ABSTRACT
Crude extracts and phytochemical compounds derived from Annona muricata leaves have been demonstrated to exert neuroprotective effects. However, the neuroprotective effects of Annona muricata leaves-derived polysaccharide extracts (ALPs) have not been investigated. ALP treatment was shown to induce concentration-dependent antioxidant activity in HT22 cells, and to increase cell viability in H2O2-treated HT22 cells. These effects were correlated with a decrease in major components of oxidation, including: Ca2+, ROS, and malondialdehyde (MDA). Mediators of the intracellular response to oxidation, including Bax, cytochrome c, and cleaved caspases-3, -8, -9, MAPKs, and NF-κB, were positively influenced by ALP treatment under conditions of H2O2-mediated oxidative stress. In addition, ALP restored the expression of superoxide dismutase (SOD) and associated signaling pathways (PARP, PI3K/AKT and Nrf2-mediated HO-1/NQO-1) following H2O2 treatment. These results provide new pharmacological evidence that ALP facilitates neuroprotection via prevention of neuronal oxidative stress and promotion of cell survival signaling pathways.
Abbreviations
ABTS: 2,2ʹ-azino-bis-(3-ethylbenzothiazoline-6-sulfonicacid); AD: Alzheimer’s disease; ALP: polysaccharide extracts isolated from Annona muricata leaves; ARE: antioxidant response element; DPPH: 1,1-diphenyl-picrylhydrazyl; DCFH-DA: 2ʹ,7ʹ-dichlorofluorescin diacetate; ECL: electrochemiluminescence; ERK: extracellular regulated kinase; FBS: Fetal bovine serum; FITC: fluorescein isothiocyanate; FRAP: ferric reducing antioxidant power; HO-1: Heme oxygenase-1; JNK: c-jun N-terminal kinase; MAPKs: mitogen-activated protein kinases; MDA: malondialdehyde; MMP: mitochondrial membrane potential; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide; NQO1: NAD(P)H:quinine oxidoreductase 1, Nrf2: nuclear factor-E2-related factor 2; PD: parkinson’s disease; PI3K: phosphatidylinositol-3kinase; PVDF: polyvinylidene difluoride; ROS: reactive oxygen species; SOD: Superoxidedismutase; TPTZ: tripydyltriazine
GRAPHICAL ABSTRACT
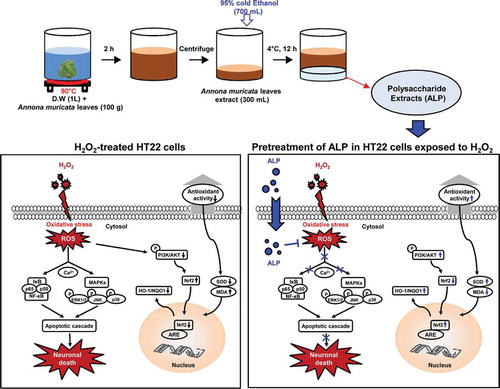
Proposed mechanism of the neuroprotective activity induced by ALP in H2O2-treated HT22 hippocampus cells.
A hallmark of neurodegenerative diseases, including Huntington’s disease (HD), Alzheimer’s disease (AD) and Parkinson’s disease (PD), is the disruption of redox (reduction/oxidation) homeostasis, triggering oxidative stress to biomolecules, thereby compromising neuronal cell function [Citation1,Citation2]. Importantly, oxidative stress arising from the excessive generation of free radicals has been demonstrated to cause cellular damage, alter calcium (Ca2+) homeostasis, and lead to mitochondrial DNA damage. The end result is neuronal dysfunction and neurodegeneration [Citation3,Citation4]. Reactive oxygen species (ROS), which is a group of free radicals, were recently implicated in the pathogenesis of HD, AD, and PD [Citation1,Citation4,Citation5]. Under normal physiological condition, ROS are essential signaling molecules that transmit signals for normal physiological processes. High levels of ROS within a nerve cell can lead to cell death by processes including apoptosis and/or necrosis [Citation6]. In particular, mitochondrial membrane injury can occur, leading to release of the pro-apoptotic proteins Bid and Bax [Citation7]. Taken together, ROS can induce various factors leading to the destruction or degeneration of nerve cells. Drug development for neurodegenerative diseases should therefore focus on the identification of compounds capable of inhibiting the ROS activity in neuronal cells.
Plant-derived compounds are a potential source for use in the fields of nutrition and medicine, and have been investigated for therapeutic potential to treat various diseases, including cardiovascular disease, cancer, asthma, rheumatoid arthritis, infectious disease, and neurodegenerative disease [Citation8–Citation11]. Crude extracts and phytochemical compounds derived from Annona muricata leaves (Annona muricata L.) have been demonstrated to promote the activity of the antioxidant enzyme superoxide dismutase (SOD). SOD neutralizes ROS to the less harmful compound hydrogen peroxide, and reduces malondialdehyde (MDA). MDA is used as a marker of oxidative stress in various disease models, including wound healing, diabetes, and arthritis [Citation12–Citation14]. Based on these results, Annona muricata L. represents a potential source of therapeutic compounds to combat neurodegenerative disease due to the presence of antioxidant activity. However, several isolated compounds, including reticuline, coreximine alkaloids, and solamin have been demonstrated to induce neurotoxicity and neurodegenerative disease [Citation15]. Thus, the selection and discovery of compounds possessing both nontoxic and antioxidant activity are critically important for effective drug development against neurodegenerative disease.
