ABSTRACT
Puerarin inhibits osteoclastogenesis and cells migration. This study aims to explore whether puerarin prevents osteoclastogenesis by inhibiting osteoclast precursors (OCPs) migration. The results showed that puerarin reduced MCP-1 production in OCPs, while inhibiting OCPs migration based on MCP-1. Puerarin reversed MCP-1-promoted osteoclastogenesis. CCR2 overexpression didn’t increase osteoclastogenesis with puerarin. Therefore, puerarin prevents OCPs migration by reducing MCP-1, whereby inhibiting osteoclastogenesis.
Bone remodeling relies on the kinetic equilibrium between osteoclast-mediated bone resorption and osteoblast-mediated osteogenesis. The imbalance of bone resorption and formation leads to the reduction in bone mass, ultimately contributing to metabolic bone diseases, such as osteoporosis [Citation1]. Pueraria lobata, a leguminous plant in China, is widely employed in the treatment of cardiovascular diseases, diabetes, osteonecrosis and neurodegeneration [Citation2]. As an extract from pueraria lobate, puerarin is of great significance in treating osteoporosis. Its therapeutic effect has been broadly reported in the prevention of bone loss [Citation3–Citation5]. Puerarin can inhibit the osteoclastogenesis, which contributes to its bone-protective effect [Citation6–Citation9]. The migration and recruitment of OCPs in the form of macrophages (bone marrow-derived macrophages, BMMs) can lead to OCPs fusion, eventually promoting the formation of osteoclasts [Citation10,Citation11]. The given chemokines play an important role in the above process [Citation12–Citation14]. Puerarin is known to inhibit the migration of various cells. Previous study showed that puerarin prevents lipopolysaccharide-induced migration, invasion and adhesion of breast cancer cells [Citation15]. Puerarin can also inhibit the migration and invasion of human retinoblastoma cells [Citation16]. In addition, the inhibitory effect of puerarin on the migration of human papillomavirus-positive cervical cancer cells was also verified [Citation17]. More importantly, Kang et al showed that puerarin attenuated the metastasis of tumor-associated macrophages from non-small cell lung cancer xenograft model, indicating that puerarin can block the migration of macrophages [Citation18]. Can puerarin also inhibit the migration of OCPs derived from bone marrow macrophages? Taken together, we hypothesized that puerarin could inhibit the migration of OCPs, resulting in the failure of OCPs recruitment, thus preventing the fusion and differentiation of osteoclasts. Our study showed a role of puerarin in inhibiting the OCPs migration due to its inhibition on the production of monocyte chemotactic protein-1 (MCP-1), which contributed to the reduction in the osteoclastogenesis. Therefore, by elucidating the significance of puerarin in the OCPs migration, the present study unmasked a novel intrinsic mechanism regarding puerarin-regulated osteoclastogenesis for the first time.
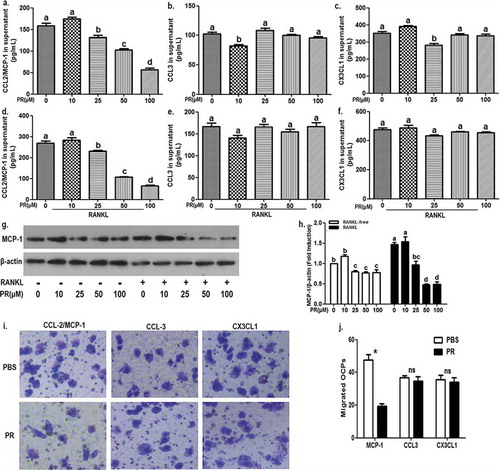
Firstly, the role of puerarin in the production of several chemokines in OCPs needs to be clarified. ELISA results showed that puerarin reduced MCP-1 secretion of supernatant in a concentration-dependent manner from 25 μM to 100 μM in the absence or presence of receptor activator of nuclear factor kappa B ligand (RANKL) ()). However, puerarin only inhibited the secretion of CCL3 or CX3CL1 at 10 or 25 μM, respectively ()). Moreover, puerarin did not affect the secretion of CCL3 or CX3CL1 in the presence of RANKL ()). In addition, Western Blotting results showed that puerarin reduced MCP-1 protein expression of OCPs at 25, 50 and 100 μM in the absence or presence of RANKL ()). It was also observed that under 0 or 10 μM puerarin intervention, MCP-1 protein level in RANKL group was higher than that in RANKL-free group (1.47-fold; 1.3-fold); however, under 50 or 100 μM puerarin intervention, MCP-1 protein level in RANKL group was significantly lower than that in RANKL-free group (0.6-fold; 0.61-fold) ()). Together, It was suggested that puerarin can inhibit the production of MCP-1 in OCPs.
