ABSTRACT
Endocrine cells in the gastrointestinal tract secrete multiple hormones to maintain homeostasis in the body. In the present study, we generated intestinal organoids from the duodenum, jejunum, and ileum of Neurogenin 3 (Ngn3)-EGFP mice and examined how enteroendocrine cells (EECs) within organoid cultures resemble native epithelial cells in the gut. Transcriptome analysis of EGFP-positive cells from Ngn3-EGFP organoids showed gene expression pattern comparable to EECs in vivo. We also compared mRNAs of five major hormones, namely, ghrelin (Ghrl), cholecystokinin (Cck), Gip, secretin (Sct), and glucagon (Gcg) in organoids and small intestine along the longitudinal axis and found that expression patterns of these hormones in organoids were similar to those in native tissues. These findings suggest that an intestinal organoid culture system can be utilized as a suitable model to study enteroendocrine cell functions in vitro.
Graphical abstract
Statistical analysis of genes preferentially expressed in Ngn3+ vs Ngn3- cells derived from intestinal organoids of Ngn3-EGFP mice.
The gastrointestinal (GI) tract has multiple functions, including absorption of nutrients, protection against foreign enemies, immune responses, and hormone secretion. These functions are performed by specialized epithelial cells that align throughout the apical surface of the GI tract. Within these epithelial cells, endocrine cells play a key role in secreting hormones to maintain homeostasis in the body [Citation1]. These endocrine cells differentiate from endocrine progenitor cells that express Neurogenin 3 (Ngn3), a basic-helix-loop-helix (bHLH) transcription factor that is known to be essential for endocrine cell differentiation in the GI epithelium as well as pancreas [Citation2,Citation3]. Therefore, Ngn3 will be a good marker with which to study enteroendocrine progenitor cells [Citation4,Citation5].
Mature endocrine cells are differentially localized within endocrine organs and possess specific functions. For example, Glucose-dependent insulinotropic polypeptide (Gip) and glucagon-like peptide-1 (Glp-1) promote insulin release in islet β-cells and maintain normal blood glucose level [Citation6]. Much attention has been paid to the dynamics of these hormones as a potential treatment for diabetes. Cholecystokinin stimulates the secretion of bile acid and pancreatic enzymes by in response to food intake [Citation7,Citation8]. Other hormones also play an important role in controlling food intake and energy balance [Citation9,Citation10]. Despite their vital roles in homeostatic control, the proportion of endocrine cells in intestinal tissue is less than 1%, making research difficult to conduct [Citation11,Citation12]. Primary intestinal cell cultures have been a challenge for researchers because they only survive for a short period of time. In addition, there are a limited number of transformed cell lines available to study the function of enteroendocrine cells (EECs) [Citation13–Citation15]. It was only in 2007 that gastrointestinal stem cells were identified [Citation16] and the new three-dimensional cells called “intestinal organoids” or “enteroids” were generated [Citation17,Citation18]. These small intestinal organoids recapitulate proliferation and differentiate similar to the native tissue. It has been reported that apart from intestines, other endoderm organs, including the stomach, pancreas, liver, and taste bud tissues, have been shown to be suitable sources for organoid culture [Citation19–Citation22]. Although an organoid culture system is reported to recapitulate in vivo epithelial cell turnover and differentiation, little is known about the properties of endocrine cells within organoids.
In the present study, we have used Ngn3-EGFP mice to investigate whether the expression profile of endocrine progenitor cells and mature EECs within intestinal organoids is similar to native tissue. We further examined whether EECs in organoids expressed endocrine hormones in a pattern similar to that in in vivo EECs.
Materials and methods
Animals
All animals used in this study were housed and cared for under the “Guiding Principles in the Care and Use of Animals,” published by the Animal Care Committee of Tokyo University of Agriculture (#150,005). Wild type (Wt) C57BL/6 mice were purchased from CLEA Japan, Inc. and Ngn3-EGFP mice were provided by Dr. Matsumoto (Juntendo University, Tokyo, Japan). Ngn3-EGFP mice express EGFP in the nucleus of their endocrine progenitor cells, because a nuclear localization signal is fused to the EGFP sequence. Animals were maintained at a temperature of 24 ± 1°C in a 12 h light cycle with food and water provided ad libitum. After mice were humanely euthanized, their peritoneal cavities were exposed, the duodenum, jejunum, and ileum were removed, and epithelial cells were exfoliated.