Although many previous reports have described the protective roles of extracts and phytochemical compounds from Annona muricata L. in various disease models, the potential neurotoxicity of polysaccharides contained in Annona muricata L remains unknown. In this study, we evaluated the neuropharmacological mechanisms of polysaccharides isolated from Annona muricata L., with the goal of identifying potential candidate compounds for the treatment of neurodegenerative disease. To identify candidate compounds, an in vitro oxidative stress model using hippocampal neuronal cells was employed. In addition, we investigated the molecular mechanisms responsible for polysaccharide-induced protection against oxidative stress.
Materials and methods
Preparation of Annona muricata L. polysaccharides
Annona muricata L. was freeze-dried (VD-800F; Taitec, Saitama-ken, Japan). The lyophilized sample was crushed into small granules for polysaccharide extraction. 100 g lyophilized powder per 1-liter deionized water (DW) was stirred at 90°C for 2 h and then vacuum filtered using Whatman No. 42 filter paper. The filtered solution was extracted using ethanol (final concentration 70%) at 4°C for 12 h. The solution was centrifuged at 10,000 × g for 15 min to obtain the precipitate, which was subsequently dialyzed (MWCO 10 ~ 12 kDa) against DW to remove monosaccharides and other components. After dialysis, the precipitate was lyophilized and designated as Annona muricata L. polysaccharide (ALP) extract for further study.
Monosaccharide composition analysis
To analyze monosaccharide composition in ALP, ALP extracts were analyzed by high-performance anion-exchange chromatography (HPAEC) after acid hydrolysis. HPAEC was performed on a Dionex ICS-5000 system (Dionex Corp., Sunnyvale, CA, USA) using a Carbo PACTM PA1 column (4.5 × 250 mm) in with a CarboPac guard column. Detection was performed by pulsed amperometric detection (PAD) detector. Monosaccharides were eluted isocratically using 16 mM NaOH.
Cell culture
Mouse hippocampal neuronal cell line (HT22) was cultured at 37°C in the presence of 5% CO2 using DMEM (GIBCO, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, GIBCO), and 1% antibiotics (penicillin/streptomycin, Lonza, Basel, Switzerland).
DPPH and ABTS radical scavenging assay
The essence of 2,2-diphenyl-1-picrylhydrazyl (DPPH, Sigma-Aldrich, St. Louis, Mo, USA) method is that the antioxidants react with the stable free radical 2,2-diphenyl-1-picrylhydrazyl (violet color) and convert it to 2,2-diphenyl-1-picrylhydrazine with discoloration. The degree of discoloration indicates the scavenging potentials of antioxidant [Citation16]. DPPH radical scavenging assay was carried out according to the manufacturer’s protocol. Briefly, 100 μL of the freshly prepared radical solution was added to 100 μL of sample extract (ALP; 0.25, 0.5, 1 and 2.5 and 5 mg/mL) and Vitamin C (1 mM, used as a positive control for antioxidant activity). The reactions were incubated at room temperature (RT) for 30 min, and samples were measured at 520 nm absorbance using a microplate reader (BioTek instruments, Winooski, VT, USA). The 2,2ʹ-azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS, Sigma-Aldrich) assay measures the relative ability of antioxidant to scavenge the ABTS generated in aqueous phase [Citation16]. ABTS radical scavenging assay was carried out according to the manufacturer’s protocol. Briefly, ABTS radicals were prepared by reacting ABTS (7 mM) with potassium persulfate (140 mM) in water. Reactions were initiated by adding 180 μL of the freshly prepared ABTS radical solution to 20 μL of sample extracts. The reactions were incubated at RT for 30 min, and absorbance was measured at 760 nm using a microplate reader.
FRAP assay
The ferric reducing/antioxidant power (FRAP, Sigma-Aldrich) assay is based on detecting the capacity of samples to reduce ferric irons, which is analyzed as an absorbance change of ferrous irons by antioxidants [Citation16]. The FRAP assay was modified as follows. The FRAP reagent consisted of 10 mM TPTZ (ferrous iron) and 40 mM HCl were added to 300 mM sodium acetate buffer (pH 3.6) at 37°C for 15 min. Reactions were initiated by adding 750 μL of the freshly prepared FRAP reagent to 50 μL sample extracts. Absorbance was measured at 593 nm.
Reducing power assay
The reducing power assay is based on analysis of the antioxidant capacity to reduce ferric ions to ferrous ions [Citation17]. The reducing power assay was carried out according to the manufacturer’s protocol. Briefly, sample extracts (100 μL) were mixed with 200 mM sodium phosphate buffer (pH 6.6) and 1% potassium ferricyanide. The mixture was incubated at 50°C for 20 min. After 10% trichloroacetic acid (w/v) was added, the mixture was centrifuged at 12,000 rpm for 10 min. The upper layer (100 μL) was mixed with 100 μL deionized water and 20 μL ferric chloride (0.1%). The absorbance was measured at 700 nm.
