ABSTRACT
Dementia and cognitive decline have become worldwide public health problems. We have previously reported that a whey-derived glycine―threonine―tryptophan―tyrosine peptide, β-lactolin, improves hippocampus-dependent memory functions in mice. The supplementation with a whey digest rich in β-lactolin improves memory retrieval and executive function in a clinical trial, but the effect of β-lactolin on prefrontal cortex (PFC)-associated cognitive function was unclear. Here we examined the effect of β-lactolin and the whey digest on PFC-associated visual discrimination (VD) and reversal discrimination (RD) learning, using a rodent touch panel-based operant system. β-Lactolin and the whey digest significantly improved the RD learning, and the whey digest enhanced the response latency during the VD task, indicating that β-lactolin and the whey digest improve PFC-associated cognitive functions. Given the translational advantages of the touch panel operant system, consumption of β-lactolin in daily life could be beneficial for improving human PFC-associated cognitive function, helping to prevent dementia.
GRAPHICAL ABSTRACT
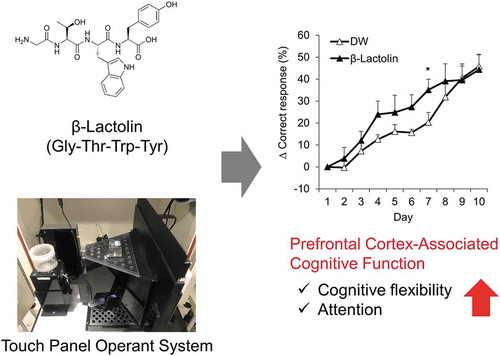
Oral administration of β-lactolin and the whey digest rich in β-lactolin improve reversal learning assessed in the rodent touch panel operant system.
Populations in many countries are aging rapidly, and cognitive decline and dementia have become worldwide public health problems. Effective therapies for dementia after onset have not been established, and strategies in daily life to prevent dementia, such as dietary habits, have received considerable attention. Several epidemiological studies have suggested that a daily intake of dairy products, including cheese and yogurt, lowers the risk of cognitive decline and dementia [Citation1–Citation3]. Intake of some dairy products has also been reported to improve cognitive decline. We have investigated mechanisms underlying the effects of dairy products, and have previously reported the preventive effects of a Penicillium candidum fermented dairy product, Camembert cheese, against Alzheimer’s disease (AD) using AD model mice [Citation4].
Spatial and episodic memory deficits are often observed as the first symptoms of AD, which are induced by the damage of medial temporal lobes including hippocampus region [Citation5]. It is known that neuronal activation in the hippocampus promotes spatial learning, spatial memory, and episodic memory. Especially, spatial learning and memory are highly dependent on the hippocampus function, mediated by location-encoding pyramidal neurons called hippocampal place cells [Citation6]. On the other hand, prefrontal cortex (PFC) plays essential roles in higher-order cognitive functions, including executive function, reward-guided learning, cognitive flexibility, or attention [Citation7]. Those PFC-associated functions are effectively assessed using visual discrimination (VD) task and reversal discrimination (RD) task. The VD learning requires the integration of perceptual learning and memory processing [Citation8], and the RD learning requires flexibility of memory function [Citation9,Citation10]. Deficits in VD and RD learning are also representative in the symptoms of AD [Citation11].
Whey is the supernatant of yogurt and a byproduct of cheese and is rich in proteins, including β-lactoglobulin and α-lactalbumin. Digestion of whey protein releases several bioactive peptides [Citation12–Citation14]. We have previously identified the glycine―threonine―tryptophan―tyrosine (GTWY) tetrapeptide β-lactolin from β-lactoglobulin, which reduces memory impairment via the activation of the dopamine system in the brain [Citation15–Citation17]. The oral administration of β-lactolin improves rodent hippocampus-dependent spatial memory impairment as assessed by the Y-maze test using scopolamine-induced amnesia model mice and aged mice. We also found that a whey digest produced by specific enzymes is rich in β-lactolin, which also reduces spatial memory impairment. Supplementation with a whey digest rich in β-lactolin improves PFC-associated working memory, memory retrieval, and executive function in healthy adults in a placebo-controlled clinical trial [Citation18,Citation19]. These findings suggest that β-lactolin is associated not only with the function of the hippocampus but also with that of the PFC. However, the effect of β-lactolin on PFC-associated cognitive function has not been previously been assessed in either humans or rodents.
