ABSTRACT
Unlike its biosynthetic mechanisms and physiological function, current understanding of riboflavin degradation in soil is limited to a few bacteria that decompose it to lumichrome. Here, we isolated six Microbacterium and three Nocardioides strains. These strains utilized riboflavin and lumichrome, respectively, as carbon sources. Among these strains, we identified Microbacterium paraoxydans R16 (R16) and Nocardioides nitrophenolicus L16 (L16), which were isolated form the same enrichment culture. Co-cultured R16 and L16 reconstituted a riboflavin-degrading interspecies consortium, in which the R16 strain degraded riboflavin to lumichrome and ᴅ-ribose. The L16 strain utilized the lumichrome as a carbon source, indicating that R16 is required for L16 to grow in the consortium. Notably, rates of riboflavin degradation and growth were increased in co-cultured, compared with monocultured R16 cells. These results indicated that a beneficial symbiotic interaction between M. paraoxydans R16 and N. nitrophenolicus L16 results in the ability to degrade riboflavin.
GRAPHICAL ABSTRACT
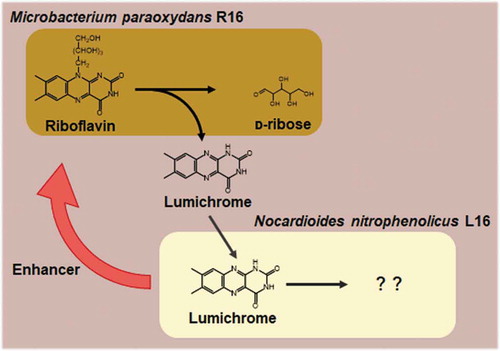
Symbiotic riboflavin-degrading consortium of M. paraoxydans R16 and N. nitrophenolicus L16 from soil.
Water- and lipid-soluble vitamins are essential nutrients for mammals. Riboflavin (vitamin B2) is a water-soluble B vitamin with a distinct yellow color that is derived from an isoalloxazine moiety with a bound ribityl base at the N10 position. After uptake by cellular organisms, riboflavin is phosphorylated to flavin mononucleotide (FMN), which is then converted to flavin adenine dinucleotide (FAD). Both flavins are essential cofactors of redox enzymes [Citation1]; thus, a riboflavin deficiency (ariboflavinosis) leads to neurodegeneration, cancer, and cardiovascular diseases [Citation2]. Plants are dietary sources of riboflavin, which they synthesize using GTP as a precursor [Citation3,Citation4]. Fungi and bacteria synthesize riboflavin by essentially the same mechanisms as plants, including the formation of an isoalloxazine ring from a guanine nucleoside that is metabolized by lumazine and riboflavin synthases to yield riboflavin [Citation4]. Other than these extensive studies of riboflavin biosynthesis, little is understood about the biological mechanisms of riboflavin degradation that should be vital for terrestrial riboflavin homeostasis.
Devosia riboflavina (formerly known as Pseudomonas riboflavina) is a soil bacterium that degrades riboflavin [Citation5,Citation6]. We found that D. riboflavina uses a two-component flavin-dependent monooxygenase to degrade riboflavin to lumichrome and ᴅ-ribose, the latter of which is utilized as a carbon source [Citation7]. The actinomyces bacteria Microbacterium maritypicum G10 and Microbacterium sp. TPU3598 isolated from soil samples produce lumichrome as an end product of riboflavin catabolism [Citation8,Citation9]. These results suggested that a relatively small population of soil bacteria degrade riboflavin via flavin-dependent monooxygenase and that the fate of lumichrome in the soil ecosystem remains obscure. Furthermore, microorganisms that can degrade lumichrome have not yet been identified.
Hundreds of millions of microorganisms are considered to inhabit 1 g of fertile soil, and increasing evidence suggests that many of them interact with plants, animals, and other microorganisms. Bacterial populations often interact symbiotically. Such interactions depend on physical contact between cells that generate biofilms [Citation10], and on interspecies transfer of metabolites and signaling molecules. Soil microbiomes could be important as factories where organic matter is degraded to maintain carbon homeostasis in the terrestrial ecosystem, but bacterial consortia that degrade released vitamins to environments, especially riboflavin, are not well understood.