Measurement of cell viability
To investigate cell viability, H2O2-treated and non-treated HT22 cells, in the absence and the presence of ALP, were analyzed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) assay (Sigma-Aldrich) and Annexin V/PI staining (BD PharMingen, San Jose, CA, USA). HT22 cells were treated with H2O2 (0 to 1000 μM) or ALP (31.2, 62.5, 125, 250 μg/mL) at various times (0 to 8 h). An additional plate of cells was treated with ALP (31.2, 62.5, 125, 250 μg/mL) for 2 h prior to treatment with H2O2 (500 μM) for 5 h at 37°C. For the MTT assay, MTT solution (1 mg/mL) was added to each well, and the plate was incubated at 37°C for 5 h. Formazan crystals were dissolved in 100 μL DMSO and the absorbance was measured at 517 nm using a microplate reader. For Annexin V/PI staining, cells were stained using the Annexin V/PI apoptosis Detection kit protocol. The stained cells were analyzed using a MACSQuant flow cytometer (Miltenyi, Bergisch-Gladbach).
Cell cycle analysis
HT22 cells were treated with ALP (250 μg/mL) for 2 h prior to treatment with H2O2 (500 μM) for 5 h at 37°C. The cells were harvested, washed twice with PBS, and fixed using 70% ethanol for 30 min. Next, cells were washed three times with PBS and stained with FxCycleTM PI/RNase Staining Solution (Thermo Scientific, Waltham, MA) for 15 min according to the manufacturer’s instructions. The cells were analyzed using a MACSQuant flow cytometer (Miltenyi, Bergisch-Gladbach).
Hoechst and JC-1 staining
HT22 cells were pretreated with ALP (250 µg/mL) for 2 h prior to H2O2 treatment for 5 h at 37°C. Cells were subsequently analyzed by flow cytometry analysis of 5,5ʹ,6,6ʹ-tetrachloro-1,1ʹ,3,3ʹ-tetraethyl-benzimidazolylcarbocyanine chloride (JC-1) and Hoechst staining and fluorescence microscopy (Etaluma, Carlsbad, CA, USA). For JC-1 staining, cells were washed three times with PBS and stained with JC-1 (Sigma-Aldrich) for 15 min at 37°C. For Hoechst staining, the cells were harvested, washed twice with PBS, and stained with the cell-permeable DNA dye Hoechst 33258 (Sigma-Aldrich) for 20 min at 37°C. The stained cells were then observed under flow cytometry and confocal laser scanning microscope (Zeiss, LSM510 Meta).
Measurement of ROS production and cytosolic calcium levels
HT22 cells were pretreated with ALP (250 µg/mL) for 2 h prior to H2O2 treatment for 5 h at 37°C. For analysis of cytosolic calcium (Ca2+) concentration, cells were loaded with 2 µM of Fluo-3/AM (Invitrogen, Carlsbad, CA, USA) in the presence of 0.02% pluronic F-127 (Sigma-Aldrich) for 30 min. For measurement of intracellular ROS production, cells were stained with 10 μM of the probe 2ʹ,7ʹ-dichlorofluorescin diacetate (DCFH-DA, Sigma-Aldrich) for 20 min. Fluorescence intensity was determined using flow cytometry and fluorescence microscopy.
Measurement of intracellular SOD and MDA levels
Cells were lysed using the freeze-thaw method (−20°C for 20 min, then 37°C bath 10 min, repeated twice). Lysates were centrifuged at 12,000 rpm for 20 min at 4°C, and protein concentration in the supernatants was analyzed using the BCA protein assay (Thermo scientific). SOD (Dojindo molecular Technologies, Rockville, MD) and MDA (OXIS Health Products, Portland, USA) levels were analyzed using quantified supernatants according to the manufacturer’s instructions.
Western blotting
Following pre-treatment with ALP for 2 h, cytosolic extracts from H2O2-treated cells were isolated using in 200 μl of lysis buffer (Thermo scientific). Nuclear extracts from cells were isolated using a Cellytic nuclear extraction kit (Sigma-Aldrich) according to the manufacturer’s protocol. The protein concentration was determined using the BCA protein assay. Proteins were separated using 10% SDS–PAGE and electrically transferred to a PVDF membrane. The membranes were blocked in 5% skim milk and incubated with the respective primary antibodies (anti-Bcl-2, anti-Bax, anti- Cytochrome C, anti-pro-caspase 3, anti-cleaved-caspase 3, anti-pro-caspase 8, anti-cleaved-caspase 8, anti-pro-caspase 9, anti-cleaved-caspase 9, anti-PARP, anti-p38, anti-ERK, anti-JNK, anti-phospho-PI3K, anti-phospho-Akt, anti-phospho-NF-κB, anti-Nrf2, anti-lamin B, anti-β-actin, anti-HO-1 and anti-NQO-1 antibody). Primary antibodies were incubated with PVDF membranes overnight at 4°C (1:1000 dilution). The following day, membranes were incubated with HRP-conjugated secondary antibody (anti-mouse IgG Ab and anti-rabbit Ab, all secondary antibodies were used in 1:2000 dilution) for 1 h at RT. The proteins were visualized using the electrochemiluminescence (ECL) advance kit (Millipore, Merck KGaA, Darmstadt, Germany). The primary and secondary antibodies were obtained from Cell signaling Technology (Danvers, MA, USA) and Calbiochem (San Diego, CA, USA), respectively.
