ABSTRACT
A novel compound, (R)-4-ethoxy-2-hydroxy-4-oxobutanoic acid (1), and six known compounds (2–7) were isolated from the fruiting bodies of the wild edible mushroom Leucopaxillus giganteus. The planar structure of 1 was determined by the interpretation of spectroscopic data analysis. The absolute configuration of 1 was determined by comparing specific rotation of the synthetic compounds. In the plant regulatory assay, the isolated compounds (1–7) and the chemically prepared compounds (8–10) were evaluated their biological activity against the lettuce (Lactuca sativa) growth. Compounds 1 and 3–10 showed the significant regulatory activity of lettuce growth. 1 showed the strongest inhibition activity among the all the compounds tested. In the lung cancer assay, all the compounds were assessed the mRNA expression of Axl and immune checkpoints (PD-L1, PD-L2) in the human A549 alveolar epithelial cell line by RT-PCR. Compounds 1–10 showed significant inhibition activity against Axl and/or immune checkpoint.
Graphical abstract
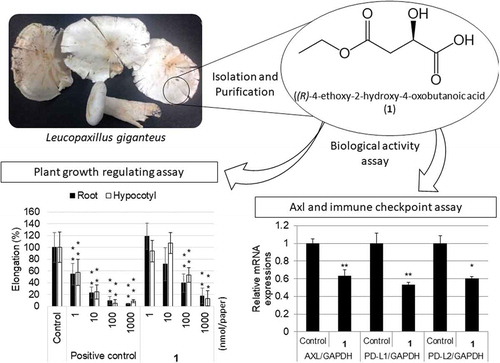
Bioactive compounds were isolated from the mushroom Leucopaxillus giganteus and evaluated their activities in the plant–regulating and Axl-immune checkpoint assays.
Higher fungi that form fruiting bodies have been attracting attention, because they produce diverse biomolecules that show various pharmaceutical and biological activities. Hence, a lot of chemical investigations of fruiting bodies as well as mycelia of higher fungi to search for new bioactive compounds have been reported. We have reported the isolation of 5,7-dimethoxy-2,4-dimethylindole, 5-methoxy-2,4-dimethylindole, and 7-acetamidophthalide from the fruiting bodies of Tricholoma flavovirens and 10-dehydroxymelleolide D and 13-hydroxymelleolide K from the culture broth of Armillaria sp. as plant growth regulators [Citation1,Citation2]. Also, 3′-deoxynisine and cordycepin from Bombyx mori inoculated with Cordyceps militaris as cytotoxic compounds against cancer cells were reported by us [Citation3]. As our continuing search for bioactive compounds from higher fungi, Leucopaxillus giganteus was targeted.
L. giganteus (giant leucopax in English, Ooichotake in Japanese) is a wild edible mushroom that belongs to Tricholomataceae family. This mushroom produces clitocine which has been previously proved as a potent anticancer agent by activating caspase-3, −8, −9, knocking-down of Mcl-1, and inhibiting transcription factor NF-κB [Citation4–Citation6].
Lung cancer is the leading cause of cancer morbidity and mortality worldwide. For this reason, molecular targeted drug discovery and drug discovery development against lung cancer are proceeding actively all over the world. Axl, a member of receptor tyrosine kinases (RTKs), has been designated as a strong candidate for targeted therapy of cancer [Citation7]. Similarly, immune-checkpoint (PD-1, PD-L1, and PD-L2) blockade has triggered a clinical reaction of people with lung cancer in latest clinical studies [Citation8]. There is ongoing research to develop possible drugs to target these signaling pathways (Axl and immune checkpoints) and treat cancers. Isolation of other therapy agents of cancer from natural sources is one of the possible contributions for drug discovery. To develop anticancer agents targeting these signaling pathways (Axl and immune checkpoints), we tried to isolated the potential Axl and immune checkpoints inhibitors from the mushroom. Herein we describe the isolation, structural determination, and biological activity of a novel compound (1) and six known compounds (2–7) from the fruiting bodies of L. giganteus. In addition, we prepared 1 and its enantiomer (8) to determine the absolute configuration of 1 and other two analogs (9 and 10) to study the structure–activity relationship.
