ABSTRACT
We evaluated the protein and energy intakes of infants fed commercial infant Formula A (protein, 2.2 g/100 kcal; energy, 68 kcal/100 mL) and examined whether changes in feeding intervals are involved in constant energy intake. Daily nutritional intake of 378 Formula A-fed infants was assessed using reference values and compared to that of infants fed Formulas B (protein: 2.3 g/100 kcal, energy: 68 kcal/100 mL) and C (protein: 2.4 g/100kcal, energy: 70 kcal/100 mL). From 15 to 149 days of age, the mean formula volume and protein intake were 758–887 mL/day and 11.4–13.3 g/day, respectively, higher than the protein intake of breast-fed infants. Daily energy intake (86–129 kcal/kg/day) was comparable to the estimated energy requirements. Feeding intervals were shorter in infants fed Formulas A and B than in those fed Formula C, whereas energy intake was similar. The protein intake of infants decreased as the protein concentration per energy in infant formula was reduced, and accordingly the protein intake of Formula A-fed infants was significantly lower than that of Formula C-fed infants. In conclusion, the new composition of Formula A is suitable in protein and energy intake of infants, and daily energy intake remains constant by shortening in feeding intervals when the energy concentration in infant formula is reduced.
Clinical Trial Registration: UMIN000023110
Graphical abstract
Daily protein and energy intakes of infants fed a commercial infant formula with a reduced protein concentration of 2.2 g/100 kcal: an impact of feeding interval on energy intake
Protein intake during infancy has a direct impact on growth and overall health at later ages as protein is an essential nutritional component involved in growth and development. To ensure adequate growth of infants, at least the required amount of protein must be ingested [Citation1–Citation4]. Insufficient protein intake can affect the growth and development of infants [Citation3]. In contrast, excessive protein intake should be avoided as it increases the burden on organs such as the kidneys and can cause excessive stimulation of metabolic regulation. Recent studies have shown that excessive intake of protein during infancy can impact future health and can cause the onset of diseases such as obesity and hypertension [Citation5,Citation6]. Therefore, while developing an infant formula, it is important to ensure that it contains the amount of protein that satisfies the minimum requirement but keeps away from deleterious levels.
Infants need to intake energy, which is neither excess nor insufficient, to balance energy expenditure for maintaining the body and optimal physical activity, as well as to support a rapid growth rate and the appropriate synthesis and deposition of body tissue. Infants ingest the required amount of energy from breast milk [Citation7]. Although previous studies have shown that formula-fed infants may consume more energy than breast-fed infants [Citation8–Citation12], we demonstrated that daily energy intake remains the same when the energy content in the formula is close to that in breast milk [Citation13]. Considering that energy intake per feeding varies depending on several factors such as the amount of breast milk secreted, amount of infant formula prepared, and energy concentration of breast milk or infant formula, we hypothesized that changes in feeding intervals plays an important role in ensuring energy intake that is neither excessive nor deficient. However, this has not been examined in any case to date.
In a novel commercial infant formula (Formula A), the protein concentration was reduced from 2.3 g/100 kcal (less than the mean plus standard deviation in breast milk) to 2.2 g/100 kcal to bring the levels closer to the mean protein concentration in breast milk (1.9 g/100 kcal) [Citation14], with energy concentration maintained at 68 kcal/100 mL. We evaluated the suitability of Formula A by conducting a 12-month follow-up study and found similar growth of infants fed Formula A and those fed breast milk as before [Citation15,Citation16]. Such an evaluation has also been recommended by some organizations [Citation17–Citation19]. However, the nutrient intake and feeding pattern of Formula A-fed infants were not examined.
Here, we evaluated the daily protein and energy intake of infants exclusively fed Formula A compared with those fed breast milk or with dietary reference intakes to assess the suitability of Formula A. Furthermore, we compared the feeding patterns of Formula A-fed infants to those of Formula B- or C-fed infants. Formula C has higher protein (2.4 g/100 kcal) and energy concentration (70 kcal/100 mL) than Formula A, whereas Formula B has higher protein concentration (2.3 g/100 kcal) than Formula A and the same energy concentration (68 kcal/100 mL) as Formula A; thus, we examined whether changes in feeding intervals are involved in constant energy intake in infants.
