ABSTRACT
Our previous study revealed that gamma-aminobutyric acid (GABA) in Earl’s muskmelon is more concentrated in the inner than the outer parts of the fruit. Here, the GABA and antioxidant capacity of the placental tissue of muskmelon, which is considered waste, were evaluated for possible use as a source of bioactive compounds. The concentrations of GABA and related substances in the placental tissue were significantly higher than in the fleshed pulp, whereas glutamic acid and sugar levels were significantly lower. The two sites showed no difference in GAD activity. Furthermore, the placental site showed high antioxidant capacities based on 2,2-diphenyl-1-picrylhydrazyl and oxygen radical absorbance capacity for hydrophilic compounds assays compared with the fleshed pulp, because of the higher levels of total phenolic and L-ascorbic acids. Therefore, the placental tissue of muskmelons may be useful for developing functional foods, which would also reduce the amount of residues during muskmelon processing.
Graphical abstract
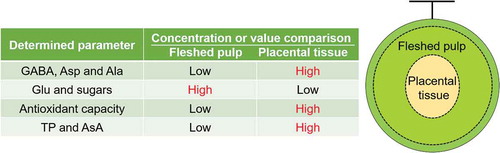
The present study found that the placental tissue in Earl’s muskmelon, has a higher GABA concentration and antioxidant capacity than the fleshed pulp.
Melon (Cucumis melo L.) is a popular dessert fruit [Citation1], and over 31 million tons of it was produced worldwide in 2016 (http://faostat3.fao.org). The melon’s morphotypes are commonly partitioned into market classes according to their culinary attributes, which separate groups such as Reticulatus (Earl’s and Andes), Cantalupensis (Galia, Charentais, and Ogen), Indodorus (Honeydew), and Conomon (Oriental) [Citation2]. The fresh raw fruits of Reticulatus, Cantalupensis, and Indodorus groups are often directly consumed due to their sweetness and flavor [Citation3]. In addition, these melons are a valuable source of gamma-aminobutyric acid (GABA) and antioxidants such as L-ascorbic acid (AsA), tocopherol, and folic acid [Citation4,Citation5] with the potential to prevent or delay lifestyle-related diseases [Citation6,Citation7].
Many studies have been conducted to evaluate the effects of GABA in the body. For example, a GABA intake of 10 mg was found to lower blood pressure in patients with mild hypertension [Citation8], while a dosage of 28 mg could induce relaxation in people experiencing stress [Citation9]. GABA is present in many foods such as fruits and vegetables that people consume every day. Plant tissues including melon produce GABA during glutamic acid (Glu) catalysis by glutamic acid decarboxylase (GAD) [Citation10], which has a calmodulin binding site [Citation11] and is thus rapidly activated in response to increased cytosolic Ca2+ concentration by hypoxia stress [Citation10,Citation12]. Glu is known to play an important role in nitrogen metabolism in plants, and it is produced from aspartic acid (Asp), alanine (Ala), and so on under transamination reaction [Citation13]. Notably, some melon cultivars have been shown to have higher GABA concentrations (0.103–4.26 mg mL−1) [Citation5,Citation12,Citation14] than other plant materials, such as tea leaves and stems, cabbage, carrot, tomato, and cucumber (ranging from 0.0012 to 0.09 mg g−1 dry weight [Citation10,Citation15] and 0.007 to 0.39 mg g−1 fresh weight [Citation16]). Therefore, melon-derived GABA is considered an important promoter of human health.
Among the many melon cultivars [Citation5,Citation14,Citation17], we have been interested in “Earl’s Favorite”. This cultivar, which came from the United Kingdom in 1925, is now the most widely produced cultivar in Shizuoka Prefecture, Japan [Citation5]. The basic cultivation method is shared among members of an agricultural cooperative (http://www.crown-melon.co.jp). It involves growing the fruit in a glass greenhouse with controlled temperature (approximately 30°C from morning to evening, and approximately 22°C at night), humidity (approximately 80% from morning to evening, and approximately 90% at night), and watering. The fruits produced by this method are called “greenhouse muskmelons.” Previously, we verified that the GABA concentration in the inner fruit part of this cultivar is higher than in the outer part [Citation5]. Therefore, we also speculate that the most internal part of the melon, namely the placenta site, may have an even higher GABA concentration, even though it is usually discarded as waste when producing cut muskmelon for market distribution. However, there has been no reported characterization of GABA or related substances such as Asp, Glu, and Ala at the melon placenta site.
On the other hand, the residues of other fruits and vegetables (apple, tomato, broccoli, and so on) have shown high amounts of phenolic compounds with antioxidant properties [Citation18]. For instance, the recycling of apple residue from pressing has been supported by reports indicating that most of the antioxidants in fresh apple are retained in the solid matter, rather than being transferred into the juice [Citation19]. Similarly, other underutilized or inedible parts including those of melons may be rich in antioxidant compounds. Characterizing the antioxidant compounds and their activity in waste melon tissue may promote its use for human health and nutrition. Recent investigation has demonstrated that the placental tissue of cantaloupe melon (of the Cantalupensis group) containing carotenoid pigments has a higher antioxidant capacity and ratio of related compounds compared to the fleshed pulp site [Citation20]. Therefore, characterization of antioxidant compounds in the placenta of Earl’s muskmelon (C. melo var. Reticulatus) in addition to GABA and related substances could lead to possible utilization of this processing residue as new functional foods and supplements.
