ABSTRACT
γ-glutamyl peptides have been suggested to impart kokumi properties to foods by activating human calcium-sensing receptor (hCaSR). In this study, the relationship between γ-glutamyl peptide structure and hCaSR activity was systematically analyzed using γ-[Glu](n=0-4)-α-[Glu](n=0-3)-Tyr. Our results suggest that N-terminal [Glu]3 moiety is very important for hCaSR activities of γ-glutamyl peptides.
In Japan, for a long time, the term koku (referring to koku attributes) has been used to describe palatable food [Citation1,Citation2]. Koku is the unique sensation given by lots of stimulation in terms of taste, aroma, and texture. Characteristics of koku attributes proposed are as follows: complexity, mouthfulness, and continuity [Citation2]. Recent studies suggested that the calcium-sensing receptor (CaSR), a G protein-coupled receptor, functions as a novel taste receptor (kokumi receptor); CaSR activators induce kokumi, which is not a taste on its own but intensifies the sweet, salty, and umami tastes, and it possesses the characteristics of koku attributes (thickness, continuity, and mouthfulness) in human sensory analysis [Citation3]. Besides Ca2+ ion, various food components possessing koku attributes have been shown to activate the receptor, e.g. L-amino acids, γ-glutamyl peptides, and protamine [Citation3–Citation7]. Although the function of CaSR does not necessarily explain all of koku attributes [Citation2,Citation3], activators for this receptor are expected to be promising taste modulators.
A lot of kokumi-active γ-glutamyl peptides have been found from cheese, soy source, and edible beans on the basis of sensory evaluation [Citation8–Citation12]. Glutathione (γ-Glu-Cys-Gly), a representative of kokumi substances [Citation9], has been shown to be a strong activator of human CaSR (hCaSR) [Citation3,Citation5]. T. Ohsu et al. screened the hCaSR activity of glutathione-related γ-glutamyl di-/tripeptides [Citation3,Citation5]. They identified many γ-glutamyl peptides including γ-Glu-Ala and γ-Glu-Val-Gly as potent hCaSR activators. As γ-Glu-Val-Gly is contained in such as soy source, the peptide is suggested to impart kokumi to these foods [Citation10]. Thus, γ-glutamyl peptides impart kokumi properties by activation of hCaSR.
Recently, glutaminase from Bacillus amyloliquefaciens and Aspergillus oryzae, used for the production of fermented foods (e.g. soy source and cheese), has been shown to catalyze the formation of a large number of γ-glutamyl peptides; the enzyme facilitated the synthesis of γ-[Glu](n)-Tyr/Phe/Val/Met with kokumi–imparting properties [Citation12–Citation14]. In sufu, a Chinese fermented bean curd, glutaminase from B. amyloliquefaciens promotes the synthesis of kokumi-active peptides, γ-[Glu](n=1-3)-Tyr and γ-[Glu](n=1,2)-Phe [Citation12]. Therefore, various structures of γ-glutamyl peptides are formed that would impart kokumi to foods. However, effect of the number of α/γ-glutamyl residues on hCaSR activity remains unclear. Thus, in the present study, the relationship between γ-glutamyl peptide structure and hCaSR activity was systematically analyzed using γ-[Glu](n=0-4)-α-[Glu](n=0-3)-Tyr. This study will not only enhance the understanding of kokumi–imparting properties of γ-glutamyl peptides to various foods but also contribute to the development of kokumi-enhanced foods.
The following materials were used in this study: γ-Glu-Ala was purchased from Anygen Co., Ltd. (Jangseong-gun, Korea); and γ-[Glu](n=1,2,3,4)-Tyr, γ-Glu-α-[Glu] (n=1,2,3)-Tyr, and α-Glu-Tyr from GenScript Japan Inc. (Tokyo, Japan). HEK293T cells were provided by the RIKEN BioResource Research Center (Ibaraki, Japan).