Materials and methods
Ethical statement
This observational study was registered at the University Hospital Medical Information Network (UMIN) Clinical Trial Registry (UMIN000023110; http://www.umin.ac.jp/). The study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the Meiji Institutional Review Board (Approval No.32). Written informed consent was obtained from the parents at one-month medical checkup before study entry.
Participants and study design
The participants and protocol, including the sample size, have been described previously [Citation15] and this study is a secondary analysis focusing on the protein and energy intakes in Formula-fed infants. In summary, infants aged 15–59 days who had been fed breast milk or Formula A (Meiji Co., Ltd., Tokyo, Japan) purchased by the parents, were recruited between September 1 2014 and March 31 2016 throughout Japan (n = 1053). The infants were followed up until 12 months of age. We conducted a questionnaire investigation at the following four time points: at the start of the study when the infants were 15–59 days old and at 90–149, 180–239, and 330–389 days of age. Generally, at these ages, infants undergo a routine medical checkup in most areas of the country; thus, accurate anthropometric data were collected. To encourage continual participation in the study, a questionnaire form was sent to the parents with baby-care goods such as toys or household items. To evaluate the nutritional intake from an infant formula, we used the data obtained until the infants were 149 days old; during this age range, infants generally intake little energy from weaning foods.
Nutritional composition of formula
The nutritional compositions of Formulas A, B, and C are shown in . We previously reported the data for Formulas B and C [Citation13,Citation20,Citation21].
Table 1. Nutritional composition of Formula A.
Data collection and calculation
We collected data using a questionnaire including questions regarding the infant’s sex, gestational age at birth, disease, and body weight at birth and the four time points described above. In addition, the amount of Formula A prepared and unfinished was recorded for one day. The ingested amount was calculated by subtracting the unfinished amount from the prepared amount. Daily energy and nutrient intakes were calculated by multiplying the concentration of energy/nutrient in the formula by the daily intake volume of the formula. The feeding intervals were estimated by dividing 24 h by the number of daily feedings.
Inclusion criteria
Inclusion criteria for analysis were as follows: no disease, gestational age not less than 37 weeks, birth weight of 2500–4000 g, and exclusively Formula A-fed at questionnaire investigation.
Evaluation of energy and nutrient intakes
Formula A-fed infants aged 15–59 days were divided into the following two groups: 15–29 and 30–59 days old. Similarly, Formula A-fed infants aged 90–149 days were divided into the following two groups: 90–119 and 120–149 days old. Energy intake was evaluated by comparing the energy intake of the infants to the estimated energy requirement as per body weight indicated by the Food and Agriculture Organization (FAO) [Citation22]. The intake of protein in Formula A-fed infants was evaluated by comparing with that in breast-fed infants. Additionally, we compared the intakes of 18 micronutrients to recommended nutrient intake (RNI) set by the World Health Organization (WHO) [Citation23].
Comparison of feeding patterns
For Formula A-fed infants aged 90–149 days, daily energy intake, volume per feeding, energy per feeding, feeding interval, and daily protein intake were calculated from the responses in the questionnaires. These data were then compared among the three groups of infants fed Formulas A, B, and C.
Statistical analysis
The values are expressed as the mean (standard deviation). We compared the differences among groups by one-way analysis of variance with a post-hoc Tukey–Kramer test. To determine the relationship between two variances, we used Pearson’s correlation coefficient. For all tests, results with P < 0.05 were considered statistically significant. Statistical analyses were performed using Bell Curve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan).
Participant involvement
Participants were not involved in the design or conduct of this study.
Results
Participants
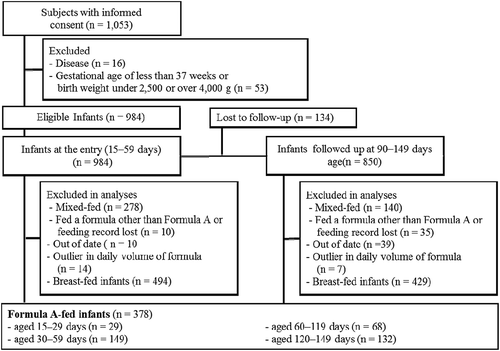
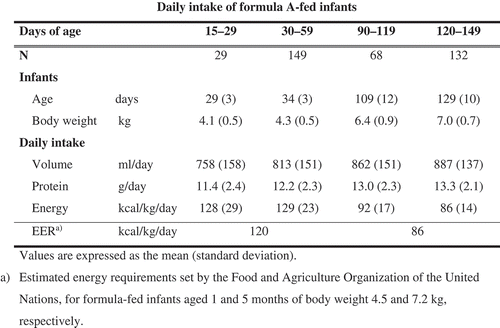
We obtained informed consent from the parents of 1053 infants. In total, 378 infants met the inclusion criteria. A detailed flow diagram and reasons for exclusion are shown in . We included 29, 149, 68, and 132 Formula A-fed infants aged 15–29, 30–59, 90–119, and 120–149 days, respectively. Participant characteristics are shown in .