Materials and methods
General experimental procedures
1H-NMR spectra (one- and two-dimensional) were recorded on the Jeol Lambda-500 spectrometer at 500 MHz, 13C-NMR spectra were recorded at 125 MHz (Jeol Ltd., Tokyo, Japan). Chemical shifts for 1H NMR and 13C NMR were reported in δ relative to 7.26 and 77.0 for CDCl3, 3.30 and 49.8 for CD3OD, respectively. HRESIMS data were measured by a JMS-T100LC mass spectrometer (Jeol Ltd., Tokyo, Japan). IR spectra were recorded on an FTIR-4100 (JASCO Co., Tokyo, Japan). The specific rotation values were measured with a Jasco DIP-1000 polarimeter (Jasco Co., Tokyo, Japan). HPLC separation was performed with a JASCO Gulliver system (Jasco Co., Tokyo, Japan) using two reverse-phase HPLC columns (Cosmosil PBr, Nacalai Tesque, Kyoto, Japan; Phenylhexyl, InertSustain, Tokyo Japan) and three normal phase HPLC columns (YMC-pack Diol-60-NP, YMC Co., Ltd., Kyoto, Japan; Cosmosil 5 SL-II, Nacalai Tesque, Kyoto, Japan; Inertsil Diol, GL Science Inc., Tokyo, Japan). Silica cartridges and C18 cartridges (Nihon Waters K.K., Tokyo, Japan) were used in the pre-processing of samples. Silica gel plate, ODS gel plate (TLC Silica gel 60 F254, Merck KGaA, Darmstadt, Germany), and silica gel 60 N (Kanto Chemical Co., Inc., Tokyo, Japan) were used for analytical TLC and for flash column chromatography, respectively.
Fungal material
Fresh fruiting bodies of L. giganteus were collected from Narusawa village, Yamanashi Prefecture in Japan.
Extraction and isolation
The fresh fruiting bodies were extracted and fractionated twice. In the first extraction, 20.6 kg of the fresh fruiting bodies were extracted with EtOH (30 L, twice) and then acetone (20 L, twice). After the solutions were combined and concentrated under reduced pressure, the concentrate was partitioned between n-hexane and H2O, EtOAc and H2O, and then n-BuOH and H2O. The EtOAc soluble part (14.8 g) was subjected to silica gel flash column chromatography (CH2Cl2; CH2Cl2/acetone = 90/10, 70/30, 50/50; CH2Cl2/MeOH = 80/20, 60/40; MeOH; 1.0 L each, 75 × 500 mm, 800 g) to obtain 15 fractions (fractions 1 to 15). Fraction 7 (146.3 mg) was further purified by reverse-phase HPLC (Cosmosil PBr, 20 × 250 mm, UV 210 nm, 5 mL/min, MeOH/H2O = 40/60) to give compound 2 (7.3 mg). Fractions 8 (82.6 mg) and 9 (57.4 mg) were separated by normal phase HPLC (Cosmosil 5 SL-II, 20 × 250 mm, UV 250 nm, 5 mL/min, CHCl3/MeOH = 97/3) to give 24 fractions (fractions 8–1 to 8–24 and fractions 9–1 to 9–24, respectively). Fractions 8–16 and 9–17 were also identified as compound 2 (18.5 mg and 6.1 mg, respectively). Fractions 8–13 (4.4 mg) and 9–14 (1.0 mg) were combined and then fractionated by reverse-phase HPLC (Inertsustain Phenylhexyl, 20 × 250 mm, UV 210 nm, 5 mL/min, MeOH/H2O = 40/60) to obtain compound 3 (2.3 mg). Fraction 15 (135.7 mg) was