hCaSR activity of the peptides was evaluated using a highly sensitive cell-based assay system, which was established in our previous study [Citation15]. hCaSR and hGα15 expression plasmids were transiently cotransfected into HEK293T cells using LipofectamineTM 2000 (Invitrogen, MD, USA). HEK cells transfected with the hGα15 expression plasmid and an empty vector (pHEK293 Ultra Expression Vector I) were used as the negative control (mock cells). The cells were loaded with 3 μM Fluo-8 AM (AAT Bioquest, CA, USA) using an assay buffer (146 mM NaCl, 5.5 mM glucose, 5 mM KCl, 1 mM CaCl2, 1 mM MgSO4, and 20 mM HEPES [pH 7.2]). The test sample was added to each well using a FlexStation II microplate reader (Molecular Devices, Inc., CA, USA), and the changes in intracellular Ca2+ levels were evaluated by a specific fluorescence signal (Ex = 490 nm, Em = 525 nm). The Ca2+ response of cells was calculated using the following equation: Response = (F − F0)/F0, where F0 (baseline) was defined as the mean fluorescence value at 0–30 s before sample addition and F (signal intensity) as the highest fluorescence value at 5–35 s after sample administration. The hCaSR response value was calculated by subtracting the response of the mock cells from that of the hCaSR and hGα15 coexpressing cells. The EC50 values of each activator were estimated by the dose–response curves created using PRISM software version 4.03 (GraphPad Software Inc., San Diego, CA). Each experiment was repeated at least two or three times, and each datapoint is represented as the mean ± S.E.
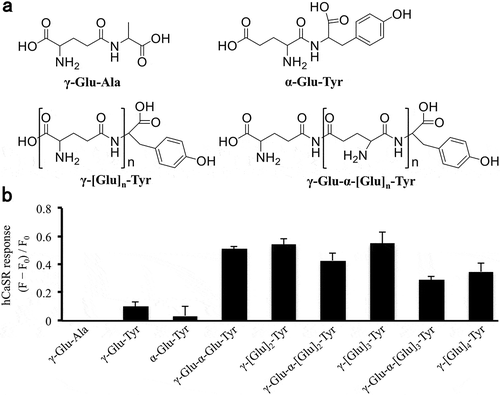
While kokumi–imparting property of γ-Glu-Tyr has been shown [Citation11,Citation12], hCaSR activity of the peptide has not been clarified. Thus, at first, the hCaSR activity of three dipeptides, γ-Glu-Tyr, α-Glu-Tyr, and γ-Glu-Ala, was analyzed (their chemical structures are shown in )). Among these three peptides, γ-Glu-Tyr induced hCaSR activation at 0.3 mM, whereas α-Glu-Tyr and γ-Glu-Ala did not activate the receptor at the same concentration with γ-Glu-Tyr ()). In agreement with previous reports [Citation3,Citation5], γ-Glu-Ala elicited an hCaSR response (data not shown) when the peptide was administered at higher concentrations (>0.6 mM). Our study using HEK293T cells suggests that γ-Glu-Tyr weakly activates hCaSR at 0.3 mM ()), whereas T. Ohsu et al. have reported that this peptide is inactive in hCaSR-expressing Xenopus oocytes [Citation5]. This inconsistency may be explained by different sensitivities of hCaSR assay system; the experimental technique using HEK cells provided higher sensitivity compared with that using Xenopus oocytes [Citation3,Citation5]. The above results indicate that γ-glutamyl moiety in the N-terminus and Tyr residue in the C-terminus of γ-Glu-Tyr are important for its hCaSR activity. This observation is consistent with previous findings; γ-glutamyl moiety is essential for potent CaSR activities of γ-glutamyl di-/tripeptides [Citation3,Citation5,Citation6]. As γ-Glu-Trp and γ-Glu-Phe have also been reported as hCaSR activators [Citation5,Citation6], aromatic amino acid in the C-terminus of the peptide would also contribute to its hCaSR activity.
We next examined the relationship between hCaSR activity and the number of α/γ-glutamyl residues of γ-glutamyl peptides using γ-[Glu](n=1-4)-α-[Glu](n=0-3)-Tyr. We found that γ-glutamyl tri-/tetra-/pentapeptides, γ-[Glu](n=2,3,4)-Tyr, and γ-Glu-α-[Glu](n=1,2,3)-Tyr were more potent hCaSR activators than γ-glutamyl dipeptides, γ-Glu-Tyr ()). Thus, γ-[Glu](n≥2) and γ-[Glu]-α-[Glu](n≥1) moieties in the N-terminus would be important for their strong hCaSR activities. As hCaSR activities of γ-[Glu](n=2,3,4)-Tyr and γ-Glu-α-[Glu](n=1,2,3)-Tyr were almost equal, both α and γ type of glutamate in peptide chain were acceptable for hCaSR activation.