Table 2. Characteristics and daily intake of formula A-fed infants.
Daily intakes of formula a-fed infants
The daily intake of formula volume, protein, and energy is shown in . The daily protein intake of Formula A-fed infants was higher than the average intake of breast-fed infants (8.8–10.8 g/day) [Citation17]. Daily energy intakes per body weight (86–128 kcal/kg/day at 15–149 days of age) were comparable to the estimated energy requirements set by the FAO (). The daily intakes of 18 micronutrients are shown in . The mean intakes of biotin and iodine did not reach the RNI recommended by the WHO. The mean intakes of other micronutrients were higher than the corresponding RNI.
Table 3. Daily intake of protein, vitamins, and minerals of Formula A-fed infants.
Feeding pattern analysis
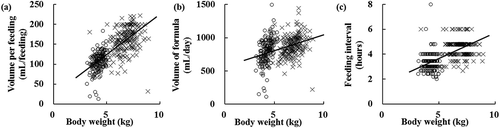
The feeding patterns of infants fed Formulas A, B, and C are shown in . Daily energy intake did not differ among the three groups. Although volume per feeding was similar among the three groups, energy intake per feeding in Formula A- and B-fed infants (68 kcal/100 mL) was significantly lower than that in Formula C-fed infants (70 kcal/100 mL; P < 0.05 based on Tukey–Kramer test). Feeding intervals were shorter in infants fed formulas A (4.5 h) and B (4.6 h) than in those fed formula C (4.9 h) (P < 0.05 based on Tukey–Kramer test). Protein intake of Formula A-fed infants was significantly lower than that of Formula C-fed infants (P < 0.05 based on the Tukey–Kramer test). Significant correlations were observed between body weight and volume per feeding, between body weight and daily volume of formula, and between body weight and feeding intervals (P < 0.05 based on Pearson’s correlation coefficient, R = 0.74, 0.36, and 0.59, respectively; ).
Table 4. Feeding pattern in infants fed Formulas A, B, and C at 90–149 days of age.
Figure 2. Relationship between body weight and each feeding variable in Formula A-fed infants.
Relationships between (a) body weight and volume per feeding, (b) body weight and volume of formula, and (c) body weight and feeding interval (○: at 15–59 days of age, ×: at 90–149 days of age). For all infants aged 15–149 days, significant correlations were observed in (a), (b), and (c) based on Pearson’s correlation coefficient (P < 0.05, R = 0.74, 0.36, and 0.59, respectively).
Discussion
The present evaluation of protein and energy intakes in Formula A-fed infants indicates that the nutritional composition of Formula A is suitable for infants. In terms of energy, we found that change in the feeding interval is a key factor for daily constant energy intake. Few studies have examined the nutritional intake or feeding patterns of infants fed a commercial infant formula.
In the present study, the protein concentration in Formula A (2.2 g/100 kcal) was found to be appropriate in terms of protein intake in Formula A-fed infants. In infants, the utilization efficiency of cow milk protein is approximately 70%, which is similar to that of breast milk [Citation24]. The protein intake of Formula A-fed infants was not less than that of breast-fed infants (8.8–10.8 g/day) [Citation17]. We previously showed that the body weight and body mass index of Formula A-fed infants were equivalent to those of breast-fed infants and that changes in body weight were in accordance with the WHO and Japan standard growth curves [Citation15]. Overall, the present study demonstrated the suitability of the protein concentration in Formula A by evaluating protein intake. Additionally, we found that the protein intake of infants decreased as the protein concentration per energy in infant formula was reduced, and accordingly the protein intake of Formula A-fed infants was significantly lower than that of Formula C-fed infants (). This is because of the constant energy intake in formula-fed infants (). These results indicate that reduced protein concentration per energy in Formula A enabled adequate reduction of protein intake while energy intake remained constant.