Statistical analysis
The levels of significance for comparison between samples were determined by Tukey’s multiple comparison test distribution and unpaired t-test using statistical software (GraphPad Prism Software, version 5; GraphPad Software, San Diego, CA). The data in the graphs are expressed as means. Each value of *p < 0.05, **p < 0.01 or ***p < 0.001 was considered to be statistically significant.
Results
Monosaccharide composition of ALP
The monosaccharide composition of ALP extracts inspected by HPAEC and their chromatographic separation is given in . Among major sugars in ALP, galactose appeared as significantly higher (64.3%), followed by glucose (25.37%), mannose (9.81%), and fucose (0.51%). Furthermore, glucosamine (0.93%) and galactosamine (0.06%), which are the amino derivatives of glucose and galactose, respectively, were also detected in ALP.
Table 1. Monosaccharide composition of ALP.
ALP increased antioxidant activity
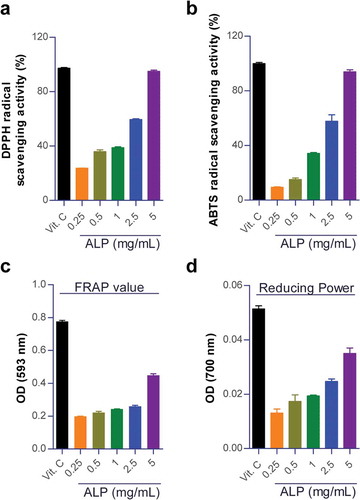
Previous studies have shown that compounds as potent free radical scavenger have neuroprotective effects in animal models of traumatic brain injury or ischemic stroke [Citation18–Citation21]. The free radicals are produced as a by-product of many cellular reactions and their excess production leads to oxidative stress [Citation22]. Importantly, antioxidant agents terminate oxidative chain reactions by eliminating free radical intermediates, thus helping to reduce oxidative tissue damages [Citation18,Citation23]. We firstly conducted DPPH free radical scavenging assay and ABTS radical cation decolonization assay to evaluate the antioxidant potential via the analysis of free radical scavenging activity of ALP. These analyzes are based on the inhibition of the DPPH and ABTS radicals by antioxidants. We also analyzed FRAP assay and reducing power assay to investigate the antioxidant capacity to reduce ferric irons to ferrous irons. As shown in , ALP (0.25 to 500 mg/mL) showed a dose-dependent induction of radical scavenging activities and ferric reducing abilities. Based on these results, we hypothesized that ALP influences would induce free radical scavenging activity in oxidative stress-induced hippocampal neuronal cells and these results indicate the protective effect against oxidative tissue damages.
ALP decreased neuronal cell death induced by H2O2 treatment in HT22 cells
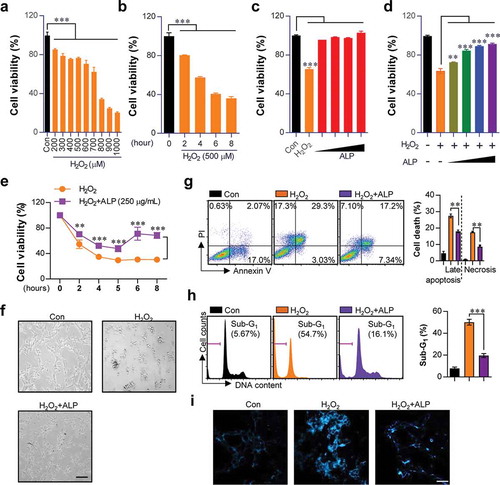
On the basis of the above results, we next investigated whether ALP that induce antioxidant activity is able to induce protective effect against ROS-induced oxidative stress. H2O2 causes neuronal cell damage following excessive ROS production [Citation24]. To confirm neuronal damage occurs following H2O2 treatment, we treated HT22 cells with a range of H2O2 concentrations (200 to 1000 μM H2O2 treatment for 5 h), and treatment durations (500 μM H2O2 treatment for 2 to 8 h). As shown in ), H2O2 concentrations over 800 μM led to pronounced cellular damage in HT22 cells. H2O2 concentrations from 500 μM were used for subsequent experiments. 500 μM H2O2 treatment for 6 h reduced cell viability by 50%. Thus, a stimulation time of 5 h was chosen for subsequent experiments. Next, we analyzed whether ALP was capable of increasing cell viability. However, no significant increase in HT22 viability was observed following dose-dependent ALP treatments (31.25, 62.5, 125, 250 μg/mL) ()). To evaluate whether ALP could increase cell viability in the context of H2O2-induced oxidative stress, HT22 cells were pre-treated with ALP at various concentrations (31.25, 62.5, 125, 250 μg/mL) and durations of treatment (2 to 8 h), and then treated with H2O2 (500 μM) for 5 h (). As shown in , time- and dose-dependent ALP treatment increased cell viability in H2O2-treated cells. The increased cell viability induced by ALP treatment was also confirmed by morphological assessment, Annexin V/PI staining (Necrotic cells; Annexin V−/PI+ cells, late apoptotic cells; Annexin V+/PI+, early apoptotic cells; Annexin V+/PI−), cell cycle analysis and Hoechst staining. Notable reductions in morphological damage ()), necrosis ()), late apoptosis ()), accumulation of cells in G1 phase ()), and nuclear fragmentation ()) were observed.