fractionated by normal phase HPLC (YMC-pack Diol-60-NP, 20 × 250 mm, UV 260 nm, 5 mL/min, CHCl3/MeOH = 90/10) to give compound 1 (3.0 mg). In the second extraction, 7.9 kg of the fruiting bodies were crushed and extracted in the same method and the EtOAc soluble part (7.4 g) was also fractionated to give 12 fractions (fractions 1′ to 12′). Fraction 3′ (95.9 mg) was separated by normal-phase HPLC (Cosmosil 5 SL-II, 20 × 250 mm, UV 250 nm, 5 mL/min, CHCl3/MeOH = 95/5) to afford 8 fractions (fractions 3′-1 to 3′-8). Fraction 3′-3 (60.2 mg) was also purified by normal phase HPLC (Cosmosil 5 SL-II, 20 × 250 mm, UV 260 nm, 5 mL/min, n-hexane/EtOAc = 40/60) to obtain compound 4 (5.8 mg). Fraction 9′ (418.0 mg) was separated by silica gel flash column chromatography (CH2Cl2/MeOH = 90/10, 80/20, 70/30, 60/40, 50/50, 40/60, 30/70; MeOH; 200 mL each, 35 × 500 mm, 400 g) to obtain 13 fractions (fractions 9′-1 to 9′-13). Fraction 9′-7 (42.9 mg) was further fractionated by reverse-phase HPLC (Cosmosil PBr, 20 × 250 mm, UV 250 nm, 5 mL/min, MeOH/H2O = 30/70) to obtain compound 5 (18.5 mg). Fraction 4′ (476.2 mg) was separated by silica gel flash column chromatography (n-hexane; n-hexane/acetone = 90/10, 80/20, 70/30, 50/50, 30/70, 20/80; acetone; MeOH; 200 mL each, 35 × 500 mm, 400 g) to obtain 18 fractions (fractions 4′-1 to 4′-18). Fraction 4′-11 (38.1 mg) was further fractionated by normal phase HPLC (Inertsil Diol, 20 × 250 mm, UV 240 nm, 5 mL/min, CHCl3/MeOH = 95/5) to give compounds 6 (6.0 mg) and 7 (8.8 mg).
Structural elucidation
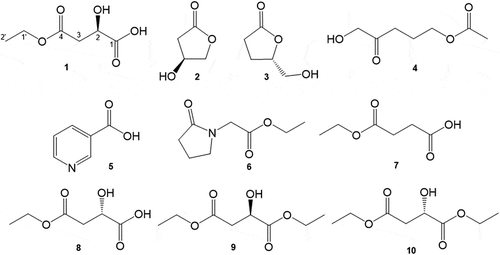
Seven compounds (1–7) were isolated from the fresh fruiting bodies of L. giganteus. The structure of each compound was confirmed by the interpretation of spectroscopic data, NMR, and HRESIMS.
Compound 1: pale yellow oil; HRESIMS m/z 185.0244 [M+ Na]+ (calcd for C6H10O5Na, 185.0239); [α]D26 + 13 (c 0.25, MeOH); 1H NMR (in CD3OD): δ 1.27 (3 H, t, J = 7.2 Hz, H-2ʹ), 2.65 (1 H, dd, J = 16.2, 7.3 Hz, H-3), 2.75 (1 H, dd, J = 16.2, 4.9 Hz, H-3), 4.19 (2 H, m, H-1ʹ), 4.46 (1 H, t, J = 6.0 Hz, H-2); 13C NMR (in CD3OD): δ 14.4, 39.9, 62.3, 68.7, 173.9, 174.7.
Compound 2: pale yellow oil; ESIMS m/z 125 [M + Na]+; [α]D26 − 23.4 (c 1.54, MeOH), lit. [α]D25 − 75.7 (c 0.86, MeOH) [Citation9]; 1H NMR (in CDCl3): δ 2.45 (1 H, d, J = 18.0 Hz), 2.70 (1 H, dd, J = 18.0, 6.1 Hz), 4.25 (1 H, d, J = 10.4 Hz), 4.37 (1 H, dd, J = 10.4, 4.3 Hz), 4.61 (1 H, dd, J = 6.1, 4.3 Hz); 13C NMR (in CDCl3): δ 37.7, 67.3, 76.3, 177.1.
Compound 3: colorless oil; ESIMS m/z 139 [M + Na]+; [α]D29 + 175 (c 0.07, in CHCl3), lit. [α]D29 + 45.0 (c 0.80, in CHCl3) [Citation10]; 1H NMR (in CDCl3): δ 2.13 (1 H, m), 2.25 (1 H, m), 2.53 (1 H, m), 2.61 (1 H, m), 3.65 (1 H, dd, J = 12.5, 4.9 Hz), 3.89 (1 H, dd, J = 12.5, 2.7 Hz), 4.61 (1 H, m); 13C NMR (in CDCl3): δ 23.2, 28.6, 64.3, 80.8, 177.2.