In the present study, we compared the concentrations of GABA and related substances as well as the antioxidant capacity between the fleshed pulp and placenta sites of greenhouse Earl’s muskmelons, as this has not been reported to the best of our knowledge. In addition, we evaluated the change in sucrose, glucose, and fructose concentrations, and GAD activity between these parts.
Materials and methods
Chemicals
Sodium acetate and ethanol were purchased from Kanto Chemical Co. (Tokyo, Japan). Fluorescein sodium salt and (±)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were from Sigma-Aldrich Co. (Milwaukee, WI, USA). Folin-Ciocalteu’s phenol reagent and 2-morpholinoethanesulfonic acid were from Merck Co. (Darmstadt, Germany) and Chemical Dojin Co. (Kumamoto, Japan), respectively. Other chemicals were from Wako Pure Chemical Industries (Osaka, Japan).
Sample preparation
Summer cropping muskmelons (Cucumis melo L. “Earl’s Favorite”) harvested in June 2018 were purchased from a melon growers’ association (Fukuroi, Shizuoka, Japan). The melons were cultivated in greenhouses at 22–30°C, and the average temperature (July 1–30) in the surrounding area in Fukuroi City was 22.5°C, based on data from 2018 (https://www.jma.go.jp/jma/index.html). The muskmelons weighed 1498 ± 155 g each. The melons were used quickly after purchase, after measuring their hardness according to acoustic impulse information (245 ± 16 Hz) derived with a hammer test (Shizuoka Seiki, Fukuroi, Shizuoka, Japan) [Citation21]. The texture of the fleshed pulp was considered firm during the early stage of ripening, as the soft-fleshed melons are considered to have a mean value below 225 Hz [Citation5].
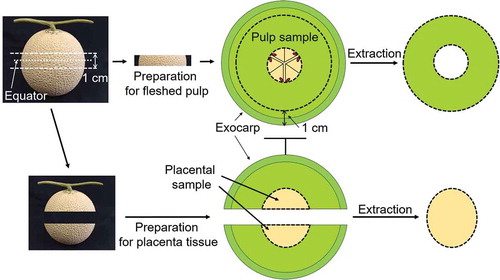
Twelve muskmelons were assigned to each of two groups, one tested for the fleshed pulp and the other for the placental tissue. All melons were cut at 0.5 cm above and below the equator. For the first group, the seeds and the placental tissue within the 1-cm slice were removed. After cutting out the rind (1 cm from the exocarp, ), the ring of fleshed pulp was squeezed using a juicer. The juice was centrifuged at 13,040 × g at 4°C. The supernatant was then passed through a 0.20-μm membrane filter and stored at −80°C until antioxidant capacity and concentrations of amino acid, total phenols, AsA, and sugars. Whereas, the pellet was used for determination of GAD activity. For the second group, the placental tissue in the upper and lower halves was obtained, the seeds were removed (4.9 ± 0.5% (w/w) of total weight of the melon), and the placental juice was obtained and processed using the same conditions as for the flesh pulp juice.
Figure 1. Method of segmenting the greenhouse muskmelons.
Muskmelons were cut 0.5 cm above and below the equator, and juice was extracted from the placental tissue in the upper and lower halves (4.9% (w/w) of the total muskmelon weight without seeds). The center slice was used as the fleshed pulp sample after cutting out the outermost ring 1 cm from the exocarp.
Determination of amino acid concentrations
The following 16 proteinogenic amino acids, as well as GABA, were separated and quantified under our HPLC method [Citation5]: GABA, Asp, Glu, serine (Ser), asparagine (Asn), glycine (Gly), glutamine (Gln), histidine (His), Ala, proline (Pro), tyrosine (Tyr), valine (Val), methionine (Met), isoleucine (Ile), leucine (Leu), lysine (Lys), and phenylalanine (Phe).
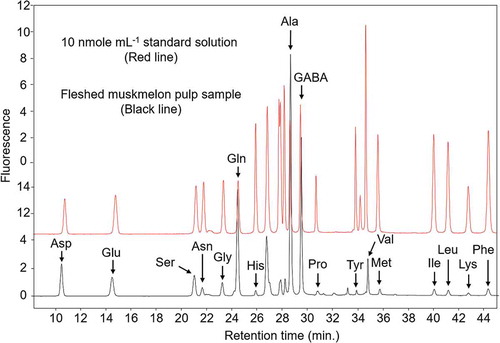
The concentrations of these amino acids were determined using AccQ-Tag high-performance liquid chromatography (Waters, Milford, MA, USA) with a fluorescence detection system (HPLC-FLD) according to a pre-column derivatization method [Citation5]. The derivatized amino acids were separated on an AccQ-Tag Column (Waters) and analyzed by HPLC-FLD according to the following conditions. The HPLC analysis was performed using a gradient method (). The column temperature was 39°C. The FLD system was set to exposure at 250 nm and emission at 395 nm. The chromatograph of amino acid separation is shown in for both 10 nmole mL−1 standard solution (red line) and fleshed muskmelon pulp sample (black line).