Generation of organoids
Isolated crypts were cultured as previously described, with minimal modifications [Citation21]. To summarize, crypts were released from murine duodenum, jejunum and ileum by incubation at 4°C for 30 min in DPBS (w/o Ca2+, Mg2+) containing 2 mM EDTA (Nacalaiteschue, Kyoto, Japan). After removal of EDTA, tissues were shaken vigorously for 1 min in 43.4 mM sucrose and 54.9 mM sorbitol mixture to obtain a crypt-enriched fraction. After size fractionation using a 70 μm filter, crypts were collected and embedded in Matrigel (Corning, NY, USA). The crypts were then plated out into 12-well plates (300 crypts per 60 μL of Matrigel per well). Basal culture media used were advanced DMEM/F12 (Thermo Fisher Scientific, Massachusetts, USA) supplemented with Gentamicin/Amphotericin B (Thermo Fisher Scientific), 10 mM HEPES (Nacalai Tesque Inc.), GlutaMAX (Thermo Fisher Scientific), 1 × N2 supplement (Thermo Fisher Scientific), and 1 × B27 supplement (Thermo Fisher Scientific). Growth factors were 50 ng/mL recombinant murine EGF (PeproTech, NJ, USA), Noggin conditional medium, R-spondin conditional medium. Media were changed every 2 or 3 days, and organoids were passaged 5 to 7 days following seeding.
Immunohistochemistry
Intestinal tissues (duodenum, jejunum, and ileum) were fixed with 4% (w/v) paraformaldehyde for 30 min at room temperature. Tissues were replaced in 30% (w/v) sucrose in PBS at 4°C, embedded in Tissue-Tek O.C.T Compound (Sakura Finetek, Tokyo, Japan), cryosections with a thickness of 12 μm were prepared using a CM1850 cryostat (Leica, Wetzlar, Germany). Sections were incubated with rabbit anti-GIP antibody (1:600 (v/v) dilution; ab22624, Abcam, Cambridge, UK), and rabbit anti-GLP-1 antibody (1:600 (v/v) dilution; ab22625, Abcam) at 4°C for 20 h. Then primary antibodies were replaced by donkey anti-rabbit IgG secondary antibody (1:1000 (v/v) dilution; Alexa Fluor 555 Thermo Fisher Scientific), and incubated for 1 h at room temperature and samples were mounted with Fluoromount (Diagnostic BioSystems, CA, USA). Stained sections were observed using a confocal laser scanning microscope (FLUOVIEW FV10i, Olympus, Tokyo, Japan).
Organoids cultured for five days were fixed with 4% (w/v) paraformaldehyde, and washed three times for 5 min in PBS. After blocking for 1 h, organoids were incubated with rabbit anti-GIP antibody (1:200) and rabbit anti-GLP-1 antibody (1:200) at 4°C for 20 h. The primary antibodies were washed out, replaced with Alexa Fluor 555 donkey anti-rabbit IgG secondary antibody (1:1000), and incubated for 1 h at room temperature. Stained organoids were observed using a confocal laser scanning microscope.
Cell sorting
Organoids generated from Ngn3-EGFP mice were digested with trypsin/EDTA to single cells and filtered through a 35 µm nylon mesh (BD Falcon no. 352,350). The EGFP positive and EGFP negative cell fractions were isolated and collected using a FACS Aria II cell sorter (BD Biosciences).