Recently, touch panel-based operant systems have been developed to assess rodent brain functions, utilizing Pavlovian operant conditioning and autoshaping [Citation20–Citation22]. These apparatuses produce results from behavioral experiments, which are more readily translated to humans because of their similarity to human cognitive tests, such as the Cambridge Neuropsychological Test Automated Battery [Citation23]. Touch panel operant systems are easily modified to assess different neuropsychological functions, including PFC-associated attention, executive functions, and cognitive flexibility [Citation24]. We have previously performed visual discrimination (VD) task in scopolamine-induced amnesia model mice and subsequent reversal discrimination (RD) task and found positive effects of food components on VD, attention, and cognitive flexibility [Citation25]. Using the touch panel operant system, we investigated the effect of β-lactolin and whey digest rich in β-lactolin on VD and RD learning and found that these peptides improve attention and executive functions.
Materials and methods
Materials
β-Lactolin (GTWY peptide; purity: 98%) was purchased from Bachem (Bubendorf, Switzerland). Whey digest was prepared by Kirin Holdings Company (Tokyo, Japan) [Citation18].Briefly, whey protein was digested with a protease from effective microbe, and 0.16% of β-lactolin was contained. (–)-Scopolamine hydrobromide trihydrate was purchased from Sigma Aldrich Co. (St. Louis, MO, USA).
Animals
Seven-week-old male C57BL/6J mice were purchased from Charles River Japan Inc. (Tokyo, Japan). Mice were maintained at room temperature (23°C ± 1°C) under a constant 12-h light/dark cycles, with a light period from 8:00 am to 8:00 pm. All mice were acclimatized by feeding a standard rodent diet, CE-2 (Clea Japan, Tokyo, Japan), for 1 week. All animal care and experimental procedures were performed according to the guidelines of the Animal Experiment Committee of Kirin Company Ltd., and all efforts were made to minimize suffering. All studies were approved by the Animal Experiment Committee of Kirin Company Ltd. and conducted from January 2016 to May 2017; the approval ID was AN10163-Z00 and AN10364-Z00.
Touch panel operant test
Apparatus
The touch panel operant test apparatus was purchased from O’HARA & Co., Ltd. (Tokyo, Japan). The apparatus is surrounded by a sound-isolated chamber with a house lamp and a speaker, and this chamber is placed in a sound-isolated room maintained at 23°C ± 1°C. A CCD camera is mounted on the apparatus. The apparatus is composed of a touch panel, a pellet dispenser delivering 10 mg reward pellets, and water bottles. The touch panel is separated into two stimulus windows by a black wooden board. The reward magazine where the pellets are delivered is placed at the opposite side of the touch panel. A picture of the apparatus was provided in a previous report [Citation25].
Animal habituation
Feeding of the mice was restricted, and their body weight was reduced to around 80% of age-matched ad libitum fed mice. The body weight was measured as frequently as possible to avoid an excessive decrease in body weight. After the body weight met the criterion, mice were acclimated to the reward food pellets (AIN-76A Rodent Tablet; TestDiet, St. Louis, MO, USA) by feeding 100 pellets per mouse for three days. Mice were placed in the apparatus for 15 minutes per day for three days, without any tasks. Fifteen reward pellets were placed in the reward magazine so that mice could learn to obtain rewards from the reward magazine.