Table 1. Gradient separation conditions used for amino acid analysis.
Evaluation of antioxidant capacity
The antioxidant capacity was determined using 2,2-diphenyl-1-picrylhydrazyl free radical scavenging (DPPH) and oxygen radical absorbance capacity for hydrophilic compounds (H-ORAC) assays.
The DPPH activity was measured according to a method described by Oki et al. [Citation22] Briefly, the sample juice, Trolox calibration solution, or a blank of 100 μL was reacted with 200 mmol L−1 2-morpholinoethanesulfonic acid buffer (pH 6.0, 50 µL) and 800 µmol L−1 DPPH solution (50 µL) for 20 min at room temperature. Then, the absorbance at 520 nm was determined by a microplate reader (Spark, Tecan Group Ltd., Männedorf, Switzerland). The DPPH activities were expressed as nanomole of Trolox equivalent (TE) per milliliter (nmol TE mL−1).
The H-ORAC value of the sample was measured according to the method described by Watanabe et al. [Citation23]. Briefly, the sample was diluted 10-fold with 75 mmol L−1 phosphate buffer solution (pH 7.4), and then further diluted with 10% (v/v) MWA (methanol:water:acetic acid = 90:9.5:0.5) in the assay buffer solution. This diluted sample, Trolox calibration solution, or a blank of 35 μL was added to the wells of a 96-well plate. Then, 115 μL of 110.7 nmol L−1 fluorescein solution and 50 μL of 31.7 mmole L−1 2,2′-azobis(2-amidinopropane) dihydrochloride solution were added to each well, and the plate was incubated at 37°C. The fluorescence intensity was monitored every 2 min for 90 min by a microplate reader, using the fluorometric condition of excitation at 485 nm and emission at 528 nm. The internal temperature of the microplate reader was 37°C. The H-ORAC values were expressed as micromole of TE per milliliter (μmol TE mL−1).
Determination of total phenolic concentration
The total phenolic (TP) concentration of the sample was determined according to the Folin-Ciocalteu method [Citation24] using gallic acid (GA) as a standard substance. Briefly, the sample, Trolox calibration solution, or a blank of 150 μL was reacted with 150 µL of Folin-Ciocalteu’s phenol reagent for 3 min at room temperature. The mixture was further mixed with 150 µL of 10% (w/v) sodium carbonate solution and incubated for 30 min at room temperature. The absorbance was measured at 760 nm, and the TP value was expressed as micrograms of gallic acid equivalent (GAE) per milliliter (µg GAE mL−1).
Determination of AsA concentration
AsA was determined according to the 2,4-dinitrophenylhydrazine (DNP) colorimetric method [Citation25], using AsA as a standard substance for calculating the concentration. Firstly, the total AsA (oxidized AsA and AsA) concentration was measured. The AsA calibration solution, a blank of 500 μL, or the sample was reacted with 0.2% (w/v) 2,6-dicholorophenol indophenol aqueous solution for 1 min at room temperature. The mixture was further mixed with 500 µL of 1% (w/v) thiourea-5% (w/v) metaphosphoric acid solution and 250 µL of 2% (w/v) DNP solution, and incubated for 70 min at 50°C. After cooling, 1.25 mL of 85% (v/v) sulfuric acid was slowly added to the reaction mixture on ice. After 30 min of reaction at room temperature, the absorbance was determined at 540 nm. The concentration of oxidized AsA was calculated in a similar manner, except that the 2,6-dicholorophenol indophenol solution was not used. Then, the AsA value was calculated from the difference between the total and oxidized AsA and expressed as milligrams of AsA equivalent per milliliter (mg AsAE mL−1).
Determination of sugar concentrations
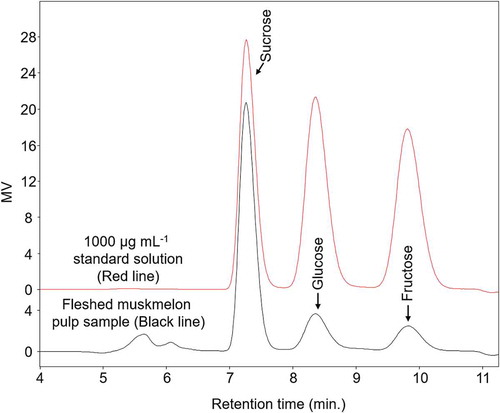
The sucrose, glucose, and fructose concentrations in the sample were determined according to our previous method [Citation26]. The three sugars were separated on a Shodex packed column for sugar (Shodex, SC1011) and analyzed by HPLC (Waters) with a refractive index detector (RID) according to the following conditions. The mobile phase was ultra-purified water. The column and RID temperatures were 90 and 50°C, respectively. The sugar separation results are shown in for both 1000 µg mL−1 standard solution (red line) and fleshed muskmelon pulp sample (black line).
