ABSTRACT
We utilized the reaction of chitosan with acetic anhydride to form a chitin gel. This gel was then dried, which formed a chitin sheet. The procedure was extremely easy for a biologist unfamiliar with materials engineering. Spheroids derived from HEK293T cells were formed on the chitin sheet, because the spheroids slightly attached and slowly moved on the chitin sheet.
3D cell aggregates such as spheroids and embryoid bodies exhibit different biological properties from cells grown in 2D cultures [Citation1–Citation3]. Spheroid size also affects the biological features of 3D cell aggregates [Citation4–Citation7]. Spheroids and embryoid bodies are usually formed on chemically modified pattern-controlled surfaces that either lack or exhibit low cell attachment [Citation4,Citation8,Citation9]. Culture dishes with very low or non-cell attachment surfaces are very expensive compared to culture dishes that allow for cell attachment and are difficult to fabricate in a biological laboratory [Citation4].
In our previous studies, we found that a chitosan solution reacted with acetic anhydride to form a gel, and the chitin-binding domain bound to the gel [Citation10]. Chitin-binding domain specifically binds to solid chitin, but not to chitosan (70% deacetylated) or soluble chitin [Citation11,Citation12]. The above gel had chitin equivalent portion, therefore, we referred to as a “chitin gel” [Citation10]. We also found that cells did not attach to the gel and instead formed cell aggregates, or spheroids. We dried the gel to form a sheet with a low cell attachment surface that was excellent material for generating spheroids. A biologist unfamiliar with chemical and materials engineering can easily fabricate this chitin sheet. The chitin sheet is constructed from chitosan and acetic anhydride, which are commonly found in many biological laboratories. Moreover, the chitin sheet is formed on a cell culture dish or plate without any special reaction apparatus. The chitin sheet was nontoxic to cells and exhibited no change in size at pH values ranging from 5 to 9 [Citation10]. In this report, we describe a method to fabricate the chitin sheet, along with a technique to generate uniform cell spheroids on the sheet.
Chitosan (Chitosan 10; 2 g; Wako, Japan) was dissolved in 100 mL of 0.1 M acetic acid with shaking overnight. Acetic anhydride (5 mL) was added to the solution (15 mL) and the mixture was thoroughly vortexed for 2 min. The resulting solution (2 mL) was quickly added to a 3.5 cm dish and allowed to form a gel. The gel was then dried at room temperature overnight, forming a chitin sheet. The chitin sheet was washed two to three times with Dulbecco’s phosphate buffered saline (PBS) in order to neutralize the pH. Next, the chitin sheet was sterilized overnight with 70% ethanol. Subsequently, the chitin sheet was washed three times with sterilized PBS to remove the remaining ethanol followed by immersion with culture medium. Using the same protocol, chitin sheets could be fabricated using other culture dish or plate sizes, including 6 cm and 10 cm dishes, as well as 12-well, 24-well, and 96-well plates.
HEK293T cells grown on a low cell attachment surface formed spheroids, but L929 cells grown on the same surface did not form spheroids. HEK293T and L929 cells were cultured on a cell attachment dish in DMEM containing 10% FBS and penicillin-streptomycin (Wako, Japan). The HEK293T and L929 cells were harvested with a conventional trypsin-EDTA method. The HEK293T cells (1.5 x 104 cells) with or without L929 cells (7.5 x 103 cells) were added to the chitin sheet in a 3.5 cm dish and were cultured in DMEM containing 10% FBS and penicillin-streptomycin with or without 2.5% gelatin (Nitta Gelatin, Japan). The cultures were observed with a time-lapse analyzer (JuLI, NanoEntek, Korea) in CO2 incubator.
In the HEK293T cell cultures that were grown on cell attachment dishes, the cells were attached and extended. In contrast, HEK293T cells grown on the chitin sheet were round-shaped and not attached to the surface of the chitin sheet. The round single HEK293T cells grown on the chitin sheet rapidly moved, exhibiting Brownian motion, and occasionally collided with other cells (Figure S1(a)). The HEK293T cells grown on the chitin sheet remained in the view area of the time-lapse camera. In contrast, HEK293T cells grown on the commercially available non-attachment dish PrimeSurface® (Sumitomo Bakelite, Japan), did not stay in the view area of the time-lapse camera because the cells moved more rapidly and went out of sight within 2 h at least. Based on these findings, we concluded that the chitin sheet is a low attachment surface that is significantly different from PrimeSurface®.