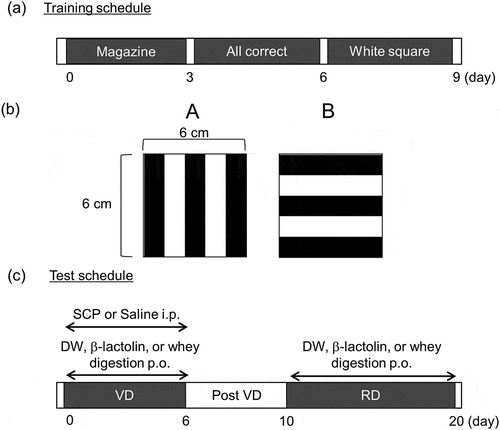
Pretraining
The pretraining schedule is shown in ). The pretraining phase consisted of three periods. (i) Reward pellets were automatically delivered every one minute for 15 minutes, accompanied by a reward tone, so that the mice could learn to seek rewards when the tone sounded. This period lasted three days. (ii) Square white pictures were presented in both stimulus windows, and reward pellets were delivered when each mouse touched any window. A session was ended following the completion of 50 trials or after 15 minutes, whichever came first. This training period was continued for three days. (iii) A square white picture was presented on one randomly chosen side of the stimulus windows, while the other side remained blank. Reward pellets were delivered with a reward tone when the mouse touched the squared white stimulus. The session was ended following the completion of 50 trials or after 15 minutes, whichever came first.
Visual discrimination task
In the VD task, a pair of stimulus pictures – a vertical stripe and a horizontal stripe ()) – appeared on the screen during each trial. Half of the mice were presented with the vertical stripe as the correct response and the horizontal stripe as the incorrect response, while the other half of the mice were presented with the opposite pairing. A trial started when the mouse touched the reward magazine. A nose poke to the correct stimulus resulted in the tone and the reward delivery, followed by a two-second intertribal interval (ITI). A nose poke to the incorrect stimulus resulted in no reward, five seconds lights out, and a five-second ITI. After each ITI, the next trial started when the mouse touched the reward magazine. A trial was omitted when a mouse did not touch any stimulus for 30 seconds. The VD task was ended following the completion of 50 trials or after 30 minutes, whichever came first. β-Lactolin (1 mg/kg body weight) dissolved in distilled water (DW), whey digest (10 mg/kg body weight) dissolved in DW, or DW alone was administered orally 60 minutes before the test, and scopolamine (0.8 mg/kg body weight) dissolved in saline or saline alone was intraperitoneally administered 30 minutes before the test. The dosages and the timings were decided according to previous reports, which expected to improve cognitive function [Citation15,Citation17].
Reversal discrimination task
To perform the RD, it is favorable that all mice successfully acquire VD learning. Before the RD task, mice performed the VD task without any drug treatment until a correct response rate of greater than 80% was achieved. In the RD task, the correct and incorrect stimuli were inverted. Mice that had been presented with the vertical stripe as the correct stimulus in the VD task were presented with the horizontal stripe as the correct stimulus in the RD task. All other conditions were the same as in the VD task. β-Lactolin (1 mg/kg body weight), whey digest (10 mg/kg body weight), or DW alone was orally administered 60 minutes before the test. Scopolamine was not used in the RD task. The testing schedule of the VD and RD experiments is shown in ).
Statistical analysis
All values are expressed as mean ± SEM. Two-group comparisons were analyzed using Student’s t-test. Correct response rates and response latency were analyzed using two-way ANOVA, followed by a Bonferroni’s test or Student’s t-test. All statistical analyses were performed using the Ekuseru–Toukei 2012 software program (Social Survey Research Information, Tokyo, Japan). Results with P < 0.05 were considered statistically significant.