Determination of GAD activity
GAD activities were measured as described previously [Citation27]. Briefly, 5 mL reaction liquid (50 mM sodium glutamate, 50 µM pyridoxal-5-phosphate, 100 mmol L−1 phosphate buffer, pH 5.5) was mixed with the pellet of fleshed pulp or placental site. The mixtures were then incubated for 1 h at 37°C and then heated for 5 min at 100°C for enzyme deactivation. In addition, protein concentrations in the mixtures were evaluated by TaKaRa BCA Protein Assay Kit (Takara, Japan) using bovine serum albumin as standard. Next, an equal volume of 16% (v/v) trichloroacetic acid aqueous solution was added, and the mixture was centrifuged at 10000 × g and 4°C for 5 min. The supernatant was mixed at equal volume with 0.4 mol L−1 lithium hydroxide aqueous solution, and then passed through a 0.20-μm membrane filter for determination of GABA using the HPLC method [Citation5]. Immediately after mixing the pellet, the enzyme was inactivated at 100°C, and then the GABA concentration was determined in the same manner. The GAD activity was converted to activity per gram of protein, assuming 1 unit (U) as the value that produces 1 μmol of GABA per minute (U g−1 protein).
Statistical analysis
Mean and standard deviation (S.D.) values were calculated from the 12 individual muskmelons. Differences in the amino acids, antioxidant compounds, sugar concentrations, antioxidant values, and GAD activities between the two regions were examined using Student’s or Welch’s t test (two-tailed), after examining the homogeneity of variances using the F test. Correlation analysis between Glu and each sugar concentration was carried out to assess the effects of anaerobic metabolism at the placental site. Each antioxidant capacity and each antioxidant compound concentration were also correlated for the fleshed pulp and placental tissues by using Spearman’s rank-order correlation. All statistical analyses were conducted in SPSS version 16.0 (Armonk, NY, USA). Differences were considered significant at a p-value less than 0.05.
Results and discussion
The purpose of this study was to clarify whether the placenta site in Earl’s muskmelon could be used as a raw material for functional foods and supplements, as in the case with cantaloupe. We found that the concentrations of GABA and its related substances were higher in the placental tissue than in the fleshed pulp. In addition, the increasing levels of GABA in the placenta were mediated by the formation of a hypoxic atmosphere inside the fruit. Furthermore, the placental site was found to have higher antioxidant capacities than the fleshed pulp because of elevated TP and AsA concentrations. These results suggest that the placenta site of muskmelon could be a useful food material owing to its high GABA concentration and antioxidant capacity, although it is presently discarded during food processing.
shows the concentrations of proteinogenic amino acids and GABA at the two sites. Compared to the fleshed pulp, the placental tissue had significantly higher levels of GABA, Asp, Ser, Asn, Gly, Gln, His, Ala, Pro, Tyr, Val, Met, Ile, Leu, Lys, and Phe, although its Glu content was significantly lower. Dissolved amino acids from the roots and leaves of melon plants pass through the vascular bundle in the following order: epicarp, mesocarp, endocarp, septum, core, and finally to the placenta [Citation5,Citation28]. This disposition allows easy accumulation of amino acids such as Asn and Glu [Citation29] in the placental tissue. Moreover, the inner part of fruits tends to contain less oxygen [Citation12], suggesting that hypoxia within the melon fruit could lead to more GABA being produced from Glu by GAD. This hypothesis would explain our results showing that GABA, Asp, and Ala accumulate in the placental tissue as compared to the fleshed pulp while the level of Glu decreases.
Table 2. Comparison of amino acid concentrations between fleshed pulp and placental tissue of muskmelon†.
Although incubated tea plant under anaerobic conditions showed increased GABA content, the Asp and Glu contents decreased [Citation10]. In contrast, we found that Asp is more concentrated in the placental tissue than in the fleshed pulp, even though metabolism via GAD is considered to occur in the inner part of the muskmelon fruit () to decrease the Glu level. Glu occupies a central position in the amino acid metabolism of plants, as a precursor for not only GABA but also arginine, α-ketoglutaric acid, Asp, Ala, and so on, as well as by undergoing some transamination reactions [Citation13]. Therefore, the Glu in the placental tissue could be rapidly utilized by GAD and other glutamate-metabolizing enzymes, while only Asp appears to accumulate there.