This study investigated riboflavin homeostasis in a soil ecosystem and found that riboflavin was generated and recycled by a consortium comprising Microbacterium and Nocardioides species that respectively metabolized riboflavin to lumichrome and consumed lumichrome for growth. The Nocardioides strain notably promoted the rate of riboflavin degradation by the Microbacterium strain in co-cultures, suggesting that their relationship is mutually beneficial.
Materials and methods
Isolation of bacteria that degrade riboflavin and lumichrome
A soil suspension was inoculated into minimal medium (MM; 10 mM NH4Cl, 10 mM potassium phosphate (pH 6.5), 0.03% MgSO4・7H2O, 0.05% KCl, 0.2% trace element solution [Citation11]) containing 50 µM riboflavin or 50 µM lumichrome, and cultured at 30°C for 72 h in air. A portion (1:100) was transferred to the same medium and cultured as described above three times to enrich riboflavin-degrading consortia. The resulting culture broths were spread over plates containing 1.5% agar and Luria-Bertani (LB) medium (1% tryptone and 0.5% yeast extract [both from Becton Dickinson, Franklin Lakes, NJ, USA], 0.5% NaCl) and bacterial colonies were isolated after incubation at 30°C for 72 h in air. Their growth was confirmed on the plates containing MM medium supplemented with riboflavin and lumichrome (50 µM each). Riboflavin and lumichrome degradation were assessed as a decrease in the amount of visible yellow pigment, and as fluorescence emission under UV light, respectively.
Nucleotide sequencing of 16S rRNA genes
Strains were cultured at 30°C for 24 h in LB medium, then total bacterial DNA was prepared using Blood & Cell Culture DNA Mini Kits (QIAGEN, Hilden, Germany). Nucleotides for the 16S rRNA genes were amplified by PCR and sequenced using a 3730xl DNA Analyzer (Thermo Fisher Scientific Inc., Waltham, MA, USA) with the following primers: 10F (5ʹ-GTTTGATCCTGGCTCA-3ʹ) and 1500R (5ʹ-TACCTTGTTACGACTT-3ʹ).
Liquid cultures of isolates
The R16 and L16 strains were precultured in LB medium at 30°C for 24 h. Portions (1 mL each) were inoculated into 100 mL of MM containing either 10 mM riboflavin or 10 mM lumichrome, respectively, and rotary cultured at 120 rpm and 30°C for 120 h. Portions (1 mL each) of precultured R16 and L16 were transferred to MM containing 10 mM riboflavin and co-cultured under the same conditions as above. Cultures with riboflavin were protected from light to prevent riboflavin photodegradation. Viable cells in flasks were periodically monitored by spreading portions of the culture broths and counting the numbers of colonies on NB agar (1.5%) plates containing 0.8% nutrient broth (Becton Dickinson). Strains R16 and L16 were distinguished by their yellow and white colonies. Cell counts are expressed as colony-forming units (cfu)/mL of culture broth.
Determination of riboflavin and lumichrome
Culture broth was diluted 10-fold with 80 mM KOH to solubilize cells and precipitates, and separated by centrifugation at 20,400 × g for 5 min at 4°C. Supernatants were analyzed by high-performance liquid chromatography (HPLC) using an Agilent 1260 Infinity system (Agilent Technologies, Santa Clara, CA, USA) equipped with a Purospher STAR RP-18 end-capped column (Merck & Co. Inc., Kenilworth, NJ, USA), and absorption was monitored at 254 nm. The mobile phase comprised 10 mM sodium acetate (pH 4.5) and methanol (1:1 [vol/vol]), with a flow rate of 0.8 mL min−1. Analysis by LC-MS proceeded using an LCMS-8030 system (Shimadzu Corp., Kyoto, Japan) with the same column. The mobile phase comprised 10 mM ammonium formate (pH 4.0) and acetonitrile (1:4 [vol/vol]), with a flow rate of 0.8 mL min−1.