Evaluation of energy intake in Formula A-fed infants indicated the suitability of the energy concentration in Formula A. The FAO indicates the estimated energy requirements for formula-fed and breast-fed infants separately, based on data showing that energy consumption in the former is higher than that in the latter [Citation22,Citation25]. In the present study, we used the estimated energy requirement of formula-fed infants. As shown in , the energy intake of Formula A-fed infants was the same as the estimated energy requirements. A previous study showed lower intake of formula (541 mL/day) in formula-fed infants aged 1 month [Citation26]. This is likely because the weight of infants included in the previous study was lower than that of the 15–29-day-old Formula A-fed infants in this study. Consistent with a previous evaluation of growth [Citation15], we found that compared to the estimated energy requirement, the energy intake of Formula A-fed infants was appropriate.
Interestingly, the daily energy intake per body weight in infants of the same age appeared to remain constant with changes in feeding intervals, regardless of the energy concentration in the infant formula. In detailed analyses, we demonstrated that energy intake per feeding depends on the energy concentration and body weight and that the change in energy intake per feeding affects the feeding interval (), resulting in a daily constant energy intake. Some studies have suggested that infants fed formula have a decreased ability to self-regulate volume per feeding [Citation27,Citation28]. However, they did not focus on the daily energy intake. Based on our findings, infants modify the feeding interval according to energy intake per feeding, resulting in a daily constant energy intake.
The intake of biotin and iodine of Formula A-fed infants may not be sufficient. These micronutrients in the formula relied only on their content in cow milk, as adding these micronutrients to the formula was not legally allowed in Japan when Formula A was released. Therefore, the present results were inevitable. However, variations in the intake of these micronutrients are relatively small (), suggesting that there would be no infants with extremely low intake. Currently (in November 2019), biotin can be legally added to infant formula. The intake of biotin by formula-fed infants in Japan is thus expected to improve in the future.
This study had some limitations. First, an observational study design always has the possibility of unknown confounders, which cannot be taken into account. Second, data were collected using a questionnaire. These methodological limitations may have resulted in overestimation or underestimation of the evaluated outcomes. Finally, evaluation of protein and micronutrient intake inherently needs comprehensive judgment based on clinical outcomes, such as frequency of deficient or excess intake-related symptoms and blood biochemical findings in clinical trials. However, clinical trials targeting healthy term infants were not feasible considering the ethical considerations. Furthermore, few studies have examined the nutritional intake of term infants fed commercial infant formula. Therefore, our results will facilitate improved infant nutrition in the future.
Conclusions
The present evaluation of energy and protein intakes indicates the suitability of the nutritional composition of Formula A, consistent with a previous evaluation of the growth of Formula A-fed infants [Citation15]. Moreover, daily energy intake remains constant by shortening in feeding intervals when the energy concentration in infant formula is reduced. Infants should consume as much milk as they want during every feeding when fed with breast milk and/or infant formula with the appropriate nutritional amount per energy. The present findings will facilitate improvements in infant nutrition.
Author contributions
S. J. and T. K. designed the study; S. J., K. Y., and T. K. conducted the study; S. J., K.Y., and Y. N. analyzed the data, and S. J., Y.N., and T. K. wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors are extremely grateful to the participants (infants and their parents) for their participation. We also thank the registered dietitians and dietitians at the head and branch offices of Meiji Co., Ltd. throughout Japan for recruiting infants for the study.
Data availability statement
The data described in this article are openly available in the Open Science Framework at DOI:10.17605/OSF.IO/TPA6U.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Fomon SJ, Ziegler EE, Nelson SE, et al. What is the safe protein-energy ratio for infant formulas? Am J Clin Nutr. 1995;62:358–363.
- Fomon SJ, Ziegler EE, Nelson SE, et al. Infant formula with protein-energy ratio of 1.7 g/100 kcal is adequate but may not be safe. J Pediatr Gastroenterol Nutr. 1999;28:495–501.
- Räihä NC, Fazzolari-Nesci A, Cajozzo C, et al. Whey predominant, whey modified infant formula with protein/energy ratio of 1.8 g/100 kcal: adequate and safe for term infants from birth to four months. J Pediatr Gastroenterol Nutr. 2002;35:275–281.
- Turck D, Grillon C, Lachambre E, et al. Adequacy and safety of an infant formula with a protein/energy ratio of 1.8 g/100 kcal and enhanced protein efficiency for term infants during the first 4 months of life. J Pediatr Gastroenterol Nutr. 2006;43:364–371.