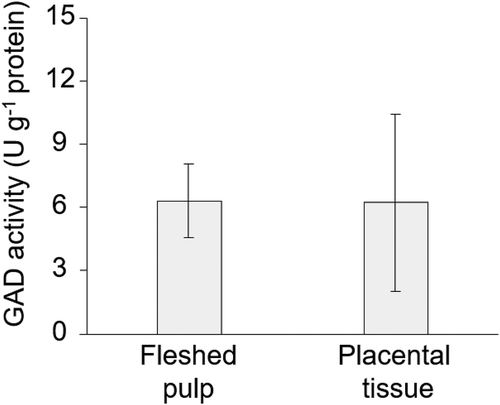
To further support the hypothesis about the inner metabolic effect, we evaluated the differences in sucrose, glucose, and fructose concentrations and GAD activity at these two sites of the fruit. Our results confirmed that all three sugar concentrations in the placental tissue decreased significantly compared to those in the fleshed pulp, in addition to a decrease in Glu, which is easily used in the nitrogen metabolism of plants () [Citation13,Citation30]. Similar trends were observed in previous reports using “Earl’s Favorite” [Citation30] and ‘Sunlady’“ [Citation20] melons. Meanwhile, according to , the GAD activities were similarly induced in the fleshed pulp (6.2 ± 4.2 U g−1 protein) and placenta site (6.3 ± 1.7 U g−1 protein), and there was no association between the GAD activity and GABA or Glu concentrations. However, there may be a difference in the GAD enzyme expression, and further verification is necessary in the future.
Table 3. Comparison of sugar concentrations between fleshed pulp and placental tissue of muskmelon†, ‡.
Figure 4. Comparison of glutamic acid decarboxylase (GAD) activity in fleshed pulp and placental tissue.
The mean values were obtained from 12 muskmelons, and the data are shown as the mean ± S.D.
Furthermore, we found that the proportion of monosaccharides in the placental tissue decreased strongly compared to that in the fleshed pulp (). Translocated sucrose from the leaves is thought to accumulate in the placental tissue as well as amino acids [Citation5,Citation28]. The sucrose would then be hydrolyzed into glucose and fructose by invertase [Citation31], as shown by an increase in the concentrations of both monosaccharides during the ripening progresses of melon fruit [Citation30]. The monosaccharides are probably consumed under the glycolysis pathway. Also, we confirmed the accumulation of proteinogenic amino acids and GABA, except for Glu, at the placental site (), which indicates an augmented amino acid metabolism there through the biosynthetic pathways of the Asp, pyruvic acid, α-ketoglutaric acid, phosphoenolpyruvate, 3-phosphoglycerate, and phosphoribosyl diphosphate families [Citation32]. In addition, the ATP/ADP ratio was found to decrease from the periphery to the center of the fruit, and the contents of ATP, ADP, and AMP conversely increased [Citation12]. This gradient suggests active anaerobic metabolism in the inner part of the fruit, which is supported by the measured substances for plant metabolism, namely the difference in both monosaccharide concentrations between the two sites in conjunction with the consumption of Glu, and a correlation between the monosaccharides and Glu (). These findings indicate that while sugars as well as amino acids enter the placental tissue from the leaf, the placental tissue consumes more glucose and fructose compared to the fleshed pulp in enhanced anaerobic reaction, due to the inner hypoxia progress.
Antioxidants deactivate radicals through two major mechanisms: single electron transfer (SET) and hydrogen atom transfer (HAT) [Citation33]. Correspondingly, we evaluated the antioxidant capacity of the muskmelon samples using DPPH and H-ORAC as SET- and HAT-based methods, respectively. and show the DPPH and H-ORAC values, TP level, and AsA concentrations determined for the fleshed pulp and placental site. The placental tissue of Earl’s muskmelon was found to have higher DPPH and H-ORAC values and TP concentration compared to the fleshed pulp. Similar results were obtained from the placenta sample of “Sunlady” melon, which has higher 2,2ʹ-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging activity (via a SET-based mechanism) and higher TP and flavonoid contents than the fleshed pulp [Citation20]. The present study showed a positive correlation between the DPPH or H-ORAC values and TP concentration, suggesting that TP could be a major contributor to the antioxidant capacity in the placental tissue of Earl’s muskmelon.
Table 4. Correlation matrix between glutamic acid and each sugar concentration in the fleshed pulp and placental tissue of muskmelon†.
Table 5. Comparison of antioxidant capacities between fleshed pulp and placental tissue of muskmelon†.
Table 6. Comparison of antioxidant compound concentrations between fleshed pulp and placental tissue of muskmelon†.
The skin of many fruits has high antioxidant capacity and TP concentration as a barrier against biotic and abiotic stress [Citation34]. However, the fleshed pulp of cantaloupe melon was reported to show higher DPPH and hydroxyl radical scavenging activities than the skin [Citation35]. In addition, the inner site of “Sunlady” melon was found to have a higher TP concentration [Citation20]. Accordingly, TP may easily accumulate at the inner site of the melon fruit.