Riboflavin-and lumichrome-degrading activity in vitro
Strains were cultured in 100 mL of MM containing riboflavin or lumichrome (0.1 mM each) at 30°C for 12 h. Cells harvested by centrifugation at 20,400 × g for 5 min were disrupted by ultrasonication, then supernatants and pellets were obtained after centrifugation at 20,400 × g for 5 min. Riboflavin and lumichrome degradation were analyzed in 50 mM potassium phosphate (pH 7.0) containing 0.1 mM riboflavin or lumichrome. Reactions were started by adding cell-free fractions and incubating them at 30°C for 1 h, then analyzing them by HPLC as described above.
Results
Isolation of riboflavin-degrading bacteria
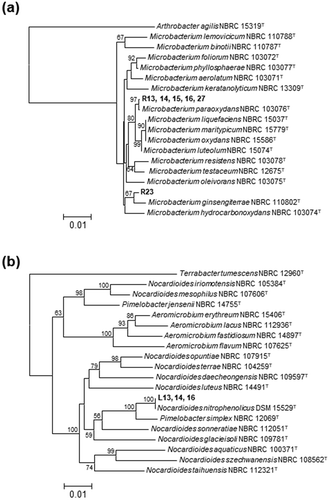
After enriching cultures in MM containing riboflavin as the sole source of carbon, culture broths were spread on MM agar plates containing riboflavin, and colonies with clear zones that were free from yellow pigments of riboflavin, were selected as being responsible for riboflavin degradation. We applied this procedure to over 30 soil samples, and isolated six bacteria that degraded and utilized riboflavin as their sole source of carbon (). Phylogenetic analyses of their 16S rRNA genes indicated that all of them were closely related to the genus Microbacterium, and that their sequences were 99.9% homologous to that of Microbacterium paraoxydans NBRC 103076T or 99.4% homologous to that of Microbacterium ginsengiterrae NBRC 110802T ()).
Table 1. Strains isolated in this study.
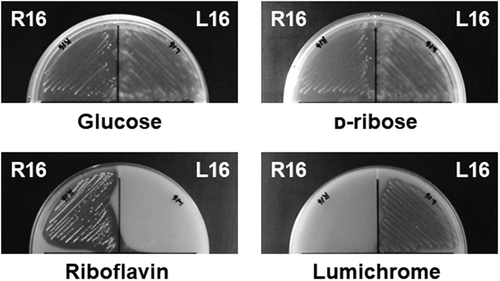
Among the strains, we selected and further analyzed R16 that comprised Gram-positive, motile rods that formed yellow colonies, and had the general properties of M. paraoxydans [Citation12]. Based on these findings, we designated strain R16 as M. paraoxydans R16. Plate assays indicated that R16 proliferated using glucose, riboflavin, and d-ribose, but not lumichrome, which is a bacterial product of riboflavin catabolism ().
Figure 2. Riboflavin and lumichrome degradation assays of M. paraoxydans R16 and N. nitrophenolicus L16 on agar plates.
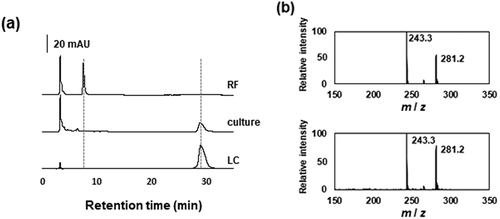
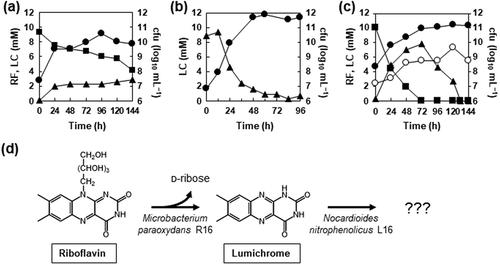
The R16 strain was cultured in flasks containing MM supplemented with 10 mM riboflavin as a sole source of carbon. During the culture period, the number of viable R16 cells increased along with the consumption of riboflavin, and the amount of an unknown compound increased and resolved as a single peak on HPLC ()) with the same retention time as commercial lumichrome. Liquid chromatography-mass spectrometry (LC-MS) detected [M + H]+ at m/z = 243.2, which matched the molecular mass of lumichrome, namely 242.2 ()), confirming that the peak was lumichrome. The culture consumed twice as much riboflavin compared with the amount of produced lumichrome, indicating that riboflavin and/or lumichrome were converted to unidentified compounds ()). Incubation of the culture medium generated < 0.1 mM lumichrome in the absence of the cells, indicating that the R16 strain degrades riboflavin to lumichrome.