CDNA synthesis and semi-quantitative RT-PCR
Total RNAs were extracted using ISOGEN (Nippon Gene, Tokyo, Japan) and were reverse transcribed using SuperScript III Reverse Transcriptase (Thermo Fisher Scientific). cDNAs were amplified by RT-PCR using GoTaq DNA Polymerase (Promega, WI, USA), using the primers described in Supplementary Table 1. The PCR conditions were as follows: 95°C for 4 min, 24–30 cycles at 95°C for 30 s, 52°C–54°C for 30 s, and 72°C for 30 s. The kinetics of amplification was studied for each combination of primers in preliminary experiments, and PCR was performed at an exponential range. PCR products were electrophoresed on 1.8% (w/v) agarose gel, stained with Midori Green Direct (Nippon Gene), and visualized using a FAS-Digi (Nippon Gene). Quantification of the bands was measured using Image J software [Citation23]. The gene expression levels were determined as relative values to those of Gapdh. Results were expressed as the means ± SE. Statistical significance was calculated by one-way ANOVA followed by Tukey’s multiple comparison test using SPSS version 22 (IBM, Armonk, NY).
RNA-seq
Total RNAs extracted were cleaned up using RNeasy Micro Kit (QIAGEN, Hilden, FRG) with DNase treatment. cDNA synthesis and preamplification were performed with total RNA using a SMART-Seq v4 Ultra Low Input RNA Kit and an Advantage 2 PCR Kit (Clontech, CA, USA), respectively. Preamplified cDNAs were supplied for RNA-Seq library preparation with Nextera XT Library Prep Kit (Illumina, CA, USA). Indexes for RNA-Seq libraries were pooled (10 nM each) and sequenced using an Illumina HiSeq 2500 System under single-end, 100 bp conditions. The read data have been deposited in the DNA Data Bank of the Japan Sequence Read Archive (accession no. DRA007308, DRA007310).
Data analysis
Single-end RNA-Seq reads for each replicate were aligned to the mouse genome (mm10, Genome Reference Consortium (GRC) Mouse Build 38) using a HiSat2 aligner. Aligned reads were quantified into RPKM and read count data for each sample using CLC Genomics Workbench and HTSeq-count. To detect differentially expressed genes (DEGs) between Ngn3-GFP+ and Ngn3-GFP−, a read count output file was input to the DESeq2 package. A comparison between expression levels of each RefSeq gene in Ngn3-GFP+ and Ngn3-GFP− was performed using the function “DESeqDataSetFromMatrix” and “DESeq.” DEGs were identified by the following two criteria: a more than two-fold change and a Wald test with Benjamin-Hochberg false discovery rate ((FDR) padj ≤ 0.01). To visualize the gene expression pattern for each RNA-Seq dataset, we generated volcano plots for each sample using the R “calibrate” package (https://cran.r-project.org/web/packages/calibrate/). To assess the biological significance of respective DEGs, the DEG list was used for Gene Ontology (GO) enrichment analysis with the DAVID web tool (http://david.abcc.ncifcrf.gov/); a background of all mouse genes was applied. Biological process terms with a significance of P < 0.05 (Fisher’s exact test) were considered significant.
Results and discussion
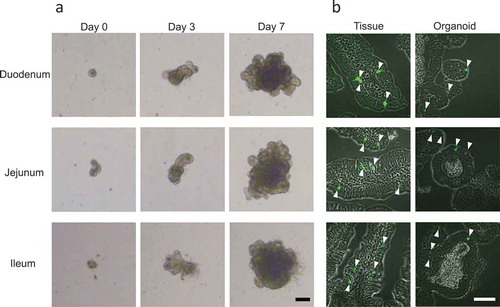
In order to focus on EECs and examine whether these cells exist in vitro in the same proportion as they do in vivo, we utilized Ngn3-EGFP mice. We succeeded in generating organoids in the duodenum, jejunum, and ileum, using a previously described method [Citation21] (). By day 7, each organoid grew to between 300 and 500 μm in size as a result of stem/progenitor cell proliferation ()), consistent with other observations [Citation17,Citation21]. EGFP signals were seen mainly in the nuclei of the intestinal tissues as well as in organoids generated from duodenum, jejunum and ileum ()), because EGFP contains a nuclear localization signal sequence [Citation4].