ALP decreased mitochondrial membrane potential (MMP) induced by H2O2 treatment in HT22 cells
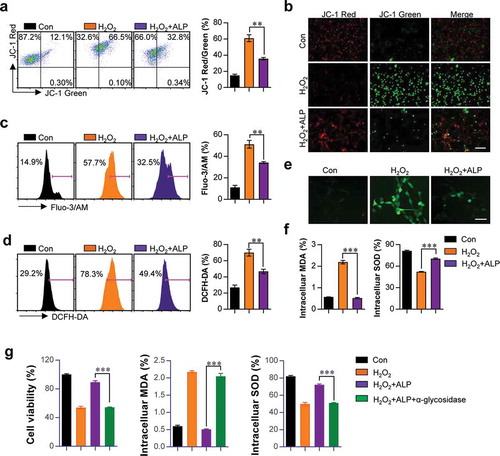
Since the loss of MMP induced by H2O2 directly leads to neuronal cell damage [Citation25], the ability of ALP to inhibit H2O2-induced upregulation of MMP was assessed using the mitochondria-specific probe JC-1. JC-1 exists as a polymer in non-apoptotic cells, and produces red fluorescence. JC-1 emits green fluorescence in the cytosol of apoptotic or necrotic cells. In HT22 cells treated with H2O2 and ALP, increased red fluorescence and decreased green fluorescence was observed compared to the positive control H2O2-treated HT22 cells ().
ALP decreased ca2+ loads, excessive ROS generation, overproduction of MDA and decreased SOD induced by H2O2 treatment in HT22 cells
Next, we confirmed the effect of ALP treatment in H2O2-induced abnormal Ca2+ levels, excessive ROS generation, MDA overproduction and decreased SOD activity. These are essential factors involved in neuronal oxidative stress, and are involved in the etiology of neurodegeneration [Citation3,Citation4,Citation12–Citation14]. Levels of intracellular Ca2+ and ROS were analyzed using Fluo-3/AM and DCFH-DA dye, respectively. ALP treatment led to reductions in Ca2+ load () and excessive ROS generation () in H2O2-treated HT22 cells. ALP pretreatment restored MDA and SOD to homeostatic levels in the presence of H2O2 ()). To investigate whether these phenomenon can be derived in presence of alpha glycosidase (inhibitor for polysaccharide activity), we examined the cell viability, intracellular MDA and SOD levels, in HT22 cells treated with H2O2 and ALP in the presence of alpha glycosidase. The increased cell viability, reduction of MDA levels, and increase in SOD levels induced by ALP were not detectable in the presence of alpha glycosidase ()). These findings suggest that ALP treatment provides a neuroprotective effect fromH2O2-induced oxidative stress in HT22 hippocampus cells.
ALP inhibited the mitochondrial apoptotic pathway and PARP activation in H2O2-treated HT22 cells
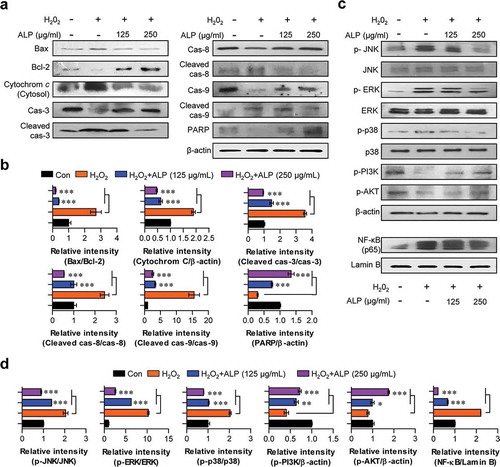
Apoptosis and necrosis signals play key roles in neuronal cell death [Citation26,Citation27]. Importantly, the predominant mechanism of neuronal cell death shifts from apoptosis to necrosis as neurons mature [Citation26]. Thus, the effective inhibition of apoptotic signals is an important step for neuroprotective strategy because inhibition of apoptosis can prevent necrotic cell death. To investigate whether ALP pretreatment blocks apoptotic signals induced by H2O2, we analyzed for the expression of various apoptotic indicators such as apoptotic proteins (Bax and cytochrome c), cleaved caspases (cleaved caspases-3, -8, and -9), and PARP protein in H2O2-treated HT22 cells pre-treated with ALP. As shown in , H2O2 triggered the up-regulation of Bax and cytosolic cytochrome c, as well as a down-regulation of Bcl-2. Expression of these proteins was restored in the presence of ALP. Next, to examine whether cleaved caspases are involved in neuroprotection following treatment with ALP, the expression levels of cleaved caspases-3, -8, and -9 were analyzed. In HT22 co-treated with H2O2 and ALP, decreased levels of cleaved caspases-3, -8, and -9 were observed relative to HT22 treated with H2O2 alone. In addition, ALP treatment restored levels of PARP, which is specifically cleaved during the induction of apoptosis, in HT22 cells. These data suggest that ALP prevents induction of cell death induced by H2O2 in HT22 cells.
