2011;43:1122–1133.
- Murillo MM, Carmona-Cuenca I, Del Castillo G, et al. Activation of NADPH oxidase by transforming growth factor-beta in hepatocytes mediates up-regulation of epidermal growth factor receptor ligands through a nuclear factor-kappaB-dependent mechanism. Biochem J. 2007;405:251–259.
- Ivanov AV, Smirnova OA, Ivanova ON, et al. Hepatitis C virus proteins activate NRF2/ARE pathway by distinct ROS-dependent and independent mechanisms in HUH7 cells. PloS One. 2011;6:e24957.
- Yang WQ, Zhu ZX, Song YL, et al. Dimeric furanocoumarins from the roots of Angelica dahurica. Nat Prod Res. 2017;31:870–877.
- Sancho R, Marquez N, Gomez-Gonzalo M, et al. Imperatorin inhibits HIV-1 replication through an Sp1-dependent pathway. J Biol Chem. 2004;279:37349–37359.
- Luszczki JJ, Glowniak K, Czuczwar SJ. Time-course and dose-response relationships of imperatorin in the mouse maximal electroshock seizure threshold model. Neurosci Res. 2007;59:18–22.
- Lin CL, Hsiao G, Wang CC, et al. Imperatorin exerts antiallergic effects in Th2-mediated allergic asthma via induction of IL-10-producing regulatory T cells by modulating the function of dendritic cells. Pharmacol Res. 2016;110:111–121.
- Wang KS, Lv Y, Wang Z, et al. Imperatorin efficiently blocks TNF-alpha-mediated activation of ROS/PI3K/Akt/NF-kappaB pathway. Oncol Rep. 2017;37:3397–3404.
- Mi C, Ma J, Wang KS, et al. Imperatorin suppresses proliferation and angiogenesis of human colon cancer cell by targeting HIF-1alpha via the mTOR/p70S6K/4E-BP1 and MAPK pathways. J Ethnopharmacol. 2017;203:27–38.
- Ye J, Piao H, Jiang J, et al. Polydatin inhibits mast cell-mediated allergic inflammation by targeting PI3K/Akt, MAPK, NF-kappaB and Nrf2/HO-1 pathways. Sci Rep. 2017;7:11895.
- Martin D, Rojo AI, Salinas M, et al. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J Biol Chem. 2004;279:8919–8929.
- Yang SH, Li P, Yu LH, et al. Sulforaphane protect against cadmium-induced oxidative damage in mouse Leydigs cells by activating Nrf2/ARE signaling pathway. Int J Mol Sci. 2019;20(3):630.
- Chen G, Zhao J, Yin Y, et al. C-type natriuretic peptide attenuates LPS-induced endothelial activation: involvement of p38, Akt, and NF-kappaB pathways. Amino Acids. 2014;46:2653–2663.
- Lee KS, Kim SR, Park HS, et al. A novel thiol compound, N-acetylcysteine amide, attenuates allergic airway disease by regulating activation of NF-kappaB and hypoxia-inducible factor-1alpha. Exp Mol Med. 2007;39:756–768.
- Liu YN, Zha WJ, Ma Y, et al. Galangin attenuates airway remodelling by inhibiting TGF-beta1-mediated ROS generation and MAPK/Akt phosphorylation in asthma. Sci Rep. 2015;5:11758.
- Holgate ST. Asthma: a simple concept but in reality a complex disease. Eur J Clin Invest. 2011;41:1339–1352.
- Tian X, Tian X, Huo R, et al. Bacillus Calmette-Guerin alleviates airway inflammation and remodeling by preventing TGF-beta1 induced epithelial-mesenchymal transition. Hum Vaccin Immunother. 2017;13:1758–1764.
- Curran DR, Cohn L. Advances in mucous cell metaplasia: a plug for mucus as a therapeutic focus in chronic airway disease. Am J Respir Cell Mol Biol. 2010;42:268–275.
- Eap R, Jacques E, Semlali A, et al. Cysteinyl leukotrienes regulate TGF-beta(1) and collagen production by bronchial fibroblasts obtained from asthmatic subjects. Prostaglandins Leukot Essent Fatty Acids. 2012;86:127–133.
- Xu GN, Yang K, Xu ZP, et al. Protective effects of anisodamine on cigarette smoke extract-induced airway smooth muscle cell proliferation and tracheal contractility. Toxicol Appl Pharmacol. 2012;262:70–79.
- Li HH, Cai Q, Wang YP, et al. [The role of transforming growth factor-beta1/connective tissue growth factor signaling pathway in paraquat-induced pulmonary fibrosis]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2016;34:484–488.
- Barbaro MP, Spanevello A, Palladino GP, et al. Exhaled matrix metalloproteinase-9 (MMP-9) in different biological phenotypes of asthma. Eur J Intern Med. 2014;25:92–96.
- Vignola AM, Paganin F, Capieu L, et al. Airway remodelling assessed by sputum and high-resolution computed tomography in asthma and COPD. Eur Respir J. 2004;24:910–917.