We documented that puerarin inhibited the expression of MCP-1 in OCPs. What is the effect of puerarin on specific chemokine-related OCPs migration? For subsequent experiments, the most effective concentration (100 μM) of puerarin was applied. As shown in ), the migration of OCPs driven by MCP-1 decreased with the addition of puerarin, but puerarin did not affect the role of CCL3 or CX3CL1 in OCPs. It was indicated that puerarin can prevent OCPs migration induced by MCP-1.
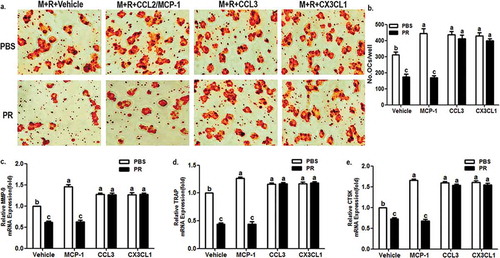
Next, it is worth to clarify that puerarin has an effect on osteoclast differentiation under the treatment of specific chemokines. As shown in ), all three chemokines can promote osteoclast differentiation under the combined intervention of RANKL and macrophage colony stimulating factor (M-CSF). However, puerarin only reduced the number of mature osteoclasts in the presence of vehicle or MCP-1 ()). Moreover, there was no significant difference in osteoclast differentiation level between vehicle and MCP-1 groups under puerarin intervention ()). The mRNA levels of the three markers (MMP-9, TRAP and CTSK) of osteoclasts showed a similar trend to the number of mature osteoclasts ()). These results revealed that puerarin can reverse the osteoclast differentiation enhanced by MCP-1.
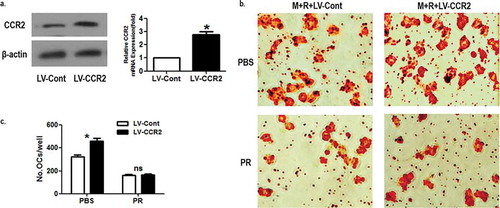
According to the above experiments, puerarin was known to reverse MCP-1-enhanced osteoclastogenesis. In order to further elucidate the effect of puerarin on the osteoclastogenesis treated by MCP-1, we employed lentivirus to overexpress CCR2 on OCPs to observe the osteoclast differentiation under puerarin intervention. The expression of viral genes was observed at protein and mRNA level ()). The results showed that although CCR2 overexpression increased the number of mature osteoclasts, there was no statistical difference between the CCR2 overexpression group and the control group in the presence of puerarin ()), proving that CCR2 overexpression is invalid in upregulating osteoclast differentiation as puerarin blocks MCP-1 production. Puerarin is known to exert an inhibitory effect on the osteoclastogenesis, yet the detailed mechanism remains unclear, which raises an interesting scientific question for investigation. The role of puerarin in inhibiting cell migration has been verified in other cells, especially macrophage [Citation15–Citation18]. Here, we provide the first evidence demonstrating the role of puerarin in inhibiting OCPs migration, which leads to the reduced osteoclastogenesis in the current study.
