ABSTRACT
To elucidate the mechanism underlying tetrahydrofolate (THF) accumulation in sake yeast strains compared with that in laboratory yeast strains, we performed a quantitative trait locus (QTL) analysis. The results revealed that the sake yeast ERC1 allele contributes to an increase in the ratio of THF to the total folate content in sake yeast.
Folate, an essential cofactor for the transfer reactions of one-carbon groups in cells (i.e., formyl, methenyl, methylene, and methyl groups), is thus important for the methionine biosynthetic pathway, amino acid metabolism, and nucleic acid biosynthesis. A sufficient intake of folate may therefore reduce the risk of neural tube defects (NTDs), cardiovascular diseases, and certain cancers [Citation1–Citation3].
Compared to laboratory yeasts, sake yeast was reported to have significantly higher accumulations of folate [Citation4], and it has been shown that several amino acids [Citation5] and genes [Citation6] are involved in the accumulation of folate and the ratio of compounds of folate in yeast. However, the details of the folate accumulation mechanism in yeast have been unclear. In this study, to elucidate the mechanism underlying the folate accumulation in sake yeast strains compared with that in laboratory yeast strains, we performed a quantitative trait locus (QTL) analysis. The main forms of folate in yeast are two types of compounds: tetrahydrofolate (THF) and 5-methyl-tetrahydrofolate (5MTHF). We therefore determined the THF and 5MTHF contents by high-performance liquid chromatography (HPLC), and we defined the total value of the THF and 5MTHF contents as the total folate content in this study.
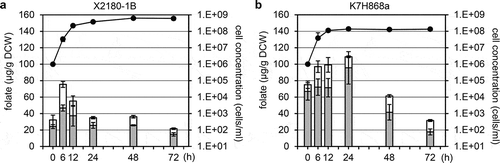
First, to develop the appropriate cultivation time for the 100 hybrid F1 segregants (the haploid sake yeast strain K7H868a × the laboratory yeast strain X2180-1B) for the QTL analysis, we determined the THF, 5MTHF, and total folate contents of the X2180-1B and K7H868a strains as parental strains during cell growth at 30°C in yeast extract peptone dextrose (YPD) medium. As shown in , the total folate content was constantly and significantly higher in the K7H868a strain than in the X2180-1B strain (p < 0.05). The 5MTHF and THF contents were significantly greater in K7H868a compared to X2180-1B strain from 0 h (incubation) to 24 h (p < 0.05) and from 12 h to 72 h (p < 0.05), respectively. We thus decided to measure the 5MTHF and THF contents of the 100 hybrid F1 segregants after 12-h incubation at 30°C in YPD medium.
Figure 1. The sake yeast strain K7H868a accumulated more THF, 5MTHF, and total folate contents compared to the laboratory yeast strain X2180-1B in YPD medium.
Next, we analyzed the THF and 5MTHF contents of the 100 F1 segregants of the hybrid yeast after 12-h incubation at 30°C in YPD medium. Because 10 of the 100 F1 segregants exhibited flocculation during preincubation, we used only the remaining 90 F1 segregants in the subsequent experiments. As a result, the total folate, THF, and 5MTHF contents of the 90 segregants showed a wide range of values from 19.9 to 148.2 (total folate), 3.5 to 40.1 (THF), and 13.4 to 125.6 (5MTHF) μg/g dry cell weight (DCW).
We then performed a QTL analysis along with a linkage analysis based on the THF, 5MTHF, and total folate contents and the DNA marker genotypes of the 90 F1 segregants. For the LOD score, the higher the numerical value, the higher the probability is that a QTL exists in that region on the chromosome. We eliminated the data for chromosomes VII and X because aneuploidy is unacceptable for the identification of QTLs [Citation7].
As shown in , we detected a significant QTL with the LOD score of 5.1 that has an additive effect on chromosome VIII in the sake yeast haplotype, and this QTL contributes to the accumulation of THF. A significant QTL contributing to the accumulation of 5MTHF was also observed on chromosome VIII, but the LOD score was low (3.2), and it was a QTL with an additive effect in a laboratory yeast haplotype. Regarding the total folate content, we detected a significant QTL with the LOD score of 4.2 that has an additive effect on chromosome XVI in the laboratory yeast haplotype. We therefore focused on the QTLs that contribute to the accumulation of THF in the sake yeast haplotype and have the highest LOD scores, and we searched for genes that contribute to the accumulation of THF in sake yeast.
Figure 2. Significant quantitative trait loci (QTLs) involved in THF, 5MTHF, and total folate contents in sake yeast strains.