- Bierer R, Nitta CH, Friedman J, et al. NFATc3 is required for chronic hypoxia-induced pulmonary hypertension in adult and neonatal mice. Am J Physiol Lung Cell Mol Physiol. 2011;301:L872–80.
- de Frutos S, Spangler R, Alo D, et al. NFATc3 mediates chronic hypoxia-induced pulmonary arterial remodeling with alpha-actin up-regulation. J Biol Chem. 2007;282:15081–15089.
- Shin JH, Shim JW, Kim DS, et al. TGF-beta effects on airway smooth muscle cell proliferation, VEGF release and signal transduction pathways. Respirology. 2009;14:347–353.
- Lee CG, Link H, Baluk P, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10:1095–1103.
- Wei Y, Gong J, Thimmulappa RK, et al. Nrf2 acts cell-autonomously in endothelium to regulate tip cell formation and vascular branching. Proc Natl Acad Sci U S A. 2013;110:E3910–8.
- Zuo L, Otenbaker NP, Rose BA, et al. Molecular mechanisms of reactive oxygen species-related pulmonary inflammation and asthma. Mol Immunol. 2013;56:57–63.
- Draberova L, Paulenda T, Halova I, et al. Ethanol inhibits high-affinity immunoglobulin E receptor (FcepsilonRI) signaling in mast cells by suppressing the function of FcepsilonRI-Cholesterol signalosome. PloS One. 2015;10:e0144596.
- Kumase F, Takeuchi K, Morizane Y, et al. AMPK-activated protein kinase suppresses Ccr2 expression by inhibiting the NF-kappaB pathway in RAW264.7 macrophages. PLoS One. 2016;11:e0147279.
- Tang L, Chen Q, Meng Z, et al. Suppression of Sirtuin-1 increases IL-6 expression by activation of the Akt pathway during allergic asthma. Cell Physiol Biochem. 2017;43:1950–1960.
- Yang B, Li JJ, Cao JJ, et al. Polydatin attenuated food allergy via store-operated calcium channels in mast cell. World J Gastroenterol. 2013;19:3980–3989.
- Zhang BF, Jiang H, Chen J, et al. KDM3A inhibition attenuates high concentration insulin-induced vascular smooth muscle cell injury by suppressing MAPK/NFkappaB pathways. Int J Mol Med. 2017;41:1265–1274.
- Peng S, Gao J, Liu W, et al. Andrographolide ameliorates OVA-induced lung injury in mice by suppressing ROS-mediated NF-κB signaling and NLRP3 inflammasome activation. Oncotarget. 2016;7(49):80262.
- Burgess JK, Lee JH, Ge Q, et al. Dual ERK and phosphatidylinositol 3-kinase pathways control airway smooth muscle proliferation: differences in asthma. J Cell Physiol. 2008;216:673–679.
- Kano G, Almanan M, Bochner BS, et al. Mechanism of Siglec-8-mediated cell death in IL-5-activated eosinophils: role for reactive oxygen species-enhanced MEK/ERK activation. J Allergy Clin Immunol. 2013;132:437–445.
- Pham CG, Papa S, Bubici C, et al. Oxygen JNKies: phosphatases overdose on ROS. Dev Cell. 2005;8:452–454.
- Yamazaki R, Kasuya Y, Fujita T, et al. Antifibrotic effects of cyclosporine A on TGF-beta1-treated lung fibroblasts and lungs from bleomycin-treated mice: role of hypoxia-inducible factor-1alpha. Faseb J. 2017;31:3359–3371.
- Lee IT, Luo SF, Lee CW, et al. Overexpression of HO-1 protects against TNF-alpha-mediated airway inflammation by down-regulation of TNFR1-dependent oxidative stress. Am J Pathol. 2009;175:519–532.
- Lee IT, Yang CM. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem Pharmacol. 2012;84:581–590.
- Kim GD. Myricetin inhibits angiogenesis by inducing apoptosis and suppressing PI3K/Akt/mTOR signaling in endothelial cells. J Cancer Prev. 2017;22:219–227.
- Ci X, Chu X, Chen C, et al. Oxytetracycline attenuates allergic airway inflammation in mice via inhibition of the NF-kappaB pathway. J Clin Immunol. 2011;31:216–227.
- Ather JL, Hodgkins SR, Janssen-Heininger YM, et al. Airway epithelial NF-kappaB activation promotes allergic sensitization to an innocuous inhaled antigen. Am J Respir Cell Mol Biol. 2011;44:631–638.
- Wang J, Le T, Wei R, et al. Knockdown of JMJD1C, a target gene of hsa-miR-590–3p, inhibits mitochondrial dysfunction and oxidative stress in MPP+-treated MES23.5 and SH-SY5Y cells. Cell Mol Biol (Noisy-le-grand). 2016;62:39–45.
- Vitek L, Vanikova J, Nachtigal P. Letter by Vitek et al regarding article, “niacin inhibits vascular inflammation via the induction of heme oxygenase-1”. Circulation. 2012;126:e99.
- Scapagnini G, Vasto S, Abraham NG, et al. Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol Neurobiol. 2011;44:192–201.