ALP reduced H2O2-induced MAPKs and NF-κB signaling and restored PI3K signaling in H2O2-treated HT22 cells
H2O2-induced ROS generation can induce tissue damages via the activation of mitogen-activated protein kinases (MAPKs) and NF-κB signaling pathways [Citation28,Citation29]. Thus, we analyzed the ROS-dependent MAPKs and NF-κB signals, in the presence and absence of ALP in H2O2-treated HT22 cells. The phosphorylation of MAPKs (p38, ERK, JNK) and the nuclear translocation of NF-κB p65 from the cytosol induced by H2O2 treatment were decreased in H2O2-treated HT22 cells pre-treated with ALP compared to cells treated H2O2 alone (). The inhibition of PI3K/AKT signaling induced by excessive ROS generation in various cell types can also induce cell death [Citation30]. As shown in , ALP pretreatment significantly restored the inhibition of PI3K/AKT signaling induced by H2O2 treatment. These data indicate that ALP reduces H2O2-induced neurotoxicity by the restoration of PI3K/AKT signaling, as well as by a decrease of MAPKs and NF-κB signals via directly canceling ROS activity in H2O2-treated cells.
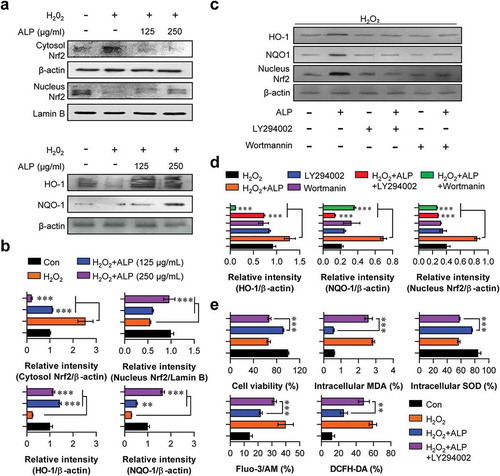
ALP treatment increased levels of nuclear Nrf2, HO-1 and NQO-1 in H2O2-treated HT22 cells
The transcription factor Nrf2, is important in the expression of antioxidant response element (ARE)-mediated cytoprotective genes (HO-1 and NQO-1) in response to oxidative stress [Citation31–Citation33]. As shown in , H2O2-treated HT22 cells pre-treated with ALP produced increased levels of nuclear Nrf2, HO-1 and NQO-1 expression in a concentration-dependent manner. Cytosolic Nrf2 levels remained constant.
PI3K/AKT signaling was essential for the neuroprotective effects induced by ALP
Based on these results, we hypothesized that ALP induces neuroprotective effects via the restoration of decreasing PI3K/AKT signals in H2O2-treated HT22 cells. To explore this hypothesis, we treated HT22 cells with pharmacological inhibitors (LY294002 or Wortmannin) of PI3K/AKT signaling for 30 min prior to treatment with ALP, and analyzed the expression of HO-1, NQO-1, nuclear Nrf2, as well as levels of intracellular MDA, SOD, Ca2+ and ROS. LY294002 and Wortmannin reduced the expression of HO-1, NQO-1, nuclear Nrf2 induced by H2O2 treatment in ALP-treated HT22 cells (). In addition, increased cell viability and SOD levels, as well as the decreased MDA, Ca2+ and ROS levels induced by ALP, were blocked in the presence of LY294002 and Wortmannin ()). From these findings, we suggest that PI3K/AKT signaling plays an important role in the neuroprotective effects of ALP.
Discussion
The isolation and functional characterization of bioactive compounds derived from phytochemical leaves is an important approach for the development of more effective therapies in neurodegenerative disease. In this study, we first evaluated the functional roles of ALP in the prevention of H2O2 induced oxidative stress. We found that ALP inhibits abnormal Ca2+ levels, NADPH oxidase activity (ROS production) and oxidative stress indicators (MDA) in HT22 hippocampal neuronal cells. ALP treatment promoted neuroprotective effects against H2O2-induced neurotoxicity through the activation of PI3K/AKT-dependent signaling. Several studies have described the functional roles of several extracts and phytochemical compounds, derived from Annona muricata L., against various diseases. To the best of our knowledge, this is the first study which describes ALP as a potential candidate extract to combat neurodegenerative diseases.
The oxidative stress induced by excessive ROS production produces mitochondrial dysfunction, and induces insulin resistance [Citation34,Citation35]. These responses are mostly observed in neurodegenerative diseases [Citation36,Citation37]. Importantly, insulin can promote ROS elimination by regulating redox regulated transcription factors such as Nrf2 and NF-κB, and redox-sensitive MAPKs signaling involved in the regulation of antioxidant defense systems [Citation38]. For example, a decline in Nrf2 signaling and HO-1/NQO-1 responding Nrf2 inhibits the production of enzymes related to antioxidation and detoxification, resulting in increased oxidative damage and neuronal cell death [Citation39]. Importantly, MAPK activation restrains Nrf2 activation [Citation39,Citation40]. In general, activation of JNK and ERK MAPKs induces Nrf2 signaling, whereas activation of p38 MAPK has both positive and negative regulatory effects [Citation40,Citation41]. However, in neurodegenerative diseases, overexpression of ERK and p38 MAPKs inhibits Nrf2 activation [Citation39,Citation40]. Furthermore, a persistent increase in NF-κB signals following exposure to oxidative insults can lead to neuroinflammation, nerve damage, and cell death [Citation39]. Interestingly, ALP-treated HT22 cells showed substantial reductions of excessive MAPKs and NF-κB signals induced by H2O2 treatment.