Compound 4: colorless oil; ESIMS m/z 183 [M+ Na]+; 1H NMR (in CD3OD): δ 1.91 (2 H, m), 2.01 (3 H, s), 2.52 (2 H, t, J = 7.2 Hz), 4.06 (2 H, t, J = 6.4 Hz), 4.19 (2 H, s); 13C NMR (in CD3OD): δ 20.7, 23.6, 35.4, 64.9, 68.7,172.9, 211.6.
Compound 5: white solid; ESIMS m/z 123 [M]+; 1H NMR (in CD3OD): δ 7.53 (1 H, dd, J = 7.9, 4.9 Hz), 8.27 (1 H, m), 8.68 (1 H, dd, J = 4.9, 1.5 Hz), 9.01 (1 H, d, J = 2.1 Hz); 13C NMR (in CD3OD): δ 125.1, 131.5, 137.3, 149.5, 152.8, 169.8.
Compound 6: pale yellow oil; ESIMS m/z 172 [M + H]+; m/z 194 [M + Na]+; 1H NMR (in CDCl3): δ 1.24 (3 H, t, J = 3.1 Hz), 2.07 (2 H, m), 2.40 (2 H, t, J = 8.2 Hz), 3.46 (2 H, t, J = 7.2 Hz), 4.10 (2 H, dd, J = 14.3, 7.3 Hz), 4.17 (2 H, dd, J = 14.3, 7.0 Hz); 13C NMR (in CDCl3): δ 14.2, 17.9, 30.3, 44.1, 47.7, 61.3, 168.7, 175.6.
Compound 7: pale yellow oil; ESIMS m/z 169 [M+ Na]+; 1H NMR (in CDCl3): δ 1.30 (3 H, t, J = 13.0 Hz), 2.60 (2 H, m), 2.70 (2 H, m), 4.14 (2 H, m); 13C NMR (in CDCl3): δ 14.1, 28.8, 28.9, 63.3, 172.1, 177.6.
Esterification of malic acid
(R) or (S)-Malic acid (0.9 g or 2 g) and EtOH (10.1 mL) were reacted in the presence of 1 M HCl (679 μL) for 15 min at room temperature [Citation11]. The reaction mixture (1.1 g or 2.0 g) was subjected to silica gel flash column chromatography followed by Sephadex LH-20 gel (GE Healthcare, Uppsala, Sweden; CHCl3/MeOH = 1/1, 35 × 500 mm, 100 g). As a result, two stereoisomers of monoethyl esters 1 (18.6 mg, 1.8% yield) and 8 (10.2 mg, 0.4% yield) along with diethyl esters 9 (12.0 mg, 1.0% yield) and 10 (98.9 mg, 3.5% yield) were isolated.
(R)-4-Ethoxy-2-hydroxy-4-oxobutanoic acid (synthetic 1). C6H10O5, ESIMS m/z 185 [M+ Na]+; [α]D28 + 12 (c 0.25, MeOH); 1H NMR (in CD3OD): δ 1.27 (3 H, t, J = 7.5 Hz, H-2ʹ), 2.65 (1 H, dd, J = 16.0, 7.5 Hz, H-3), 2.75 (1 H, dd, J = 16.0, 4.5 Hz, H-3), 4.19 (2 H, m, H-1ʹ), 4.46 (1 H, t, J = 6.0 Hz, H-2).
(S)-4-Ethoxy-2-hydroxy-4-oxobutanoic acid (8). C6H10O5, ESIMS m/z 185 [M+ Na]+; [α]D26 −15 (c 0.24, MeOH); 1H NMR (500 MHz, in CD3OD): δ 1.27 (3 H, t, J = 7.3 Hz, H-2ʹ), 2.65 (1 H, dd, J = 16.0, 7.3 Hz, H-3), 2.75 (1 H, dd, J = 16.0, 4.5 Hz, H-3), 4.19 (2 H, m, H-1ʹ), 4.46 (1 H, t, J = 6.0 Hz, H-2).