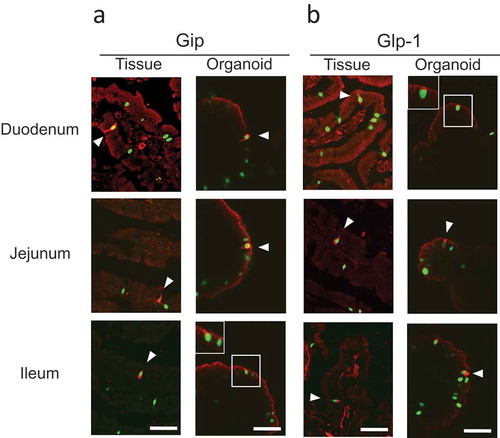
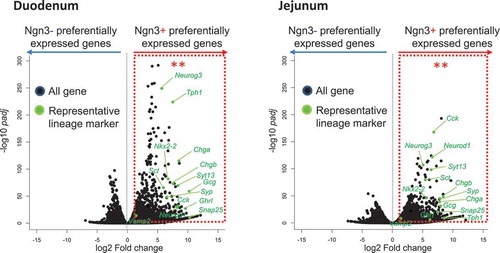
We then used immunohistochemistry to compare incretin expression in organoids and native tissues, by using tissue sections and organoids from Ngn3-EGFP mice. The results showed that Gip and Glp-1 were present in all intestinal tissues and organoids (, Supplementary Figure 1). Some Ngn3-EGFP-positive cells were colocalized with cells that expressed Gip or Glp-1 in both tissues and organoids (arrowheads in ). In order to confirm that Ngn3-positive cells in organoids are committed to the enteroendocrine lineage, both EGFP-positive (+) and EGFP-negative (−) cells from intestinal organoids were purified using a cell sorter and subjected to RNA-Seq analysis (Supplementary Figure 2). We identified 1172 and 1028 genes that were significantly upregulated in Ngn3+ fractions purified from organoids derived from the duodenum and jejunum, respectively (, Supplementary Figure 3). We determined GO terms that were enriched in DEGs using DAVID. Within both sets of organoids generated from jejunum and ileum tissue, the GO terms “exocytosis,” “regulation of exocytosis,” “neurotransmitter secretion,” “chemical synaptic transmission,” and “insulin secretion” were significantly enriched within the top 20 GO terms, suggesting that EGFP-positive cells represent endocrine cells (Supplementary Figure 4). We also identified transcripts of endocrine hormone genes (Ghrl, Cck, Gip, Sct, Gcg); enterochromaffin cell markers (Tph1, ChgA, ChgB); transcription factors that regulate enteroendocrine differentiation (Neurod1, Ngn3, Nkx2-2); and SNARE proteins (Snap25, Syt13, Syp, VAMP2), which are known to have functions in enteroendocrine lineages (). In the present study, we detected much less number of differentially expressed genes in Ngn3 negative cells (). The reason for this is that Ngn3 negative fraction contain heterologous cell types and differentially expressed genes were averaged and not being detected in volcano plot.
Figure 3. Statistical analysis of genes preferentially expressed in Ngn3+ vs Ngn3- cells derived from intestinal organoids of Ngn3-EGFP mice.