Results
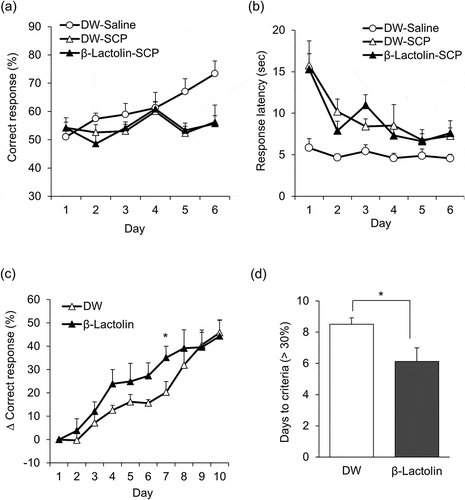
Oral administration of β-lactolin improves reversal learning
Using the touch panel operant system, we examined the effects of β-lactolin on VD and RD learning. The VD task was performed with three experimental groups: DW and saline (DW–Saline), DW and scopolamine (DW–SCP), and β-lactolin and scopolamine (β-lactolin–SCP). A significant main effect of session days (F5,100 = 4.442, P = 0.001) and treatment (F2,20 = 3.884, P = 0.037), and a significant session × treatment interaction (F10,100 = 2.186, P = 0.024) were observed. Correct response rates were significantly decreased in scopolamine-treated mice on days five and six compared with the control group, while treatment with β-lactolin did not influence correct response rates ()). The response latency was longer in the DW–SCP and β-lactolin–SCP groups than in the DW–Saline group ()). These results indicate that treatment with β-lactolin does not improve VD learning in the scopolamine-induced amnesia model.
After the VD task, we performed the RD task in order to evaluate the effects of β-lactolin on cognitive flexibility. Mice in the DW–SCP group and the β-lactolin–SCP group continued the VD task without any drug treatment so that all mice successfully learned the VD task. Mice were further treated with DW ((DW–SCP)–DW group) or β-lactolin (β-lactolin–SCP)–β-lactolin group), without scopolamine treatment and subjected to the RD task. A significant main effect of session days was observed (F9,126 = 39.95, P < 0.001), but there was no significant main effect of treatment (F1,14 = 1.250, P = 0.283) and no significant session × treatment interaction (F9,126 = 1.370, P = 0.208). Comparison of each session day revealed that oral administration of β-lactolin significantly increased the changes of correct response rate on day seven, compared with the control group ()). The time taken to reach the criterion, Δ correct response > 30%, was significantly decreased in the β-lactolin-treated group compared with the DW-treated group ()). These results indicate that β-lactolin improves cognitive flexibility as assessed by the RD task.
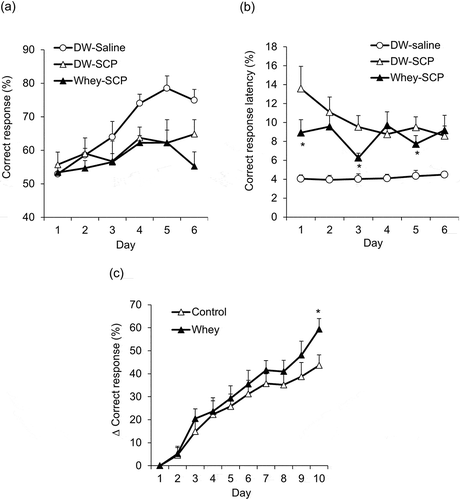
Oral administration of whey peptide improves reversal learning
We investigated the effect of the whey digest peptide-rich in β-lactolin on VD and RD learning. The VD task was performed by three groups: DW–Saline, DW–SCP, and whey peptide and scopolamine (Whey–SCP). A significant main effect of session days (F5,95 = 7.191, P < 0.001) and treatment (F2,19 = 10.48, P < 0.001) was detected, but there was no significant session × treatment interaction (F10,95 = 1.647, P = 0.105). Post-hoc comparison between the groups showed that the correct response rate in the DW–SCP group was significantly decreased compared with that of the DW–Saline group and treatment with whey peptide did not improve the correct response rate ()). We also evaluated the response latency during the sessions. A significant main effect of session days was observed (F2,21 = 17.94, P < 0.001), but there was no significant main effect of treatment (F5,105 = 8.629, P = 0.132) and no significant session × treatment interaction (F10,105 = 1.617, P = 0.112). Treatment with scopolamine significantly prolonged response latency compared with saline treatment. Comparisons of each session day revealed that the response latency in the Whey–SCP group was significantly shorter than that of the DW–SCP group on days one, three, and five ()). These results indicate that treatment with whey peptide does not improve scopolamine-induced VD learning impairment, but does improve scopolamine-induced attention deficits.