In the next experiment, we compared the hCaSR activity of four γ-glutamyl peptides, γ-[Glu](n=1,2,3,4)-Tyr (Supplemental ). All activated hCaSR in a concentration-dependent manner, whereas they did not elicit response in the mock cells. EC50 values of γ-Glu-Tyr and γ-[Glu]2-Tyr were 508 µM and 219 µM, respectively (Supplemental )). Compared with these two peptides, γ-[Glu](n=3,4)-Tyr showed smaller EC50 values: γ-[Glu]3-Tyr, 180 µM and γ-[Glu]4-Tyr, 140 µM (Supplemental Figure 1(c,d)). Moreover, maximal responses of γ-[Glu](n=3,4)-Tyr were 1.5 to 2 times higher than those of γ-[Glu](n=1,2)-Tyr (Supplemental Table 1). Consequently, [Glu](n≥3) structure in the N-terminus was indicated to be important for potent hCaSR activity of γ-glutamyl peptides. In the hCaSR assay system, EC50 values of γ-[Glu]3-Tyr (180 µM) and γ-[Glu]4-Tyr (140 µM) were comparable with those of spermine (198 µM) and protamine (231 µM), the representative hCaSR-active peptides, and lysozyme (590 µM), a protein-type hCaSR agonist identified in our previous work [Citation3,Citation4,Citation7,Citation15]. As γ-[Glu](n=3,4)-Tyr showed smaller EC50 values and larger maximal responses than γ-[Glu](n=1,2)-Tyr, the formers would possess more effective kokumi–imparting properties than the laters.
The hCaSR responses by γ-[Glu]3-Tyr were attenuated by the coadministration of NPS 2143, a selective inhibitor for the receptor [Citation4], in a concentration-dependent manner (data not shown), thereby showing that γ-[Glu]3-Tyr provoked Ca2+ responses via hCaSR activation. CaSR activators are classified into two types: orthosteric agonists and positive allosteric modulators. Orthosteric agonists (e.g. Ca2+ and Mg2+) are capable of activating CaSR on their own [Citation4,Citation15], whereas positive allosteric modulators (e.g. cinacalcet and L-amino acids) bind to allosteric sites on CaSR and enhance the activity of orthosteric agonists [Citation3,Citation4]. hCaSR response by γ-[Glu]3-Tyr was evaluated in the buffer without Ca2+ and Mg2+ to clarify whether the peptide is an orthosteric agonist or a positive allosteric modulator. It was found that γ-[Glu]3-Tyr activated hCaSR in the absence of orthosteric agonists Ca2+ and Mg2+, indicating that the peptide itself is an orthosteric agonist of the receptor (Supplemental Figure 2(a)). γ-[Glu]3-Tyr showed stronger hCaSR responses in the Ca2+ and Mg2+-containing buffer than in the buffer without Ca2+ and Mg2+ (Supplemental Figure 2(b)). These results suggest the possibility that γ-[Glu]3-Tyr also functions as a positive allosteric modulator; the peptide enhances hCaSR activity of Ca2+ and Mg2+, orthosteric agonists, present in the buffer. Because Ca2+ and Mg2+ are not positive allosteric modulators but orthosteric agonists, it is unlikely that hCaSR activity of γ-[Glu]3-Tyr was intensified by Ca2+ and Mg2+. Although activation mode of known CaSR activators was not completely investigated, γ-[Glu]3-Tyr was found to be a unique activator, functioning as both an orthosteric agonist and a positive allosteric modulator.
In the present study, hCaSR activity of γ-[Glu](n=0-4)-α-[Glu](n=0-3)-Tyr was systematically analyzed. The [Glu]3 moiety in the N-terminus is suggested to be an important structure for strong hCaSR activity of γ-glutamyl peptides. The results of this study will not only facilitate further understanding of kokumi–imparting properties of γ-glutamyl peptides to foods but also contribute to the production of kokumi-enhanced foods.