Various chemokines are responsible for cell migration. MCP-1 is mainly involved in the recruitment and fusion of OCPs [Citation12]. Other chemokines include CCL3 and CX3CL1 [Citation13,Citation14,Citation19]. Our ELISA studies showed that puerarin decreased the secretion of MCP-1 but not CCL3 and CX3CL1 in OCPs. Through Western Blotting analysis, it was concluded that puerarin-inhibited MCP-1 secretion is owing to its inhibition on MCP-1 production in OCPs. What’s more, among the three chemokines, only MCP-1-regulated OCPs migration was inhibited by puerarin. These results indicate that puerarin can inhibit the migration of OCPs by blocking the production of MCP-1. The underlying mechanisms of puerarin on MCP-1 production requires further investigation. In addition, we observed that RANKL could induce MCP-1 expression, which is consistent with previous studies [Citation20,Citation21]. As the osteoclastogenic cytokine, RANKL plays a crucial role in inducing osteoclastogenesis under the aid of M-CSF [Citation22–Citation24]. Previous study also suggested that RANKL can induce the production of MCP-1, which is involved in osteoclast differentiation at the stage of multinucleation of osteoclast precursors [Citation20,Citation21]. However, with the elevation of puerarin concentration, the decline of MCP-1 level under RANKL intervention was more significant. Accordingly, the effect of puerarin on RANKL-regulated MCP-1 expression also needs more explorations in the future.
Furthermore, it was found that although the above three chemokines can promote osteoclast formation, only MCP-1-promoted osteoclastogenesis was inhibited by puerarin application. It was suggested that puerarin can reduce the osteoclastogenesis by inhibiting MCP-1 production. It is clear that there is an essential relationship among puerarin-inhibited MCP-1 expression, OCPs migration and osteoclast formation. In addition, under puerarin intervention, CCR2 overexpression using lentivirus transduction could not enhance the osteoclastogenesis. Given the inhibition of puerarin on MCP-1, CCR2 overexpression lost the capacity in upregulating osteoclast formation. The above data further confirmed the role of MCP-1 inhibition in the osteoclastogenesis regulated by puerarin.
Puerarin is well considered an osteoclastogenesis inhibitor and a cell (especially macrophages) migration inhibitor. However, the role of OCPs migration in puerarin-regulated osteoclastogenesis still keep vague. Our study elucidated the underlying mechanism of puerarin-regulated osteoclastogenesis from the angle of cell migration. The above data proved the role of puerarin in inhibiting the OCPs migration owing to its inhibitory effect on MCP-1 production, which is responsible for puerarin-inhibited osteoclastogenesis. The present study not only explores the novel potential mechanism regarding puerarin-inhibited osteoclastogenesis, but also sheds light on optimizing the application of puerarin in the treatment of metabolic bone diseases (such as osteoporosis).
Author contribution
D.K. and Y.Y. conceived and designed experiments; S.L. and D.K. performed experiments, analyzed data, prepared figures, and helped with writing of the manuscript; X.F. performed experiments; Y.L. helped with data analysis; S.L. and Y.Y. wrote the manuscript; D.K. provided technical expertise and edited the article.
Supporting_information_YT.doc
Download MS Word (234.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Datta HK, Ng WF, Walker JA, et al. The cell biology of bone metabolism. J Clin Pathol. 2008;61:577–587.
- Fu R, Zhang Y, Guo Y, et al. Digital gene expression analysis of the pathogenesis and therapeutic mechanisms of ligustrazine and puerarin in rat atherosclerosis. Gene. 2014;552(1):75–80.
- Cho HJ, Jun HJ, Lee JH, et al. Acute effect of high-dose isoflavones from Pueraria lobata (Willd.) Ohwi on lipid and bone metabolism in ovariectomized mice. Phytother Res. 2012;26(12):1864–1871.
- Guo CJ, Xie JJ, Hong RH, et al. Puerarin alleviates streptozotocin (STZ)-induced osteoporosis in rats through suppressing inflammation and apoptosis via HDAC1/HDAC3 signaling. Biomed Pharmacother. 2019;115:108570.
- Urasopon N, Hamada Y, Cherdshewasart W, et al. Preventive effects of Pueraria mirifica on bone loss in ovariectomized rats. Maturitas. 2008;59(2):137–148.
- Park KH, Gu DR, Jin SH, et al. Pueraria lobate inhibits RANKL-mediated osteoclastogenesis via downregulation of CREB/PGC1β/c-Fos/NFATc1 signaling. Am J Chin Med. 2017;45(8):1725–1744.