Mice in the DW–SCP group and Whey–SCP group were subsequently subjected to the RD task. A significant main effect of session days was observed (F9,144 = 52.34, P < 0.001), but there was no significant main effect of treatment (F1,16 = 0.856, P = 0.369) and no significant session × treatment interaction (F9,144 = 1.035, P = 0.415). Comparisons of each session day revealed that the changes in correct response rates in whey peptide-treated mice increased significantly compared with those of DW-treated mice on day 10 ()), indicating that treatment with whey peptide improved cognitive flexibility as assessed by the RD task.
Discussion
We have previously reported that β-lactolin and a whey digest rich in β-lactolin improves hippocampus-dependent spatial memory as assessed by the Y-maze test using a scopolamine-induced amnesia mouse model [Citation15]. However, the effect of these peptides on PFC-associated cognitive function had not been investigated until now. In the study presented here, we showed that treatment with β-lactolin and whey digest improved correct response rates in the RD task, suggesting that β-lactolin improves cognitive flexibility which is the function of PFC. The results presented here show that β-lactolin and whey digest improved PFC-associated cognitive function in addition to hippocampus-dependent memory. However, these peptides did not improve the correct response rates in the VD task under the present conditions. β-Lactolin and whey digest may not affect the learning of the VD but may enhance the retrieval or changing of acquired memories. Acquisition of memory is associated with both the PFC and the hippocampus, while memory retrieval is more closely associated with the functioning of the PFC. In clinical trials, consumption of whey digest improved memory retrieval in a verbal fluency test and in a visual paired associated working memory test but did not improve memory acquisition in a verbal immediate recall test. These results are consistent with those of the present study.
Previous reports have shown that dopaminergic system is involved in the effects of β-lactolin. Pharmacokinetic studies have revealed that β-lactolin and its core sequence, tryptophan―tyrosine (WY) peptide are effectively distributed to PFC and inihibit the activity of monoamine oxidase-B [Citation15,Citation26]. Since various WY-related peptides enhance memory functions, β-lactolin or its digested peptides would be the active compounds. The dopaminergic system, particularly in the frontal cortex, is crucial for cognitive functions, including memory retrieval, attention, and cognitive flexibility [Citation27,Citation28]. Touch panel operant experiments using rodents and marmosets have revealed that dopamine signaling is required for RD learning [Citation29,Citation30]. The improvement in RD learning induced by whey peptide and β-lactolin observed in the present study could be induced by the enhancement of dopamine signaling. Further studies are needed to investigate the involvement of the dopamine system in the improvement of RD learning induced by β-lactolin.
We demonstrated that administration of whey peptide, reduced the response latency in the VD task, which may be associated with attention [Citation31,Citation32]. Attention deficits are induced by a cholinergic neuronal loss [Citation33,Citation34], or treatment with scopolamine [Citation35]. Though the VD task was not specifically designed to evaluate attention, this result suggests that whey peptide improves scopolamine-induced attention deficits. In clinical trials, consumption of whey peptide improved attention in a visual cancellation test, consistent with the results of the present study. On the other hand, β-lactolin did not affect the response latency in the VD task. There is a possibility that unidentified peptides other than β-lactolin might contribute to the attention improvement.
Executive function is defined as a set of higher order cognitive functions, including inhibition, updating, and shifting, that enables individuals to successfully achieve goal-directed behavior [Citation7]. We used rodent touch panel operant systems and found that intake of β-lactolin and the whey digest improved cognitive flexibility. Response inhibition is also required in reversal learning [Citation36]. In clinical trials, whey digest also improved the executive function in the Stroop test. These results support the contention that β-lactolin improves PFC-associated cognitive function. Executive function deficits have been observed in neurodegenerative diseases, including AD [Citation37], Huntington’s disease (HD) [Citation38], and schizophrenia [Citation39]. Cognitive flexibility deficits in the RD task have been detected in touch panel operant systems using AD [Citation40,Citation41], HD [Citation42], and schizophrenia [Citation43] model rodents. Whey peptide and β-lactolin might be effective in treating these diseases.