Wang et al. have reported that treatment with 20 mM GABA by vacuum infiltration promoted the antioxidant potential and TP contents in banana peel [Citation36]. In addition, the GABA has been associated with the scavenging ability of reactive oxygen species [Citation37]. When the measured GABA concentrations were converted from mg mL−1 to mM, the pulp and placental site contain 14.9 and 29.8 mM of GABA, respectively (). So, at both sites of the muskmelon, GABA may have improved the antioxidant capacity and TP content in the same manner, especially at the placenta.
Since AsA also contributes to the antioxidant capacity, we evaluated its concentration across the two parts of Earl’s muskmelon. The results revealed that the placental tissue was also richer in AsA than the fleshed pulp, showing a positive correlation between H-ORAC values and AsA concentration ( and ). It is known that AsA is actively produced in the leaf blades of herbaceous plants, accumulated in the phloem of source leaves, and transported to sink tissues such as seeds, flowers, and buds [Citation38,Citation39]. This effect could be responsible for the high AsA concentrations in the placental tissue of muskmelons, allowing AsA to contribute strongly to the high H-ORAC values there.
Table 7. Correlation matrix between antioxidant capacity and antioxidant compounds in fleshed pulp and placental tissue of muskmelon†.
Different melon cultivars show similar trends in the in situ anaerobic conditions [Citation12] and nutrient flow [Citation5,Citation28], and so they may also have similar differences in the metabolizing capacity via GAD and Glu contents between the fleshed pulp and placental tissue. We therefore consider that other melon cultivars could resemble Earl’s muskmelon in terms of the different levels of GABA and other amino acids between these two parts, in both the concentration and absolute quantity. Meanwhile, the cultivars may differ from each other in their antioxidant components [Citation20,Citation40]. Further studies are needed to evaluate the antioxidant property at different sites in other melon cultivars.
The present study clarified that the GABA concentration of placental tissue was 2.0 times higher than that in the fleshed pulp. In climacteric melon fruits, respiration and ethylene production increase during the ripening progress, resulting in various physiological changes [Citation41]. For instance, according to Lyons et al., the internal oxygen level in cantaloupe melon steadily decreases during the ripening, concomitant with a gradual increase in carbon dioxide concentration [Citation42]. In addition, a previous study has revealed that the contents of Asp and Glu, which are substrates of GABA production, decrease in the fleshed pulp in association with the melon ripening progress [Citation30]. Further, since the melons used in this study are harder (245 ± 16 Hz) than those in our previous study (<220 Hz) [Citation5], the different maturity stages could affect the degree of GAD activity and the amount of GABA produced. Indeed, our previous study found a GABA concentration range of 2.41–3.00 mg mL−1 in the horizontal section [Citation5], which is higher than our results here using summer croppings (1.53 mg mL−1). From these considerations, the ripening progress probably increases the GABA production from Glu by GAD, because the placental site is exposed to a more hypoxic condition. This effect could widen the difference in GABA concentration between the fleshed pulp and placenta samples. However, an opposite trend was observed in tomatoes, with increasing Glu and decreasing GABA contents toward maturity [Citation43]. Thus, further studies are needed to confirm the increased GABA level in muskmelons during the maturity progression.
Meanwhile, we found that the DPPH and H-ORAC values of the placenta sample were 1.2 and 2.7 times higher than those in the fleshed pulp, respectively. The ripening process could induce oxidative stress in fruits by reactive oxygen species (ROS), while the antioxidant enzyme activities increase for better ROS scavenging [Citation44]. For this reason, the antioxidant capacity in the fleshed pulp and placenta samples could show a decreasing tendency, even if the decrease is not dramatic. We think that the ripening progress induces GABA accumulation in the placental tissue by anaerobic fermentation, while the antioxidant capacity decreases gradually due to increased ROS production.
Research has shown that GABA intakes of 10 and 28 mg from chocolate and fermented milk products could lower the blood pressure in patients with mild hypertension and induce relaxation in people experiencing stress, respectively [Citation8,Citation9]. Melon juice has different contents of dietary fiber, sugar, and protein from those of chocolate and fermented milk products, and this may affect the GABA absorption rate in the living body. In our study, the placental juice contained 3.07 mg mL−1 GABA, and thus ingesting more than 3.3 or 9.1 mL of the juice could have the same effect as chocolate and fermented milk products, respectively. Also, our results suggest that GABA concentration and antioxidant capacity at the placental site can be expected to increase by food processing and harvesting considering ripeness based on the above characteristics. Thus, the desired functionality could be achieved with even less placental juice. Therefore, the placenta tissue is a promising material for developing powder and pastes with high GABA concentration and antioxidant capacity.
As mentioned earlier, the placental site of Earl’s muskmelon is often discarded during food processing. Here, we report high GABA concentrations and antioxidant capacity in this site, which accounts for 4.9% (w/w) of the total weight of the melon except seeds. Although the ratio would differ depending on the melon cultivar and differential morphology [Citation2], the present results indicate that the placental site of melons in general could be a useful raw material for developing functional foods, rather than being discarded as residue in processing.