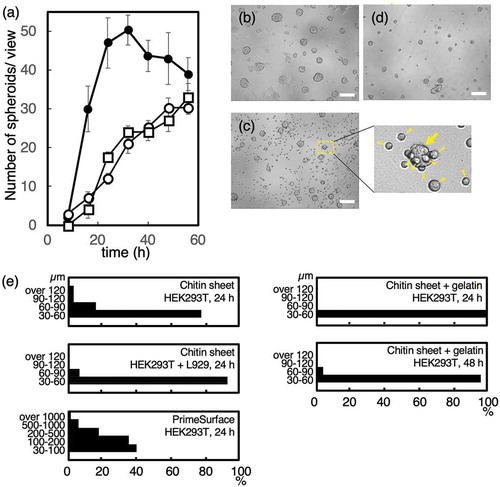
The time-lapse observation of HEK293T cells grown on the chitin sheet revealed that cell-cell binding occurred in the culture at approximately 1 h. These cells began to form spheroids at 6 to 12 h. At 16 h, several round HEK293T spheroids with a smooth surface were observed (Figure S1(a)). All of the HEK293T spheroids were attached to the chitin sheet within a limited area (Figure S1(b)). Therefore, the spheroids could be detached by tapping the culture dish. Because the spheroids were attached to the chitin sheet, cultivation medium was easily changed in comparison of non-attached spheroids culture. However, all spheroids could be harvested by tapping. We thought that the chitin sheets are good materials for spheroids cultivation. In contrast to the HEK293T cells, the L929 cells were not attached to the chitin sheet. Time-lapse imaging of L929 cells revealed that round single cells were observed at any time (Figure S1(c)). We did not find any L929 spheroids on the chitin sheet. This showed that spheroids formation on chitin sheet was strictly depend on spontaneous cell to cell interaction although L929 cells were reported to form spheroids by forced cell to cell collision using hanging drop system [Citation13].
HEK293T cells and L929 cells were co-cultivated on the chitin sheet (Figure S3). Spheroid formation in both the mixed culture and in the culture only containing HEK293T cells was observed at 8 h. At 16 h, the number of spheroids over 30 µm in diameter in the mixed culture was less than 25% of those found in the culture only containing HEK293T cells. The number of spheroids in the mixed culture increased incrementally up to 56 h. In contrast, the number of spheroids in the culture only containing HEK293T cells rapidly increased by 24 h and then decreased due to the fusion between spheroids to form larger spheroids (Figure S1(a)). In the mixed culture, the HEK293T spheroids were accompanied by round L929 cells ()). The L929 cells inhibited the formation of HEK293T spheroids by interfering with the collision between HEK293T cells. At 48 h, the L929 cells also inhibited the collision between the spheroids, resulting in a smaller spheroid size compared to cultures only containing HEK293T cells (,)).
We hypothesized that inhibiting the movement of cells and spheroids would reduce both the collision between cells and spheroids as well as the spheroid growth. Thus, in order to inhibit the movement of cells and spheroids, we introduced 2.5% gelatin to the culture media, which will increase its viscosity. At 37°C, gelatin does not form a gel and is nontoxic to cells. Spheroid formation of HEK293T cells grown with or without gelatin medium was observed at 8 h. On early phase of culture at 16 h, the number of HEK293T spheroids over 30 µm in diameter in the gelatin medium was less than 15% of those found in the culture medium lacking gelatin. The number of spheroids from mixed cultures (HEK293T and L929) or from cultures only containing HEK293T cells, both grown in the gelatin medium, increased incrementally up to 56 h ()). In the gelatin medium, the HEK293T spheroids were small ()). In addition, the HEK293T spheroids were slow-growing and large spheroids (over 60 µm in diameter) were not observed at 24 h and only 4% of large spheroids were observed at 48 h ()). A single spheroid remained at or near the same position during the 56 h time-lapse observation (Figure S4). Several spheroids also remained at or near the same position when viewed over a 24 h period. Our data revealed that the gelatin-induced viscosity of the culture medium restricted the movement of spheroids and cells, which resulted in a reduction in spheroid size.
On PrimeSurface®, HEK293T cell spheroids were rapidly formed, exhibited accelerated growth, and the fusion between spheroids occurred frequently. At 24 hr, extra-large spheroids with a long axis length > 1 mm, were observed (Figure S5). Interestingly, in the same dish, small spheroids (< 100 µm) comprised approximately 40% of the total spheroids ()). Since PrimeSurface® is a non-cell attachment surface, cells and spheroids moved rapidly and frequently fused to form spheroids more varied in size. Therefore, the shape of the spheroids was not round and instead was distorted (Figure S5).
In order to form small uniform spheroids, a fixed number of cells are often cultivated in a U bottom well plate. The cells do not attach to the bottom of the plate and form a single spheroid in a well that has a fixed size. However, the number of spheroids are restricted by the number of wells. Moreover, these plates are very expensive and difficult for biologists to fabricate.