In conclusion, we evaluated the effects of β-lactolin and whey peptide on PFC-associated cognitive functions using a touch panel-based operant system and revealed that treatment with β-lactolin and whey peptide improves cognitive flexibility. Since whey is a natural compound that has been consumed for centuries, a daily intake of β-lactolin-rich whey peptide might be a safe and effective approach to assist with the prevention of cognitive decline and dementia.
Author contributions
T.A. and Y.A. designed the most of experiments. T.A. and R.O. performed the experiments and analyzed the data. T.A. wrote the most of paper and Y.A. conceived and supervised the paper.
Disclosure statement
All authors are employed by Kirin Holdings Company, Ltd.
References
- Camfield DA, Owen L, Scholey AB, et al. Dairy constituents and neurocognitive health in ageing. Br J Nutr. 2011 Jul;106(2):159–174. PubMed PMID: 21338538.
- Crichton GE, Murphy KJ, Bryan J. Dairy intake and cognitive health in middle-aged South Australians. Asia Pac J Clin Nutr. 2010;19(2):161–171. PubMed PMID: 20460228; eng.
- Ozawa M, Ninomiya T, Ohara T, et al. Dietary patterns and risk of dementia in an elderly Japanese population: the Hisayama Study. Am J Clin Nutr. 2013 May;97(5):1076–1082. PubMed PMID: 23553168.
- Ano Y, Ozawa M, Kutsukake T, et al. Preventive effects of a fermented dairy product against Alzheimer’s disease and identification of a novel oleamide with enhanced microglial phagocytosis and anti-inflammatory activity. PLoS One. 2015;10(3):e0118512. PubMed PMID: 25760987; PubMed Central PMCID: PMCPMC4356537. eng.
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002 Aug 15;35(4):625–641. PubMed PMID: 12194864; eng.
- O’Keefe J. Do hippocampal pyramidal cells signal non-spatial as well as spatial information? Hippocampus. 1999;9(4):352–364. PubMed PMID: 10495018; eng. DOI:10.1002/(sici)1098-1063(1999)9:4<352::Aid-hipo3>3.0.Co;2-1.
- Logue SF, Gould TJ. The neural and genetic basis of executive function: attention, cognitive flexibility, and response inhibition. Pharmacol Biochem Behav. 2014 Aug;123:45–54. PubMed PMID: 23978501; PubMed Central PMCID: PMCPMC3933483.
- Gilbert CD, Sigman M, Crist RE. The neural basis of perceptual learning. Neuron. 2001 Sep 13;31(5):681–697. PubMed PMID: 11567610; eng.
- Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol. 2010 Apr;20(2):199–204. PubMed PMID: 20167474.
- Klanker M, Feenstra M, Denys D. Dopaminergic control of cognitive flexibility in humans and animals. Front Neurosci. 2013 Nov 5;7:201. PubMed PMID: 24204329; PubMed Central PMCID: PMCPMC3817373.
- Freedman M, Oscar-Berman M. Spatial and visual learning deficits in Alzheimer’s and Parkinson’s disease. Brain Cogn. 1989 Sep;11(1):114–126. PubMed PMID: 2789813; eng.
- Song JJ, Wang Q, Du M, et al. Identification of dipeptidyl peptidase-IV inhibitory peptides from mare whey protein hydrolysates. J Dairy Sci. 2017 Sep;100(9):6885–6894. PubMed PMID: 28711271; eng.
- Pandey M, Kapila R, Kapila S. Osteoanabolic activity of whey-derived anti-oxidative (MHIRL and YVEEL) and angiotensin-converting enzyme inhibitory (YLLF, ALPMHIR, IPA and WLAHK) bioactive peptides. Peptides. 2018 Jan;99:1–7. PubMed PMID: 29122669; eng.
- Yamada A, Mizushige T, Kanamoto R, et al. Identification of novel beta-lactoglobulin-derived peptides, wheylin-1 and −2, having anxiolytic-like activity in mice. Mol Nutr Food Res. 2014 Feb;58(2):353–358. PubMed PMID: 24039078; eng.