Author contribution
Y. T., T. M., T. S., and K. I. designed the study. M. Y. and Y. T. wrote the initial draft of the manuscript, and K. I., T. M., M. S., and T. S. revised the manuscript. All authors contributed to analysis and interpretation of data.
200312_supplemental_materials_PDF.pdf
Download PDF (302.2 KB)Acknowledgments
We acknowledge the generous support from Council for Science, Technology and Innovation (CSTI) and Cross-ministerial Strategic Innovation Promotion Program (SIP) “Technologies for creating next-generation agriculture, forestry and fisheries” to Keisuke Ito.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplementary data for this article can be accessed here.
Additional information
Funding
References
- Nishimura T, Egusa AS, Nagao A, et al. Phytosterols in onion contribute to a sensation of lingering of aroma, a koku attribute. Food Chem. 2016;192:724–728.
- Nishimura T, Kuroda M. Koku in food science and physiology. Singapore: Springer; 2019.
- Ohsu T, Amino Y, Nagasaki H, et al. Involvement of the calcium-sensing receptor in human taste perception. J Biol Chem. 2010;285(2):1016–1022.
- Saidak Z, Brazier M, Kamel S, et al. Agonists and allosteric modulators of the calcium-sensing receptor and their therapeutic applications. Mol Pharmacol. 2019;76(6):1131–1144.
- Amino Y, Nakazawa M, Kaneko M, et al. Structure–CaSR–activity relation of kokumi γ-glutamyl peptides. Chem Pharm Bull (Tokyo). 2016;64(8):1181–1189.
- Yang J, Bai W, Zeng X, et al. γ-[Glu](n=1,2)-Phe/-Met/-Val stimulates gastrointestinal hormone (CCK and GLP-1) secretion by activating the calcium-sensing receptor. Food Funct. 2019;10(7):4071–4080.
- Muramatsu M, Hira T, Mitsunaga A, et al. Activation of the gut calcium-sensing receptor by peptide agonists reduces rapid elevation of plasma glucose in response to oral glucose load in rats. Am J Physiol Gastrointest Liver Physiol. 2014;306(12):G1099–G1107.
- Zhao CJ, Schieber A, Gänzle MG. Formation of taste-active amino acids, amino acid derivatives and peptides in food fermentations - A review. Food Res Int. 2016;89:39–47.
- Ueda Y, Yonemitsu M, Tsubuku T, et al. Flavor characteristics of glutathione in raw and cooked foodstuffs. Biosci Biotech Biochem. 1997;61(12):1977–1980.
- Kuroda M, Kato Y, Yamazaki J, et al. Determination and quantification of the kokumi peptide, γ-glutamyl-valyl-glycine, in commercial soy sauces. Food Chem. 2013;141(2):823–828.
- Shibata M, Hirotsuka M, Mizutani Y, et al. Isolation and characterization of key contributors to the “kokumi” taste in soybean seeds. Biosci Biotechnol Biochem. 2017;81(11):2168–2177.
- Yang J, Sun-Waterhouse D, Cui C, et al. Gamma-glutamylation of the white particulates of sufu and simultaneous synthesis of multiple acceptor amino acids-containing γ-glutamyl peptides: favorable catalytic actions of glutaminase. LWT. 2018;96:315–321.
- Yang J, Sun-Waterhouse D, Cui C, et al. Synthesis and sensory characteristics of kokumi γ-[Glu]n-Phe in the presence of glutamine and phenylalanine: glutaminase from Bacillus amyloliquefaciens or Aspergillus oryzae as the catalyst. J Agric Food Chem. 2017;65(39):8696–8703.
- Yang J, Sun-Waterhouse D, Xie J, et al. Comparison of kokumi γ-[Glu](n>1)-Val and γ-[Glu](n>1)-Met synthesized through transpeptidation catalyzed by glutaminase from Bacillus amyloliquefaciens. Food Chem. 2018;247:89–97.
- Yamamoto M, Terada Y, Motoyama T, et al. Sweet proteins lysozyme and thaumatin are protein-type agonists for the calcium-sensing receptor. Biochem Biophys Res Commun. 2020;521(1):227–231.