We assessed riboflavin degradation activity in vitro using cell-free culture supernatants and cell extracts prepared from the six isolates including R16. The results indicated that the culture supernatants and the soluble fractions of the cell extracts contained < 0.5 nmol min−1 mg−1 of riboflavin degradation activity, whereas the pellet fractions of the cell extract, which presumably contain cytoplasmic membrane, cell wall, and other insoluble materials, degraded riboflavin at a specific activity of 9.3 ± 1.8 nmol min−1 mg−1 protein (). The reaction contains lumichrome as a degradation product of riboflavin while d-ribose is likely to be another degradation product, which agrees to d-ribose utilization by the R16 strain ().
Isolation of lumichrome-degrading bacteria
Broths from enriched cultures in MM containing lumichrome spread on agar plates containing the same medium generated several colonies. Ultraviolet irradiation showed that strains L13 and L14 exhibited a fluorescence-free clear zone of lumichrome, suggesting that they degraded lumichrome (). In addition, culture broths derived from the cultures enriched with riboflavin-degrading bacteria resulted in strain L16 that also had a clear zone on plates containing lumichrome (). The pellet fractions of the cell extracts prepared from the three isolates cultured in MM supplemented with lumichrome, degraded lumichrome with activity of 6.3- to 14.7- nmol min−1 mg−1 protein (). We selected strain L16 for further characterization.
Strain L16 comprised Gram-positive, motile rods that formed white colonies. These characteristics agreed with those of Nocardioides species in general [Citation13]. Phylogenetic analyses of the 16S rRNA gene sequences of strain L16 and the other isolates revealed that all lumichrome-degrading isolates were located in the genus Nocardioides in the phylum Actinobacteria, and had sequences that were homologous (100%) to that of Nocardioides nitrophenolicus DSM 15529T ()). Based on these findings, we designated strain L16 as N. nitrophenolicus L16. This strain thrived on plates containing MM supplemented with lumichrome, with the simultaneous disappearance of lumichrome (), but not in MM supplemented with riboflavin, indicating that it utilizes lumichrome but not riboflavin. Strain L16 used d-ribose as a sole source of carbon (). We cultured N. nitrophenolicus L16 in MM supplemented with lumichrome as the sole carbon source. This strain thrived, and growth was monitored by counting the numbers of cells that visibly increased during the culture period, along with lumichrome consumption ()). Control incubation in the absence of the cells degraded < 0.1 mM lumichrome, indicating that N. nitrophenolicus L16 consumed lumichrome.
Co-cultured M. paraoxydans R16 and N. nitrophenolicus L16
Strains R16 and L16 originating from the same soil sample were isolated from the same enrichment culture using riboflavin as the sole carbon source. Strain L16 could not utilize riboflavin (), indicating that its growth depended on a compound produced by the R16 strain in the enrichment culture, and that these bacteria constitute a symbiotic consortium that degrades and consumes riboflavin. We reconstructed the consortium by co-culturing R16 and L16 in MM containing riboflavin and monitoring their growth ()). During the initial 72 h, riboflavin in the culture was consumed and lumichrome accumulated. After all the riboflavin was exhausted, lumichrome decreased over the next 72 h in culture. The numbers of cells in both strains increased during these reactions ()). Considering their substrate preferences, R16 and L16 concomitantly degrade and consume riboflavin in vitro for growth. That is, R16 converted riboflavin to lumichrome, and L16 catabolized lumichrome in this interspecies consortium ()).
Co-cultured R16 and L16 converted riboflavin to lumichrome at a higher rate than monocultured R16 ()). The amount of R16 cell growth estimated as cfu was also enhanced, indicating that riboflavin utilization by R16 was upregulated in co-culture with L16. These results indicate that M. paraoxydans R16 and N. nitrophenolicus L16 interacted to mutually promote their degradative activities. This is the first symbiotic bacterial consortium for riboflavin degradation, which might contribute to soil ecosystems.