Ethyl (R)-4-ethoxy-3-hydroxypent-4-enoate (9). C8H14O5, ESIMS m/z 213 [M+ Na]+; [α]D27 + 5.0 (c 1.08, MeOH); 1H NMR (in CD3OD): δ 1.24 (3 H, t, J = 6.0 Hz), 1.27 (3 H, t, J = 6.3 Hz), 2.69 (1 H, dd, J = 16.0, 7.0 Hz), 2.77 (1 H, dd, J = 15.5, 5.0 Hz), 4.14 (2 H, m), 4.19 (2 H, m), 4.47 (1 H, t, J = 6.0 Hz).
Ethyl (S)-4-ethoxy-3-hydroxypent-4-enoate (10). C8H14O5, ESIMS m/z 213 [M+ Na]+; [α]D25 −5.2 (c 1.00, MeOH); 1H NMR (in CD3OD): δ 1.24 (3 H, t, J = 6.3 Hz), 1.27 (3 H, t, J = 6.3 Hz), 2.69 (1 H, dd, J = 15.5, 7.0 Hz), 2.77 (1 H, dd, J = 15.5, 5.0 Hz), 4.14 (2 H, m), 4.19 (2 H, m), 4.47 (1 H, t, J = 6.0 Hz).
Biological activity assay
Plant growth–regulating assay
Lettuce seeds (Lactuca sativa L. cv. Cisko; Takii Co., Ltd., Tokyo, Japan) were used in this bioassay. Suitable amount of lettuce seeds were put on filter paper (Advantec No. 2, ϴ 55 mm; Toyo Roshi Kaisha, Ltd., Japan), soaked in distilled water in a Petri dish (ϴ 60 × 20 mm), and incubated in a dark growth chamber at 20°C for 24 h. Compounds 1–10 and 2,4-dichlorophenoxyacetic acid (2,4-D, positive control) were dissolved in 1 mL of MeOH (1, 10, 102, and 103nmol/mL) and allowed permeating on filter paper (ϴ 55 mm) in a Petri dish (ϴ 60 × 20 mm). After the sample-loaded paper was dried, 1 mL of distilled water was poured on the paper or intact filter paper (control). The pre-incubated lettuces (n = 9 in each Petri dish) were transferred onto the filter paper and incubated in a dark growth chamber at 20°C for 3 d. The length of the root and the hypocotyl was measured using a digimatic caliper (Mitutoyo Corporation CD-15AXR, Japan). Data collected were analyzed statistically using Student’s t-test to determine the significant difference with P values was considered significant.
Axl and immunce checkpoint assay
The human A549 alveolar epithelial cell line was purchased from the American Type Culture Collection (Rockville, MD, USA) and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM), supplemented with 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine and 100 U/mL penicillin plus 100 U/mL streptomycin. All cells were cultured at 37°C in 75 cm2 flasks in an atmosphere composed of 5% CO2 and 95% air. Confluent cells were passaged after 5–7 d. A549 cells in 0.1% Bovine Serum Albumin (BSA) and DMEM were seeded in 24-well plates. Each of compounds 1–10 (20 μg/mL) was added to the wells, and the plates were incubated for 24 h. Total RNA was extracted using Sepasol®-RNA I Super G (Nacalai) following the instructions of the manufacturer. One μg of total RNA was denatured at 65°C for 10 min, and then reverse-transcribed using ReverTra Ace Reverse Transcriptase (TOYOBO) and oligo (dT) primer in a volume of 20 μL according to the manufacturer’s protocol. Each gene contains forward and reverse sequence (5΄ > 3΄) as GGAGCGAGATCCCTCCAAAAT and GGCTGTTGTCATACTTCTCATGG for GADPH gene, TGCCATTGAGAGTCTAGCTGAC and TTAGCTCCCAGCACCGCGAC for Axl gene, GGACAAGCAGTGACCATCAAG and CCCAGAATTACCAAGTGAGTCCT for PD-L1 gene, ACCGTGAAAGAGCCACTTTG and GCGACCCCATAGATGATTATGC for PD-L2 gene, respectively. The cDNA was amplified by PCR and the conditions were as follows: 94°C, 1 min; 60°C, 1 min; and 72°C, 1 min for 28–35 cycles. PCR products were electrophoresed on a 1.5% agarose gel and then stained with ethidium bromide solution. Semi-quantitative RT-PCR results were quantified by using ImageJ software. The statistical difference was calculated by analysis of variance with post hoc analysis using Fisher’s protected least significant difference test.