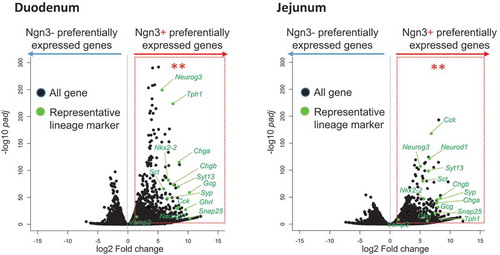
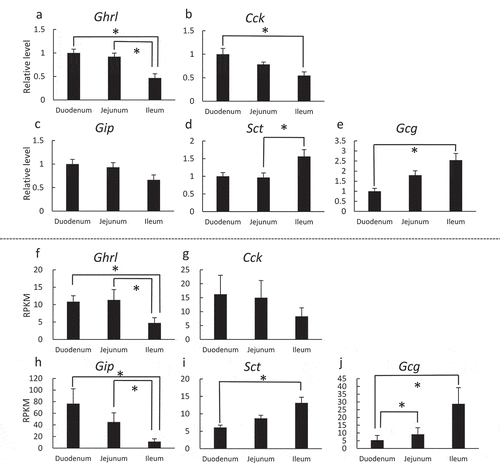
To further investigate whether the expression of hormones in organoids also mimics native tissues, we compared the mRNA expression of major peptide hormones in intestinal organoids and the epithelium of their native tissues by RT-PCR () and RNA-Seq (), respectively. Five peptide hormones, namely ghrelin (Ghrl), cholecystokinin (Cck), secretin (Sct), Gip, and Glp-1 were examined, and all were detected in both epithelial tissues and organoids, with similar expression patterns, as previously reported [Citation24–Citation29]. In both tissues and organoids, expression patterns of Glp-1 and Gip, known as incretins, were comparable to what has previously been reported – Gip is abundant in the upper portion whereas Glp-1 is abundant in the lower portion of the gastrointestinal tract () [Citation24,Citation25,Citation30]. Expression of Ghrl was significantly higher in the duodenum and jejunum than in the ileum in both intestinal tissues and in organoids (). Our result is similar to the previous report that Ghrl is mainly localized in the stomach but is also localized to some extent in the upper intestinal tract [Citation26]. Cck mRNA levels were significantly higher in the duodenum than in the ileum in the intestinal tissue ()) but not significant in the organoids ()). Cck has been reported to be synthesized and secreted mainly from the duodenum and jejunum [Citation24,Citation27], which is consistent with the results of the present study (). Sct is, historically, the first hormone to be discovered and has since been believed to be expressed in the upper part of the intestines [Citation24,Citation27,Citation31]. From our present study as well as others, Sct expression has also been detected mainly in the lower intestines (), raising the possibility that Sct is widely expressed throughout the intestines [Citation28,Citation29]. Overall, the gene expression patterns of Ghrl, Cck, Gip, Sct and Gcg mRNAs showed similarities when compared between native tissues and organoids. Measurement of hormones at protein level also must to be done to thoroughly understand whether individual organoid mimics their tissue origin.
Figure 4. Comparison of mRNA expression patterns of five major peptide hormones in intestines or intestinal organoids.
Although there are multiple peptide hormones in the gut that are important for food intake, the mechanism by which these hormones are released is not well understood. In this study we confirmed that endocrine progenitor cells, marked by Ngn3-EGFP, are committed to endocrine lineages. Since Ngn3 is a marker of endocrine precursor, we could utilize Ngn3-EGFP organoids to examine how endocrine cells get matured and secrete hormones in vitro. Replating Ngn3-EGFP cells into matrigel with various culture condition after cell sorting might be a way to examine terminal differentiation of endocrine cells.
To summarize, we have demonstrated that the intestinal organoid culture system resembles endocrine cells and their hormone expression profiles of native tissues. Therefore, we conclude that intestinal organoids can be suitable for examining the function of EECs, such as sensing nutrients and secreting various hormones.
Author Contribution
JO, YN and KI designed the research. JO, AS, EA, AI, HU, MM and KI performed the research. JO, AS and KI analyzed the data. JO, TY, YO and KI wrote the paper. All authors read and approved the final manuscript.
2019-12-27acceptedSuppl_figure.pdf
Download PDF (998.5 KB)Acknowledgments
We thank Dr. Clevers for Wnt3a and Noggin-producing cell lines and Dr. Jeffery Whitsett for the R-spondin-producing cell line. We also thank the Genome Research Center at Tokyo University of Agriculture for performing RNA-Seq and bioinformatic analyses.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Mace OJ, Tehan B, Marshall F. Pharmacology and physiology of gastrointestinal enteroendocrine cells. Pharmacol Res Perspect. 2015;3:e00155.
- Jenny M, Uhl C, Roche C, et al. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. Embo J. 2002;21:6338–6347.
- Schonhoff SE, Giel-Moloney M, Leiter AB. Minireview: development and differentiation of gut endocrine cells. Endocrinology. 2004;145:2639–2644.