Discussion
Vitamins are essential nutrients, as well as popular commercial and medicinal dietary supplements. Therefore, their biosynthetic mechanisms and physiological functions have been extensively studied, whereas their natural degradation in ecosystems such as soil, is not fully understood. Here, we isolated two actinomycetes bacteria, M. paraoxydans R16 and N. nitrophenolicus L16 and determined their involvement in riboflavin degradation. Bacteria that can degrade riboflavin include M. maritypicum G10 and Microbacterium sp. TPU3598 [Citation5–Citation9]. Together with our finding that M. paraoxydans R16 degraded riboflavin, Microbacterium strains apparently decompose riboflavin in soil. Xu et al. indicated that the two-component flavin-dependent monooxygenase and flavin reductase system of M. marytipicum G10 converts riboflavin to lumichrome and ᴅ-ribose [Citation9]; however, the enzymatic counterparts produced by other Microbacterium strains remain unknown. Nevertheless, Microbacterium strains are responsible for the initial reaction in riboflavin-degrading ecosystem since they produce lumichrome as the final product of riboflavin degradation.
As far as we can ascertain, this is the first study to identify bacteria that could degrade lumichrome, but were unable to utilize riboflavin as a carbon source. This indicated that such bacteria acquire lumichrome produced from natural riboflavin by other bacteria such as Microbacterium and by photolysis [Citation14]. The former is highly plausible since we reconstituted a symbiotic riboflavin-degradation process using co-cultured M. paraoxydans R16 that proliferated by degrading riboflavin and utilizing the resulting ᴅ-ribose as a carbon source, while excreting lumichrome. Nocardioides nitrophenolicus L16 then utilized lumichrome as a carbon/energy sources for growth. Thus, N. nitrophenolicus L16 degrades lumichrome dependently on the amount of riboflavin degraded by M. paraoxydans R16.
Notably, co-culture with N. nitrophenolicus L16 accelerated M. paraoxydans R16 riboflavin degradative activity and cell growth, indicating a mutually beneficial symbiotic relationship with respect to riboflavin degradation. The mechanism(s) through which L16 promotes M. paraoxydans R16 activities remains unknown. One explanation could be that N. nitrophenolicus L16 produces a metabolite(s) that diffuses into culture medium and stimulates M. paraoxydans R16 to degrade riboflavin. Another is that N. nitrophenolicus L16 diminishes a metabolite(s) that inhibits the growth of M. paraoxydans R16, as symbiotic mechanisms are often mediated by metabolite transfer between bacterial species [Citation15]. One example of metabolite interaction is the promotion of Symbiobacterium thermophilum strain TT growth by Bacillus strain S [Citation16]. Besides, this study is the first to report bacterial symbiotic relationship mediated by interspecies transfer of lumichrome.
Nocardioides bacteria include organisms that can decompose scarcely degradable polyaromatic, nitroaromatic, and heterocyclic compounds, and the enzymes involved in these degradative processes have been extensively studied [Citation17–Citation19]. Our novel finding that Nocardioides bacteria decomposed lumichrome, a tricyclic alloxazine of biological origin comprising benzene, pyrazine and pyrimidine rings, indicates that Nocardioides are important for maintaining biogeochemical cycles in soil ecosystems. The mechanism of lumichrome degradation by Nocardioides might be comparable to those of other known aromatic/cyclic compounds. Identifying the N. nitrophenolicus L16 genes that are involved in lumichrome metabolism will facilitate tests of this hypothesis.
Author contribution
H.K. and N.T. designed and conducted experiments; H.K. and S.O. performed culture experiments; Y.D. analyzed bacterial phylogeny; S.M. performed mass spectrometry; H.K., Y.D. and N.T. wrote the paper.
Acknowledgments
We thank Norma Foster for critical reading of the manuscript. This study was partly supported by JSPS Grant-in-Aid for Scientific Research on Innovative Areas (Post-Koch Ecology) Grant Number JP19H0587.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data described in this article are openly available in the Open Science Framework at DOI:10.17605/OSF.IO/TPA6U.