Results and discussion
Structural determination
The fresh fruiting bodies of L. giganteus were extracted with EtOH and then acetone. The extract was divided into n-hexane, EtOAc, and n-BuOH soluble parts. The EtOAc soluble part was fractionated by repeated chromatography. As a result, a novel compound (1) and six known compounds (2–7) were isolated ().
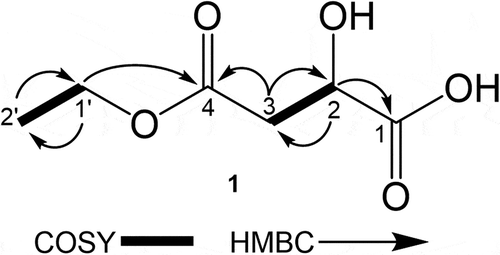
Compound 1 was isolated as pale yellow oil. The molecular formula was determined as C6H10O5 by HRESIMS (m/z 185.0244 [M+ Na]+; calcd for C6H10O5Na, 185.0239), indicating two degrees of unsaturation in the molecule. The structure of 1 was elucidated by interpretation of NMR spectra including DEPT, COSY, HMQC, and HMBC (). The DEPT experiment indicated the presence of one methyl, two methylenes, one methine, and two tetrasubstituted carbons. The presence of malic-acid moiety was constructed by a COSY correlation (H-2/H-3) and the HMBC correlations (H-2/C-1, C-3; H-3/C-2, C-4). The COSY correlation (H-1ʹ/H-2ʹ) and the HMBC correlations (H-1ʹ/C-4, 2ʹ; H-2ʹ/C-1ʹ) suggested that the carboxylic acid at C-4 was esterified. The absolute configuration of 1 was determined by comparing its specific rotation {[α]D26 + 13 (c 0.25, MeOH)} with that of synthetic one {[α]D28 + 12 (c 0.25, MeOH)} and its enantiomer (8) {[α]D26 −15 (c 0.24, MeOH)}. The compound that has the same planar structure to 1 has been reported as one of the chemical constituents from the seeds of Morinda citrifolia, the whole plant of Lobelia chinensis, and the dried roots of Ampelopsis japonica. However, specific rotation and CD data have not been reported in the literatures; therefore, the absolute configuration of the isolated compound has not been determined yet [Citation12–Citation14]. Thus, our finding allowed us to conclude that compound 1 was a novel compound, (R)-4-ethoxy-2-hydroxy-4-oxobutanoic acid ().
Compound 6 was identified as ethyl 2-(2-oxopyrrolidin-1-yl)acetate that has been synthesized as a fungicide [Citation15]. However, it was isolated from a natural source for the first time. Compound 3 was identified as (S)-5-(hydroxymethyl)-dihydrofuran-2(3 H)-one, which has been isolated from a plant, Clematis hirsuta [Citation10]. Its biological activity and isolation of it from fungi have not been reported yet. Compound 2, (S)-3-hydroxy-4-butanolide, has been isolated from a mushroom, Climacodon septentrionalis, and was evaluated for cytotoxicity against human lung cancer cells, but no effects were exhibited [Citation16]. Compound 4 was identified as catathelasmol D, which has been isolated from the fruiting bodies of Catathelasma imperiale, and it has inhibitory activities against two isozymes, 11β-hydroxysteroid dehydrogenases (11β -HSD1 and 11β -HSD2) [Citation17]. Compound 5 has been isolated from an edible mushroom Astraeus odoratus, which has been used to help in the fight against various diseases such as elevated fasting glucose, diabetes, metabolic syndrome, and the treatment of dyslipidemia [Citation18]. Compound 7, monoethyl succinate, was isolated from a marine fungus, Cladosporium cladosporioides, and it was reported that 7 inhibited stem elongation on the grown dwarf peas [Citation19,Citation20].