Conclusions
The present study found that the placental tissue in Earl’s muskmelon, which is conventionally discarded during processing, has a higher GABA concentration and antioxidant capacity than the fleshed pulp. These changes are due to variances in the cumulative amounts of amino acids and antioxidants, as well as the metabolization effects. This is the first report on the functionality of the placental tissue of this melon cultivar. This tissue can be useful as raw food material for developing functional foods while reducing the residues of muskmelon fruit processing.
Author contribution
TT was responsible for the research design, drafted the manuscript. TT participated in paper writing, reviewed the literature, simultaneously did the data examining. TN did some experiment work. All authors read and approved the final manuscript.
Acknowledgments
The authors would like to thank Dr. Yuya Deguchi at Nagasaki International University for his suggestions and reviews. No potential conflict of interest was reported by the authors.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
- Nuñez-Palenius HG, Gomez-Lim M, Ochoa-Alejo N, et al. Melon fruits: genetic diversity, physiology, and biotechnology features. Crit Rev Biotechnol. 2008;28:13–55.
- Staub JE, Danin-Poleg Y, Fazio G, et al. Comparative analysis of cultivated melon groups (Cucumis melo L.) using random amplified polymorphic DNA and simple sequence repeat markers. Euphytica. 2000;115:225–241.
- McCreight JD, Nerson H, Grumet R. Melon Cucumis melo L. In: Kalloo G, Berch BO, editors. Genetic improvement of vegetable crops. Oxford (UK): Pergamon Press; 1993. p. 267–294.
- MLS DM, Narain N, Bora PS. Characterisation of some nutritional constituents of melon (Cucumis melo hybrid AF-522) seeds. Food Chem. 2000;68:411–414.
- Toyoizumi T, Ohba S, Fujii KS, et al. Differential GABA concentration gradients are present in the edible parts of greenhouse melon (Cucumis melo L.) during all four seasonal croppings. Biosci Biotechnol Biochem. 2019;83:330–338.
- Boeing H, Bechthold A, Bub A, et al. Critical review: vegetables and fruit in the prevention of chronic diseases. Eur J Nutr. 2012;51:637–663.
- Diana M, Quílez J, Rafecas M. Gamma-aminobutyric acid as a bioactive compound in foods: a review. J Funct Foods. 2014;10:407–420.
- Inoue K, Shirai T, Ochiai H, et al. Blood-pressure-lowering effect of a novel fermented milk containing γ-aminobutyric acid (GABA) in mild hypertensives. Eur J Clin Nutr. 2003;57:490–495.
- Nakamura H, Takishima T, Kometani T, et al. Psychological stress-reducing effect of chocolate enriched with γ-aminobutyric acid (GABA) in humans: assessment of stress using heart rate variability and salivary chromogranin A. Int J Food Sci Nutr. 2009;605:106–113.
- Sawai Y, Yamaguchi Y, Miyama D, et al. Cycling treatment of anaerobic and aerobic incubation increases the content of γ-aminobutyric acid in tea shoots. Amino Acids. 2001;20:331–334.
- Baum G, Lev-Yadun S, Fridmann Y, et al. Calmodulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. Embo J. 1996;15:2988–2996.
- Biais B, Beauvoit B, Allwood JW, et al. Metabolic acclimation to hypoxia revealed by metabolite gradients in melon fruit. J Plant Physiol. 2010;167:242–245.
- Forde BG, Lea PJ. Glutamate in plants: metabolism, regulation, and signalling. J Exp Bot. 2007;58:2339–2358.
- Kondo K, Zushi K, Wajima T, et al. Analysis of the sugar, amino acid, ascorbic acid, and β-carotene in a fruit of ‘Prince’ melon. J Jpn Soc Agric Technol Manag. 2012;19:1–5. (published in Japanese).
- Oh SH, Moon YJ, Oh CH. γ-Aminobutyric acid (GABA) content of selected uncooked foods. Prev Nutr Food Sci. 2003;8:75–78.
- Yaghi S, Otoguro C, Sumino T, et al. Effect of low-temperature steam heating on the free amino acids in various vegetables. J Cook Sci Jpn. 2008;41:42–48. (published in Japanese).
- Nhi PTP, Akashi Y, Hang TTM, et al. Genetic diversity in Vietnamese melon landraces revealed by the analyses of morphological traits and nuclear and cytoplasmic molecular markers. Breed Sci. 2010;60:255–266.
- Peschel W, Sánchez-Rabaneda F, Diekmann W, et al. An industrial approach in the search of natural antioxidants from vegetable and fruit wastes. Food Chem. 2006;97:137–150.
- Van der Sluis AA, Dekker M, Skrede G, et al. Activity and concentration of polyphenolic antioxidants in apple juice. 1. Effect of existing production methods. J Agric Food Chem. 2002;50:7211–7219.
- Nattaporn W, Pranee A. Effect of pectinase on volatile and functional bioactive compounds in the flesh and placenta of ‘Sunlady’ cantaloupe. Int Food Res J. 2011;18:819–827.