Additional information
Funding
References
- Paine MJ, Garner AP, Powell D, et al. Cloning and characterization of a novel human dual flavin reductase. J Biol Chem. 2000 Jan;275(2):1471–1478.
- Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr. 2003 Jun;77(6):1352–1360.
- Herz S, Eberhardt S, Bacher A. Biosynthesis of riboflavin in plants. The ribA gene of Arabidopsis thaliana specifies a bifunctional GTP cyclohydrolase II/3,4-dihydroxy-2-butanone 4-phosphate synthase. Phytochemistry. 2000 Apr;53(7):723–731.
- Schwechheimer SK, Park EY, Revuelta JL, et al. Biotechnology of riboflavin. Appl Microbiol Biotechnol. 2016;100(5):2107–2119.
- Foster JW. Microbiological aspects of riboflavin: I. Introduction. II. Bacterial oxidation of riboflavin to lumichrome. J Bacteriol. 1944 Jan;47(1):27–41.
- Nakagawa Y, Sakane T, Yokota A. Transfer of Pseudomonas riboflavina (Foster 1944), a gram-negative, motile rod with long-chain 3-hydroxy fatty acids, to Devosia riboflavina gen. nov., sp. nov., nom. rev. Int J Syst Bacteriol. 1996 Jan;46(1):16–22.
- Kanazawa H, Shigemoto R, Kawasaki Y, et al. Two-component flavin-dependent riboflavin monooxygenase degrades riboflavin in Devosia riboflavina. J Bacteriol. 2018 Jun;200(12):e00022–18.
- Yamamoto K, Asano Y. Efficient production of lumichrome by Microbacterium sp. strain TPU 3598. Appl Environ Microbiol. 2015 Nov;81(21):7360–7367.
- Xu H, Chakrabarty Y, Philmus B, et al. Identification of the first riboflavin catabolic gene cluster isolated from Microbacterium maritypicum G10. J Biol Chem. 2016 Nov;291(45):23506–23515.
- Nadell CD, Xavier JB, Foster KR. The sociobiology of biofilms. FEMS Microbiol Rev. 2009 Jan;33(1):206–224.
- Hunter SH, Provasoli L, Schatz A, et al. Some approaches to the study of the role of metals in the metabolism of microorganisms. Proc Am Philos Soc. 1950;94:152–170.
- Laffineur K, Avesani V, Cornu G, et al. Bacteremia due to a novel Microbacterium species in a patient with leukemia and description of Microbacterium paraoxydans sp nov. J Clin Microbiol. 2003 May;41(5):2242–2246.
- Yoon JH, Cho YG, Lee ST, et al. Nocardioides nitrophenolicus sp. nov., a p-nitrophenol-degrading bacterium. Int J Syst Bacteriol. 1999;49(2):675–680.
- Ahmad I, Fasihullah Q, Vaid FHM. A study of simultaneous photolysis and photoaddition reactions of riboflavin in aqueous solution. J Photochem Photobiol B Biol. 2004 Jul;75(1–2):13–20.
- Schink B. Synergistic interactions in the microbial world. Antonie Van Leeuwenhoek. 2002;81(1–4):257–261.
- Ohno M, Okano I, Watsuji TO, et al. Establishing the independent culture of a strictly symbiotic bacterium Symbiobacterium thermophilum from its supporting Bacillus strain. Biosci Biotechnol Biochem. 1999;63(6):1083–1090.
- Saito A, Iwabuchi T, Harayama S. A novel phenanthrene dioxygenase from Nocardioides sp. strain KP7: expression in Escherichia coli. J Bacteriol. 2000 Apr;182(8):2134–2141.
- Hanne LF, Kirk LL, Appel SM, et al. Degradation and induction specificity in actinomycetes that degrade p-nitrophenol. Appl Environ Microbiol. 1993 Oct;59(10):3505–3508.
- Piutti S, Semon E, Landry D, et al. Isolation and characterisation of Nocardioides sp. SP12, an atrazine-degrading bacterial strain possessing the gene trzN from bulk- and maize rhizosphere soil. FEMS Microbiol Lett. 2003 Apr;221(1):111–117.