- Jiang FX, Li K, Archer M, et al. Differentiation of islet progenitors regulated by nicotinamide into transcriptome-verified beta cells that ameliorate diabetes. Stem Cells. 2017;35:1341–1354.
- Nakajima C, Kamimoto K, Miyajima K, et al. A method for identifying mouse pancreatic ducts. Tissue Eng Part C Methods. 2018;24:480–485.
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157.
- Hughes CA, Bates T, Dowling RH. Cholecystokinin and secretin prevent the intestinal mucosal hypoplasia of total parenteral nutrition in the dog. Gastroenterology. 1978;75:34–41.
- Fried M, Erlacher U, Schwizer W, et al. Role of cholecystokinin in the regulation of gastric emptying and pancreatic enzyme secretion in humans. Studies with the cholecystokinin-receptor antagonist loxiglumide. Gastroenterology. 1991;101:503–511.
- Monteiro MP, Batterham RL. The importance of the gastrointestinal tract in controlling food intake and regulating energy balance. Gastroenterology. 2017;152:1707–1717 e1702.
- Goldspink DA, Reimann F, Gribble FM. Models and tools for studying enteroendocrine cells. Endocrinology. 2018;159:3874–3884.
- Moran GW, Leslie FC, Levison SE, et al. Enteroendocrine cells: neglected players in gastrointestinal disorders? Therap Adv Gastroenterol. 2008;1:51–60.
- May CL, Kaestner KH. Gut endocrine cell development. Mol Cell Endocrinol. 2010;323:70–75.
- Park JG, Oie HK, Sugarbaker PH, et al. Characteristics of cell lines established from human colorectal carcinoma. Cancer Res. 1987;47:6710–6718.
- Rindi G, Grant SG, Yiangou Y, et al. Development of neuroendocrine tumors in the gastrointestinal tract of transgenic mice. Heterogeneity of hormone expression. Am J Pathol. 1990;136:1349–1363.
- Drucker DJ, Jin T, Asa SL, et al. Activation of proglucagon gene transcription by protein kinase-A in a novel mouse enteroendocrine cell line. Mol Endocrinol. 1994;8:1646–1655.
- Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007.
- Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265.
- Stelzner M, Helmrath M, Dunn JC, et al., N.I.H.I.S.C. Consortium. A nomenclature for intestinal in vitro cultures. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1359–1363.
- Huch M, Bonfanti P, Boj SF, et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. Embo J. 2013;32:2708–2721.
- Huch M, Dorrell C, Boj SF, et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250.
- Mahe MM, Aihara E, Schumacher MA, et al. Establishment of gastrointestinal epithelial organoids. Curr Protoc Mouse Biol. 2013;3:217–240.
- Aihara E, Mahe MM, Schumacher MA, et al. Characterization of stem/progenitor cell cycle using murine circumvallate papilla taste bud organoid. Sci Rep. 2015;5:17185.
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675.
- Egerod KL, Engelstoft MS, Grunddal KV, et al. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology. 2012;153:5782–5795.
- Reimann F, Habib AM, Tolhurst G, et al. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8:532–539.
- Date Y, Kojima M, Hosoda H, et al. Ghrelin, A novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261.
- Roth KA, Hertz JM, Gordon JI. Mapping enteroendocrine cell populations in transgenic mice reveals an unexpected degree of complexity in cellular differentiation within the gastrointestinal tract. J Cell Biol. 1990;110:1791–1801.
- Kopin AS, Wheeler MB, Leiter AB. Secretin: structure of the precursor and tissue distribution of the mRNA. Proc Natl Acad Sci U S A. 1990;87:2299–2303.
- Habib AM, Richards P, Cairns LS, et al. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology. 2012;153:3054–3065.
- Polak JM, Bloom SR, Kuzio M, et al. Cellular localization of gastric inhibitory polypeptide in the duodenum and jejunum. Gut. 1973;14:284–288.
- Bloom SR. Hormones of the gastrointestinal tract. Br Med Bull. 1974;30:62–67.