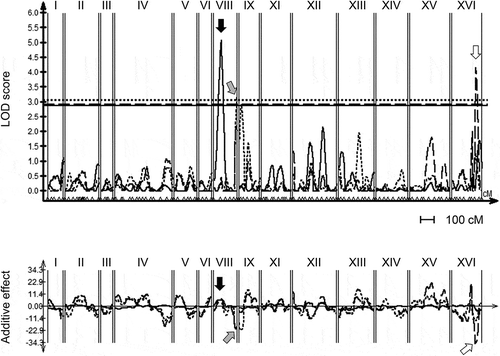
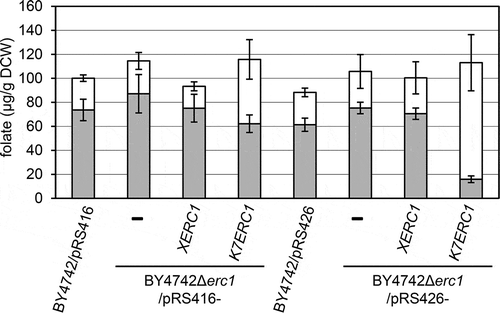
Surprisingly, the QTL and the DNA marker nearest the QTL on chromosome VIII, G1HH1 that contributed to the accumulation of THF identified in this study were identical to the QTL and DNA marker that were demonstrated to contribute to the high accumulation of S-adenosylmethionine (SAM) in sake yeast and led to enhanced stress resistance and longevity [Citation8,Citation9]. Therefore, to clarify whether the sake yeast ERC1 allele (K7ERC1) gene also contributes to the accumulation of THF in sake yeast strains, we determined the THF, 5MTHF, and total folate contents of yeasts transformed with the K7ERC1 and the laboratory yeast ERC1 allele (XERC1) genes by using a low- (pRS416) and a high-copy number plasmid (pRS426) in BY4742 and BY4742Δerc1.
These yeasts were cultivated at 30°C for 22 h in SD-Ura medium, which consisted of a 0.67% yeast nitrogen base without amino acids, 1% glucose, and 0.077% uracil dropout supplements (Takara Bio, Mountain View, CA). As shown in , compared with the BY4742Δerc1 with a vector (pRS416 or 426), the expression of the XERC1 gene using a low- and a high-copy number plasmid in BY4742Δerc1 had no significant effect on the accumulation of THF. On the other hand, the expression of the K7ERC1 gene using a low- and high-copy number plasmid in BY4742Δerc1 resulted in a significant intracellular accumulation of THF (p < 0.05), and no significant change in total folate content was observed. However, the 5MTHF content was significantly decreased with the increase of THF content using a high-copy number plasmid in BY4742Δerc1 (p < 0.001). These results suggest that the sake yeast ERC1 allele contributes to an increase in the ratio of THF to the total folate content rather than an increase in the THF content in the folate synthetic pathway in sake yeast. We predict that the functions of multiple genes with K7ERC1 contribute to the accumulation of the THF content in K7H868a because the folate content in yeast is a quantitative trait, but the details of the mechanism remain unclear, thus warrant further study.
Figure 3. The sake yeast ERC1 allele contributed to an increase in the ratio of THF to the total folate content in sake yeast.
SAM plays an important role in vivo mainly as a methyl group donor, and the methyl group is derived from the metabolism of 5MTHF to THF in the folate synthesis pathway. We thus speculate that (i) the metabolism reaction of 5MTHF to THF (MET6: methyltransferase) in the folate synthesis pathway was activated by the expression of K7ERC1 in sake yeast, and then (ii) the metabolism of the homocysteine to methionine, which is the precursor of SAM in the methionine synthesis pathway, was activated by the action of Met6p using a methyl group, resulting in (iii) increased SAM content in sake yeast. In future analyzes, if the responsible genes and mutations of QTLs contributing to high accumulations of 5MTHF and total folate in sake yeast are identified, we expect that greater amounts of total folate in sake yeast can be accumulated. Chemically synthesized folate is currently widely used, but for pregnant women who are sensitive to the effects on the fetus, there is a tendency to demand safe, natural folate. Thus, our findings might be useful in the breeding of high folate-accumulating yeast strains for industrial uses such as dietary supplements, sake cakes and so on.
Author contributions
H.I. and M.K. designed the study. T.K. performed almost all the experiments. T.M. supported the experiments. M.K. wrote the draft of the manuscript and revised the final manuscript. M.M., D.W., T.A., T.F. and H.I. discussed the results and contributed to the improvement of the manuscript. All authors have approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data described in this article are openly available in the Open Science Framework at DOI:10.17605/OSF.IO/TPA6U.
References
- De-Regil LM, Pena-Rosas JP, Fernandez-Gaxiola AC, et al. Effects and safety periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2015;12:CD007950
- Li Y, Huang T, Zheng Y, et al. Folic acid supplementation and the risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2016;5:e003768.
- Pieroth R, Paver S, Day S, et al. Folate and its impact on cancer risk. Curr Nutr Rep. 2018;7:70–84.
- Izu H, Shobayashi M, Manabe Y, et al. S-adenosylmethionine (SAM)-accumulating sake yeast suppresses acute alcohol-induced liver injury in mice. Biosci Biotechnol Biochem. 2006;70:2982–2989.
- Hjortmo S1, Patring J, Andlid T. Growth rate and medium composition strongly affect folate content in Saccharomyces cerevisiae. Int J Food Microbiol. 2008;123:93–100.
- Shibata Y, Yamada T, Morimoto T, et al. Mechanism of high folate accumulation in a sake yeast other than Kyokai yeasts. J Biosci Bioeng. 2020;129:1–5.
- Katou T, Namise M, Kitagaki H, et al. QTL mapping of sake brewing characteristics of yeast. J Biosci Bioeng. 2009;107:383–393.
- Kanai M, Kawata T, Yoshida Y, et al. Sake yeast YHR032W/ERC1 haplotype contributes to high S-adenosylmethionine accumulation in sake yeast strains. J Biosci Bioeng. 2017;123:8–14.
- Ogawa T, Tsubakiyama R, Kanai M, et al. Stimulating S-adenosyl-l-methionine synthesis extends lifespan via activation of AMPK. Proc Natl Acad Sci USA. 2016;113:11913–11918.