- Yuan SY, Sheng T, Liu LQ, et al. Puerarin prevents bone loss in ovariectomized mice and inhibits osteoclast formation in vitro. Chin J Nat Med. 2016;14(4):265–269.
- Zhang Y, Yan M, Yu QF, et al. Puerarin prevents LPS-induced osteoclast formation and bone loss via inhibition of Akt activation. Biol Pharm Bull. 2016;39(12):2028–2035.
- Zhang G, Wang Y, Tang G, et al. Puerarin inhibits the osteoclastogenesis by inhibiting RANKL-dependent and -independent autophagic responses. BMC Complement Altern Med. 2019;19(1):269.
- Ishii M, Egen JG, Klauschen F, et al. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458(7237):524–528.
- Ha J, Choi HS, Lee Y, et al. CXC chemokine ligand 2 induced by receptor activator of NF-kappa B ligand enhances osteoclastogenesis. J Immunol. 2010;184(9):4717–4724.
- Lu Y, Cai Z, Xiao G, et al. Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption. Cancer Res. 2007;67(8):3646–3653.
- Vallet S, Raje N, Ishitsuka K, et al. MLN3897, a novel CCR1 inhibitor, impairs osteoclastogenesis and inhibits the interaction of multiple myeloma cells and osteoclasts. Blood. 2007;110(10):3744–3752.
- Matsuura T, Ichinose S, Akiyama M, et al. Involvement of CX3CL1 in the migration of osteoclast precursors across osteoblast layer stimulated by interleukin-1β. J Cell Physiol. 2017;232(7):1739–1745.
- Liu X, Zhao W, Wang W, et al. Puerarin suppresses LPS-induced breast cancer cell migration, invasion and adhesion by blockage NF-κB and Erk pathway. Biomed Pharmacother. 2017;92:429–436.
- Yang C, Fan X, Fan S. Effects and mechanism of puerarin on the human retinoblastoma cells. J Cell Biochem. 2018;119(6):4506–4513.
- Jia L, Hu Y, Yang G, et al. Puerarin suppresses cell growth and migration in HPV-positive cervical cancer cells by inhibiting the PI3K/mTOR signaling pathway. Exp Ther Med. 2018;18:543–549.
- Kang H, Zhang J, Wang B, et al. Puerarin inhibits M2 polarization and metastasis of tumor-associated macrophages from NSCLC xenograft model via inactivating MEK/ERK 1/2 pathway. Int J Oncol. 2017;50(2):545–554.
- Koizumi K, Saitoh Y, Minami T, et al. Role of CX3CL1/fractalkine in osteoclast differentiation and bone resorption. J Immunol. 2009;183(12):7825–7831.
- Kim MS, Day CJ, Morrison NA. MCP-1 is induced by receptor activator of nuclear factor-{kappa}B ligand, promotes human osteoclast fusion, and rescues granulocyte macrophage colony-stimulating factor suppression of osteoclast formation. J Biol Chem. 2005;280(16):16163–16169.
- Huh JE, Lee WI, Kang JW, et al. Formononetin attenuates osteoclastogenesis via suppressing the RANKL-induced activation of NF-κB, c-Fos, and nuclear factor of activated T-cells cytoplasmic 1 signaling pathway. J Nat Prod. 2014;77(11):2423–2431.
- Xiu Y, Xu H, Zhao C, et al. Chloroquine reduces osteoclastogenesis in murine osteoporosis by preventing TRAF3 degradation. J Clin Invest. 2014;124(1):297–310.
- Chai L, Zhou K, Wang S, et al. Psoralen and bakuchiol ameliorate M-CSF plus RANKL-induced osteoclast differentiation and bone resorption via inhibition of AKT and AP-1 pathways in vitro. Cell Physiol Biochem. 2018;48(5):2123–2133.
- Ke D, Ji L, Wang Y, et al. JNK1 regulates RANKL-induced osteoclastogenesis via activation of a novel Bcl-2-Beclin1-autophagy pathway. FASEB J. 2019;33(10):11082–11095.