- Weber M, Grote V, Closa-Monasterolo R, et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am J Clin Nutr. 2014;99:1041–1051.
- Koletzko B, von Kries R, Closa R, et al. Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr. 2009;89:1836.
- Dewey KG, Lönnerdal B. Infant self‐regulation of breast milk intake. Acta Paediatr. 1986;75:893–898.
- Fomon SJ, Filmer JL, Thomas LN, et al. Influence of formula concentration on caloric intake and growth of normal infants. Acta Paediatr Scand. 1975;64:172–181.
- Heinig MJ, Nommsen LA, Peerson JM, et al. Energy and protein intakes of breast-fed and formula-fed infants during the first year of life and their association with growth velocity: the DARLING Study. Am J Clin Nutr. 1993;58:152–161.
- Baird J, Poole J, Robinson S, et al. Southampton women’s survey study group. Milk feeding and dietary patterns predict weight and fat gains in infancy. Paediatr Perinat Epidemiol. 2008;22:575–586.
- Kramer MS, Guo T, Platt RW, et al. Feeding effects on growth during infancy. J Pediatr. 2004;145:600–605.
- Dewey KG. Growth characteristics of breast-fed compared to formula-fed infants. Neonatol. 1998;74:94–105.
- Kanno T, Jinno S, Kaneko T. Survey of the anthropometric growth, nutritional intake, fecal properties, and morbidity of infants, in relation with feeding methods (XI) (in Japanese). J Child Health. 2013;72:253–260.
- Yamawaki N, Yamada M, Kan-no T, et al. Macronutrient, mineral and trace element composition of breast milk from Japanese women. J Trace Elem Med Biol. 2005;19:171–181.
- Jinno S, Yamazaki K, Nakamura Y, et al. Growth of term infants fed a commercial infant formula with a protein content of 2.2 g/100 kcal: an observational follow-up study (Published online). Biosci Biotechnol Biochem. 2020;84:633–639.
- Jinno S. Infant growth evaluation: essential for appropriate design of infant formula (in Japanese). Milk Sci. 2015;64:35–42.
- Scientific Committee on Food. Report of the scientific committee on food on the revision of essential requirements of infant formulae and follow-on formulae. 2003.
- Codex Alimentarius Commission, Codex Alimentarius Commission. Standard for infant formula and formulas for special medical purposes intended for infants. Codex Stan. 2007;72:1–17.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the essential composition of infant and follow‐on formulae. Efsa J. 2014;12:3760.
- Jinno S, Nakamura Y, Kanno T, et al. Intake of energy and nutrients in exclusively formula-fed infants. Milk Sci. 2014;63:63–69.
- Yonekubo A, Kanno T. A survey of physical growth, nutritional intake, fecal properties and morbidity of infants as related to feeding methods (VIII) (in Japanese). J Child Health. 1999;58:93–103.
- Food and agricutural organization. Human energy requirements: report of a Joint FAO/WHO/UNU Expert Consultation. FAO Food Nutr Tech Rep Ser. 2001;96.
- Food and Agriculture Organization of the United Nations, World Health Organization. Vitamin and mineral requirements in human nutrition. Joint FAO/WHO Consultation on Human Vitamin and Mineral Requirements. Geneva: FAO/WHO; 2004. [cited 2019 Oct 29]. Available from: https://www.who.int/nutrition/publications/micronutrients/9241546123/en/
- Joint WH. Protein and amino acid requirements in human nutrition. World Health Organ Tech Rep Ser. 2007;935:1.
- Butte NF, Wong WW, Ferlic L, et al. Energy expenditure and deposition of breast-fed and formula-fed infants during early infancy. Pediatr Res. 1990;28:631.
- Isomura H, Takimoto H, Miura F, et al. Type of milk feeding affects hematological parameters and serum lipid profile in Japanese infants. Pediatr Int. 2011;53:807–813.
- Li R, Fein SB, Grummer-Strawn LM. Do infants fed from bottles lack self-regulation of milk intake compared with directly breastfed infants? Pediatrics. 2010;125:e1386–e1393.
- Ventura AK, Beauchamp GK, Mennella JA. Infant regulation of intake: the effect of free glutamate content in infant formulas. Am J Clin Nutr. 2012;95:875–881.