- Ano Y, Ayabe T, Kutsukake T, et al. Novel lactopeptides in fermented dairy products improve memory function and cognitive decline. Neurobiol Aging. 2018 Dec;72:23–31. PubMed PMID: 30176402; eng.
- Ano Y, Ayabe T, Ohya R, et al. Tryptophan-tyrosine dipeptide, the core sequence of beta-lactolin, improves memory by modulating the dopamine system. Nutrients. 2019 Feb 6;11(2):348. PubMed PMID: 30736353; PubMed Central PMCID: PMCPMC6412195. eng.
- Ayabe T, Ano Y, Ohya R, et al. The lacto-tetrapeptide gly-thr-trp-tyr, beta-lactolin, improves spatial memory functions via dopamine release and D1 receptor activation in the hippocampus. Nutrients. 2019 Oct 15;11(10). PubMed PMID: 31618902; PubMed Central PMCID: PMCPMC6835598. eng. DOI:10.3390/nu11102469.
- Kita M, Kobayashi K, Obara K, et al. Supplementation with whey peptide rich in β-lactolin improves cognitive performance in healthy older adults: a randomized, double-blind, placebo-controlled study [clinical trial]. Front Neurosci. 2019 April 24;13(399). English. DOI:10.3389/fnins.2019.00399.
- Kita M, Obara K, Kondo S, et al. Effect of supplementation of a whey peptide rich in tryptophan-tyrosine-related peptides on cognitive performance in healthy adults: a randomized, double-blind, placebo-controlled study. Nutrients. 2018 Jul 13;10(7). PubMed PMID: 30011836; PubMed Central PMCID: PMCPMC6073406. DOI:10.3390/nu10070899.
- Lopez JC, Karlsson RM, O’Donnell P. Dopamine D2 modulation of sign and goal tracking in rats. Neuropsychopharmacology. 2015 Aug;40(9):2096–2102. PubMed PMID: 25759299; PubMed Central PMCID: PMCPMC4613614. eng. .
- Parkinson JA, Willoughby PJ, Robbins TW, et al. Disconnection of the anterior cingulate cortex and nucleus accumbens core impairs Pavlovian approach behavior: further evidence for limbic cortical-ventral striatopallidal systems. Behav Neurosci. 2000 Feb;114(1):42–63. PubMed PMID: 10718261; eng.
- Tomie A, Di Poce J, Aguado A, et al. Effects of autoshaping procedures on 3H-8-OH-DPAT-labeled 5-HT1a binding and 125I-LSD-labeled 5-HT2a binding in rat brain. Brain Res. 2003 Jun 13;975(1–2):167–178. PubMed PMID: 12763605; eng.
- Barnett JH, Blackwell AD, Sahakian BJ, et al. The Paired Associates Learning (PAL) test: 30 years of CANTAB translational neuroscience from laboratory to bedside in dementia research. Curr Top Behav Neurosci. 2016;28:449–474. PubMed PMID: 27646012; eng. .
- Brigman JL, Rothblat LA. Stimulus specific deficit on visual reversal learning after lesions of medial prefrontal cortex in the mouse. Behav Brain Res. 2008 Mar 5;187(2):405–410. PubMed PMID: 18022704; eng.
- Ayabe T, Ohya R, Ano Y. Hop-derived iso-alpha-acids in beer improve visual discrimination and reversal learning in mice as assessed by a touch panel operant system. Front Behav Neurosci. 2019;13:67. PubMed PMID: 31001094; PubMed Central PMCID: PMCPMC6454052. eng. .
- Ano Y, Yoshino Y, Kutsukake T, et al. Tryptophan-related dipeptides in fermented dairy products suppress microglial activation and prevent cognitive decline. Aging (Albany NY). 2019 May 23;11(10):2949–2967. PubMed PMID: 31121563; PubMed Central PMCID: PMCPMC6555451. eng.
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005 Jun 1;57(11):1377–1384. PubMed PMID: 15950011; eng.
- Puig MV, Rose J, Schmidt R, et al. Dopamine modulation of learning and memory in the prefrontal cortex: insights from studies in primates, rodents, and birds. Front Neural Circuits. 2014;8:93. PubMed PMID: 25140130; PubMed Central PMCID: PMCPMC4122189. eng.