The chitin sheet was easily fabricated with reagents from a biological laboratory and was capable of generating small uniform spheroids compared to PrimeSurface®. The low cell attachment property of the chitin sheet reduced cell and spheroid movement. Therefore, the collision of cells and spheroids was limited. On the other hand, the non-cell attachment property of PrimeSurface® permitted free and rapid cell movement that promoted cell collision and spheroid growth. Only cells seeded on the chitin sheet generated uniform spheroids. In addition, co-cultivation of L929 cells, which inhibit cell and spheroid collision, promoted the formation of more uniform spheroids compared to a culture containing only HEK293T cells. The L929 cells do not form spheroids, but they do interact with the HEK293T spheroids. Thus, we hypothesize that these properties are required for L929 cell-mediated inhibition of collision between cells and spheroids. In addition, L929 cells are known to produce extracellular matrix such as collagen [Citation14], therefore, the spheroids surface will be covered with collagen. The collagen covered spheroids are under investigation. The addition of gelatin into the cultivation medium restricted the movement of cells and spheroids, thus inhibiting their collision, which generated more uniformly sized spheroids. In conclusion, we proposed the cultivation of cells in gelatin medium or co-culture of L929 cells on chitin sheet for uniform spheroid formation. However, the cultivation on chitin sheet were sufficient to form uniform spheroids, because near 80% of spheroids were 30 to 60 µm in diameter at 24 h ()), even if without gelatin or co-culture of L929 cells.
Author contributions
YI performed all experiments. AT analyzed the data and wrote this paper. All authors approved the final version of the manuscript.
3supporting_infomation_Nd.pdf
Download PDF (8.7 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data described in this article are openly available in the Open Science Framework at DOI:http//:10.17605/OSF.IO/TPA6U.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Choi SC, Lee H, Choi JH, et al. Cyclosporin A induces cardiac differentiation but inhibits hemato-endothelial differentiation of P19 cells. PLoS One. 2015;10:e0117410.
- Ohkura T, Ohta K, Nagao T, et al. Evaluation of human hepatocytes cultured by three-dimensional spheroid systems for drug metabolism. Drug Metab Pharmacokinet. 2014;29:373–378.
- Sasai Y. Next-generation regenerative medicine: organogenesis from stem cells in 3D culture. Cell Stem Cell. 2013;12:520–530.
- Arai K, Eguchi T, Rahman MM, et al. A novel high-throughput 3D screening system for EMT inhibitors: a pilot screening discovered the EMT inhibitory activity of CDK2 inhibitor SU9516. PLoS One. 2016;11:e0162394.
- Kelm JM, Timmins NE, Brown CJ, et al. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol Bioeng. 2003;83:173–180.
- Landry J, Bernier D, Ouellet C, et al. Spheroidal aggregate culture of rat liver cells: histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J Cell Biol. 1985;101:914–923.
- Mittler F, Obeïd P, Rulina AV, et al. High-content monitoring of drug effects in a 3D spheroid model. Front Oncol. 2017;7:293.
- Napolitano AP, Dean DM, Man DM, et al. Scaffold-free three-dimensional cell culture utilizing micromolded nonadhesive hydrogels. Biotechniques. 2007;43:496–500.
- Shi W, Kwon J, Huang Y, et al. Facile tumor spheroids formation in large quantity with controllable size and high uniformity. Sci Rep. 2018;8:6837.
- Tachibana A, Yasuma D, Takahashi R, et al. Chitin degradation enzyme-responsive system for controlled release of fibroblast growth factor-2. J Biosci Bioeng. 2020;129:116–120.
- Watanabe T, Ito Y, Yamada T, et al. The roles of the C-terminal domain and type III domains of chitinase A1 from Bacillus circulans WL-12 in chitin degradation. J Bacteriol. 1994;176:4465–4472.
- Hardt M, Laine RA. Mutation of active site residues in the chitin-binding domain ChBDChiA1 from chitinase A1 of Bacillus circulans alters substrate specificity: use of a green fluorescent protein binding assay. Arch Biochem Biophys. 2004;426:286–297.
- Neto AI, Correia CR, Oliveira MB, et al. A novel hanging spherical drop system for the generation of cellular spheroids and high throughput combinatorial drug screening. Biomater Sci. 2015;3:581–585.
- Ozaki C, Somamoto S, Kawabata S, et al. Effect of an artificial silk elastin-like protein on the migration and collagen production of mouse fibroblasts. J Biomater Sci Polym Ed. 2014;25:1266–1277.