- Iglesias BD, Ruiz-Altisent M, Jancsók P. Vibrational analysis of seedless watermelons: use in the detection of internal hollows. Span J Agric Res. 2005;3:52–60.
- Oki T, Kobayashi M, Nakamura T, et al. Changes in radical-scavenging activity and components of mulberry fruit during maturation. J Food Sci. 2006;71:C18–C22.
- Watanabe J, Oki T, Takebayashi J, et al. Extraction efficiency of hydrophilic and lipophilic antioxidants from lyophilized foods using pressurized liquid extraction and manual extraction. J Food Sci. 2014;79:C1665–C1671.
- Islam MS, Yoshimoto M, Yahara S, et al. Identification and characterization of foliar polyphenolic composition in sweetpotato (Ipomoea batatas L.) genotypes. J Agric Food Chem. 2002;50:3718–3722.
- Arakawa N, Tsutsumi K, Sanceda NG, et al. A rapid and sensitive method for the determination of ascorbic acid using 4,7-diphenyl-l,10-phenanthroline. Agric Biol Chem. 1981;45:1289–1290.
- Toyoizumi T, Yamamoto H, Sasaki M. Explore of steaming condition for elderly people on carrot using low temperature steam cooking. Nippon Shokuhin Kagaku Kogaku Kaishi. 2015;62:341–348. (published in Japanese).
- Ohno T, Takahashi S. Changes in glutamate decarboxylase activity and distribution during storage of rice. Nippon Shokuhin Kagaku Kogaku Kaishi. 2014;61:552–554. (published in Japanese).
- Okumoto S, Pilot G. Amino acid export in plants: a missing link in nitrogen cycling. Mol Plant. 2010;4:453–463.
- Lea PJ, Sodek L, Parry MA, et al. Asparagine in plants. Ann Appl Biol. 2007;150:1–26.
- Mizuno T, Kato K, Harada M, et al. Studies on the free sugars and amino acids in a fruit of muskmelon. Nippon Shokuhin Kogyo Gakkaishi. 1971;18:319–325. (published in Japanese).
- Agravante JU, Matsui T, Kitagawa H. Starch breakdown in ethylene-and ethanol-treated bananas: changes in phosphorylase and invertase activities during ripening. Nippon Shokuhin Kogyo Gakkaishi. 1990;37:911–915.
- Kusano M, Tabuchi M, Fukushima A, et al. Metabolomics data reveal a crucial role of cytosolic glutamine synthetase 1;1 in coordinating metabolic balance in rice. Plant J. 2011;66:456–466.
- Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856.
- Gancel AL, Alter P, Dhuique-Mayer C, et al. Identifying carotenoids and phenolic compounds in naranjilla (Solanum quitoense Lam. var. Puyo hybrid), an Andean fruit. J Agric Food Chem. 2008;56:11890–11899.
- Ismail HI, Chan KW, Mariod AA, et al. Phenolic content and antioxidant activity of cantaloupe (Cucumis melo) methanolic extracts. Food Chem. 2010;119:643–647.
- Wang Y, Luo Z, Huang X, et al. Effect of exogenous γ-aminobutyric acid (GABA) treatment on chilling injury and antioxidant capacity in banana peel. Sci Hortic. 2014;168:132–137.
- Liu C, Zhao L, Yu G. The dominant glutamic acid metabolic flux to produce γ‐amino butyric acid over proline in Nicotiana tabacum leaves under water stress relates to its significant role in antioxidant activity. J Integr Plant Biol. 2011;53:608–618.
- Kitagawa Y. Distribution of vitamin C related to the growth of some vegetable fruits (cucumbers, princemelons and okra). J Jap Soc Food Nutr. 1972;25:436–442. (published in Japanese).
- Franceschi VR, Tarlyn NM. L-Ascorbic acid is accumulated in source leaf phloem and transported to sink tissues in plants. Plant Physiol. 2002;130:649–656.
- Kolayli S, Kara M, Tezcan F, et al. Comparative study of chemical and biochemical properties of different melon cultivars: standard, hybrid, and grafted melons. J Agric Food Chem. 2010;58:9764–9769.
- Seymour GB, McGlasson WB. Melons. In: Seymour GB, Taylor JE, Tucker G, editors. Biochemistry of fruit ripening. London: Chapman & Hall; 1993. p. 273–290.
- Lyons JM, McGlasson WB, Pratt HK. Ethylene production, respiration, & internal gas concentrations in cantaloupe fruits at various stages of maturity. Plant Physiol. 1962;37:31–36.
- Nagata M, Saijo R. Changes in free amino acid contents of tomato fruits during ripening, especially changes in glutamine. Nippon Shokuhin Kogyo Gakkaishi. 1992;39:64–67. (published in Japanese).
- Jimenez A, Creissen G, Kular B, et al. Changes in oxidative processes and components of the antioxidant system during tomato fruit ripening. Planta. 2002;214:751–758.