PI3K and AKT are the major pathways of insulin signaling; a lack of these signals may lead to diabetes and insulin resistance, ultimately leading to Alzheimer’s disease [Citation42]. Thus, compounds that can activate PI3K/AKT signaling are also considered as potential candidates for drug development to combat neurodegeneration [Citation43–Citation45]. Given that PI3K/AKT signaling plays a key role in insulin sensitivity and resistance [Citation46], we found that the neuroprotective effect of ALP was induced by the restoration of decreasing PI3K/AKT signals in H2O2-treated HT22 cells, and that ALP-induced antioxidant molecules (Nrf2-mediated HO-1/NQO-1, MDA) were also stimulated through the activation of PI3K/AKT signaling.
Recent studies suggest that several compounds isolated from Annona muricata L. can paradoxically induce neurotoxicity and neurodegenerative disease [Citation15]. Our data provide mechanistic evidence for a neuroprotective role of polysaccharides extracts derived from Annona muricata L. We demonstrated that the protective roles of ALP against oxidative stress are mediated through direct inhibition of H2O2-induced ROS generation, resulting in the inhibition of excessive MAPKs, NF-κB signals and the activation of PI3K/AKT-mediated Nrf2 signaling indirectly. Consequently, our study demonstrates that ALP represents a novel candidate for pharmacological or therapeutic strategies to treat neurodegenerative diseases.
Author contributions
Woo Sik Kim, Yi-Eun Kim: methodology, data analysis, and writing original draft; Yi-Eun Kim, Eun-Ji Cho, Eui-Baek Byun, Woo Yong Park, Ha-Yeon Song, Kwangwook Kim, Sang-Hyun Park: methodology and data analysis; Woo Sik Kim: review and editing; Eui-Hong Byun: conceptualization, supervision, review, and editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data described in this article are openly available in the Open Science Framework at DOI:10.17605/OSF.IO/TPA6U.
Additional information
Funding
References
- Liu Z, Zhou T, Ziegler AC, et al. Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxid Med Cell Longev. 2017;2017:2525967.
- Kim GH, Kim JE, Rhie SJ, et al. The role of oxidative stress in neurodegenerative diseases. Exp Neurobiol. 2015;24(4):325–40.
- Bezprozvanny IB. Calcium signaling and neurodegeneration. Acta Naturae. 2010;2(1):72–82.
- Nissanka N, Moraes CT. Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett. 2018;592(5):728–742.
- Mitra S, Nguyen LN, Akter M, et al. Impact of ROS generated by chemical, physical, and plasma techniques on cancer attenuation. Cancers (Basel). 2019;11(7):1030.
- Noctor G, Foyer CH. Intracellular redox compartmentation and ROS-related communication in regulation and signaling. Plant Physiol. 2016;171(3):1581–1592.
- Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863(12):2977–2992.
- Abdul Wahab SM, Jantan I, Haque MA, et al. Exploring the leaves of annona muricata L. as a source of potential anti-inflammatory and anticancer agents. Front Pharmacol. 2018;9:661.
- Vasanthi HR, ShriShriMal N, Das DK. Phytochemicals from plants to combat cardiovascular disease. Curr Med Chem. 2012;19(14):2242–2251.
- Barbieri R, Coppo E, Marchese A, et al. Phytochemicals for human disease: an update on plant-derived compounds antibacterial activity. Microbiol Res. 2017;196:44–68.
- Taur DJ, Patil RY. Some medicinal plants with antiasthmatic potential: a current status. Asian Pac J Trop Biomed. 2011;1(5):413–418.
- Moghadamtousi SZ, Rouhollahi E, Hajrezaie M, et al. Annona muricata leaves accelerate wound healing in rats via involvement of Hsp70 and antioxidant defence. Int J Surg. 2015;18:110–117.
- Moghadamtousi SZ, Fadaeinasab M, Nikzad S, et al. Annona muricata (Annonaceae): a review of its traditional uses, isolated acetogenins and biological activities. Int J Mol Sci. 2015;16(7):15625–15658.
- Florence NT, Benoit MZ, Jonas K, et al. Antidiabetic and antioxidant effects of Annona muricata (Annonaceae), aqueous extract on streptozotocin-induced diabetic rats. J Ethnopharmacol. 2014;151(2):784–790.
- Gavamukulya Y, Wamunyokoli F, El-Shemy HA. Annona muricata: is the natural therapy to most disease conditions including cancer growing in our backyard? A systematic review of its research history and future prospects. Asian Pac J Trop Med. 2017;10(9):835–848.