- Morita M, Wang Y, Sasaoka T, et al. Dopamine D2L receptor is required for visual discrimination and reversal learning. Mol Neuropsychiatry. 2016 Oct;2(3):124–132. PubMed PMID: 27867937; PubMed Central PMCID: PMCPMC5109995.
- Takaji M, Takemoto A, Yokoyama C, et al. Distinct roles for primate caudate dopamine D1 and D2 receptors in visual discrimination learning revealed using shRNA knockdown. Sci Rep. 2016 Nov 2;6:35809. PubMed PMID: 27805010; PubMed Central PMCID: PMCPMC5090965.
- Dickson PE, Calton MA, Mittleman G. Performance of C57BL/6J and DBA/2J mice on a touchscreen-based attentional set-shifting task. Behav Brain Res. 2014 Mar 15;261:158–170. PubMed PMID: 24361287; PubMed Central PMCID: PMCPMC4060595. eng.
- Dickson PE, Cairns J, Goldowitz D, et al. Cerebellar contribution to higher and lower order rule learning and cognitive flexibility in mice. Neuroscience. 2017 Mar;14(345):99–109. PubMed PMID: 27012612; PubMed Central PMCID: PMCPMC5031514. eng.
- Voytko ML, Olton DS, Richardson RT, et al. Basal forebrain lesions in monkeys disrupt attention but not learning and memory. J Neurosci. 1994 Jan;14(1):167–186. PubMed PMID: 8283232; eng.
- Bucci DJ, Holland PC, Gallagher M. Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. J Neurosci. 1998 Oct 1;18(19):8038–8046. PubMed PMID: 9742170; eng.
- Dillon GM, Shelton D, McKinney AP, et al. Prefrontal cortex lesions and scopolamine impair attention performance of C57BL/6 mice in a novel 2-choice visual discrimination task. Behav Brain Res. 2009 Dec 1;204(1):67–76. PubMed PMID: 19416740; eng.
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013 Sep;108:44–79. PubMed PMID: 23856628; eng.
- Kirova AM, Bays RB, Lagalwar S. Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. Biomed Res Int. 2015; 2015:748212. PubMed PMID: 26550575; PubMed Central PMCID: PMCPMC4624908. eng. .
- Maurage P, Heeren A, Lahaye M, et al. Attentional impairments in Huntington’s disease: a specific deficit for the executive conflict. Neuropsychology. 2017 May;31(4):424–436. PubMed PMID: 28240935; eng.
- Zai G, Robbins TW, Sahakian BJ, et al. A review of molecular genetic studies of neurocognitive deficits in schizophrenia. Neurosci Biobehav Rev. 2017 Jan;72:50–67. PubMed PMID: 27866942; eng.
- Romberg C, Horner AE, Bussey TJ, et al. A touch screen-automated cognitive test battery reveals impaired attention, memory abnormalities, and increased response inhibition in the TgCRND8 mouse model of Alzheimer’s disease. Neurobiol Aging. 2013 Mar;34(3):731–744. PubMed PMID: 22959727; PubMed Central PMCID: PMCPMC3532594. eng.
- Romberg C, Mattson MP, Mughal MR, et al. Impaired attention in the 3xTgAD mouse model of Alzheimer’s disease: rescue by donepezil (Aricept). J Neurosci. 2011 Mar 2;31(9):3500–3507. PubMed PMID: 21368062; PubMed Central PMCID: PMCPMC3066152. eng.
- Piiponniemi TO, Parkkari T, Heikkinen T, et al. Impaired performance of the Q175 mouse model of Huntington’s disease in the touch screen paired associates learning task. Front Behav Neurosci. 2018;12:226. PubMed PMID: 30333735; PubMed Central PMCID: PMCPMC6176131. eng.
- Brigman JL, Padukiewicz KE, Sutherland ML, et al. Executive functions in the heterozygous reeler mouse model of schizophrenia. Behav Neurosci. 2006 Aug;120(4):984–988. PubMed PMID: 16893304; eng.