- Ozgen M, Reese RN, Tulio AZ Jr., et al. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (abts) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2ʹ-diphenyl-1-picrylhydrazyl (DPPH) methods. J Agric Food Chem. 2006;54(4):1151–1157.
- Gulcin I. Fe(3+)-Fe(2+) transformation method: an important antioxidant assay. Methods Mol Biol. 2015;1208:233–246.
- Simonyi A, Wang Q, Miller RL, et al. Polyphenols in cerebral ischemia: novel targets for neuroprotection. Mol Neurobiol. 2005;31(1–3):135–147.
- Wang CX, Shuaib A. Neuroprotective effects of free radical scavengers in stroke. Drugs Aging. 2007;24(7):537–546.
- Clausen F, Marklund N, Lewen A, et al. The nitrone free radical scavenger NXY-059 is neuroprotective when administered after traumatic brain injury in the rat. J Neurotrauma. 2008;25(12):1449–1457.
- Shichinohe H, Kuroda S, Yasuda H, et al. Neuroprotective effects of the free radical scavenger edaravone (MCI-186) in mice permanent focal brain ischemia. Brain Res. 2004;1029(2):200–206.
- Tan BL, Norhaizan ME, Liew WP, et al. Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Front Pharmacol. 2018;9:1162.
- Pizzino G, Irrera N, Cucinotta M, et al. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev. 2017;2017:8416763.
- Ismail N, Ismail M, Azmi NH, et al. Modulation of hydrogen peroxide-induced oxidative stress in human neuronal cells by thymoquinone-rich fraction and thymoquinone via transcriptomic regulation of antioxidant and apoptotic signaling genes. Oxid Med Cell Longev. 2016;2016:2528935.
- Park WH. Effects of antioxidants and MAPK inhibitors on cell death and reactive oxygen species levels in H2O2-treated human pulmonary fibroblasts. Oncol Lett. 2013;5(5):1633–1638.
- Yuan J. Neuroprotective strategies targeting apoptotic and necrotic cell death for stroke. Apoptosis. 2009;14(4):469–477.
- Fricker M, Tolkovsky AM, Borutaite V, et al. Neuronal cell death. Physiol Rev. 2018;98(2):813–880.
- Karin M, Shaulian E. AP-1: linking hydrogen peroxide and oxidative stress to the control of cell proliferation and death. IUBMB Life. 2001;52(1–2):17–24.
- Ho JQ, Asagiri M, Hoffmann A, et al. NF-kappaB potentiates caspase independent hydrogen peroxide induced cell death. PLoS One. 2011;6(2):e16815.
- Milkovic L, Cipak Gasparovic A, Cindric M, et al. Short overview of ROS as cell function regulators and their implications in therapy concepts. Cells. 2019;8:8.
- Chan K, Kan YW. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc Natl Acad Sci USA. 1999;96(22):12731–12736.
- Chan K, Han XD, Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci USA. 2001;98(8):4611–4616.
- Itoh K, Chiba T, Takahashi S, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236(2):313–322.
- Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102(4):401–414.
- Turner N, Heilbronn LK. Is mitochondrial dysfunction a cause of insulin resistance? Trends Endocrinol Metab. 2008;19(9):324–330.
- De Felice FG. Alzheimer’s disease and insulin resistance: translating basic science into clinical applications. J Clin Invest. 2013;123(2):531–539.
- Bhatti JS, Bhatti GK, Reddy PH. Mitochondrial dysfunction and oxidative stress in metabolic disorders - a step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis. 2017;1863(5):1066–1077.
- McDonnell C, Leanez S, Pol O. The induction of the transcription factor Nrf2 enhances the antinociceptive effects of delta-opioid receptors in diabetic mice. PLoS One. 2017;12(7):e0180998.
- Ganesh Yerra V, Negi G, Sharma SS, et al. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-kappaB pathways in diabetic neuropathy. Redox Biol. 2013;1:394–397.
- Huang Y, Li W, Su ZY, et al. The complexity of the Nrf2 pathway: beyond the antioxidant response. J Nutr Biochem. 2015;26(12):1401–1413.
- Singh S, Vrishni S, Singh BK, et al. Nrf2-ARE stress response mechanism: a control point in oxidative stress-mediated dysfunctions and chronic inflammatory diseases. Free Radic Res. 2010;44(11):1267–1288.
- Pugazhenthi S, Qin L, Reddy PH. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim Biophys Acta Mol Basis Dis. 2017;1863(5):1037–1045.
- Shah SA, Lee HY, Bressan RA, et al. Novel osmotin attenuates glutamate-induced synaptic dysfunction and neurodegeneration via the JNK/PI3K/Akt pathway in postnatal rat brain. Cell Death Dis. 2014;5:e1026.
- Bagli E, Goussia A, Moschos MM, et al. Natural compounds and neuroprotection: mechanisms of action and novel delivery systems. In Vivo. 2016;30(5):535–547.
- Kim KC, Lee IK, Kang KA, et al. 7,8-dihydroxyflavone suppresses oxidative stress-induced base modification in DNA via induction of the repair enzyme 8-oxoguanine DNA glycosylase-1. Biomed Res Int. 2013;2013:863720.
- Peltier J, O’Neill A, Schaffer DV. PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev Neurobiol. 2007;67(10):1348–1361.