Plant growth–regulating activity
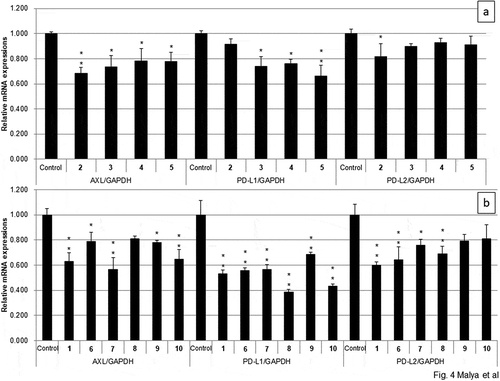
Plant growth–regulating activity of 1–7 was evaluated using lettuce. 2,4-Dichlorophenoxyacetic acid was used as positive control. As shown in , 5 promoted the root growth at 1 nmol/paper and 6 showed the promotion effect at 10 and 100 nmol/paper against hypocotyl growth. Among 3, 4, and 7 showing inhibition activity at 1000 nmol/paper, 4 showed the strongest activity. In order to study the structure–activity relationship of 1, 9 and 10 were chemically prepared, and the activity of 1, its enantiomer (8), and di-esters (9, 10) was evaluated (). The inhibition activity of the novel compound 1 was the strongest among all the compounds tested. The antipode of 1 (8) showed much less activity than 1.
Figure 3. Growth-regulating activity against lettuce of compounds 1–10 against root (a) or hypocotyl (b). 2,4-Dichlorophenoxyacetic acid (2,4-D) was used as positive control. Results are the mean ± standard deviation (n = 9). [*p < 0.05, **p < 0.01 (growth inhibition); +p < 0.05, ++p < 0.01 (growth promotion)].
![Figure 3. Growth-regulating activity against lettuce of compounds 1–10 against root (a) or hypocotyl (b). 2,4-Dichlorophenoxyacetic acid (2,4-D) was used as positive control. Results are the mean ± standard deviation (n = 9). [*p < 0.05, **p < 0.01 (growth inhibition); +p < 0.05, ++p < 0.01 (growth promotion)].](/cms/asset/53574512-a733-46de-9a3c-ae02ea460e2f/tbbb_a_1743170_f0003_b.gif)
Axl and immune checkpoint assay
The human A549 alveolar epithelial cell lines were treated with each compound from 1 to 10. As shown in , among compounds 2–7,compounds 6 and 7 inhibited expressions of all the three genes, 2 significantly suppressed the expressions of Axl and PD-L2, and 3–5 showed suppressing activity against Axl and PD-L1 expressions. Among malic-acid esters (1, 8–10), only the isolated compound 1 showed the effects on all the gene expressions. The results indicated that the carboxylic acid moiety played an important role in the suppression of PD-L2 and the natural product 1 was the most promising candidate for cancer therapy. To our knowledge, it was the first time that Axl and immune checkpoint inhibitors were isolated from higher fungi.
Figure 4. Effect of compounds 1–10 on expressions of Axl and immune checkpoints (PD-L1 and PD-L2) on lung cancer cell line A549 cells. Values indicate means with standard deviation from three independent triplicate experiments. Statistical analysis was performed using Fisher’s test (*p < 0.05, **p < 0.01 vs control, n = 3).
Author contribution
H. K. conceived the project and designed the experiments. I. Y. M., J. W., E. H., E. C. G., M. T., T. Y., and C. N. D. performed the experiments. J. C., H. H., and H. K. contributed to discussions. I. Y. M., J. W., and H. K. wrote the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Qiu W, Kobori H, Wu J, et al. Plant growth regulators from the fruiting bodies of Tricholoma flavovirens. Biosci Biotechnol Biochem. 2017;81:441–444.
- Kobori H, Sekiya A, Suzuki T, et al. Bioactive sesquiterpene aryl esters from the culture broth of Armillaria sp. J Nat Prod. 2015;78:163–167.
- Qiu W, Wu J, Choi J, et al. Cytotoxic compounds against cancer cells from Bombyx mori inoculated with Cordyceps militaris. Biosci Biotechnol Biochem. 2017;81:1224–1226.
- Ren G, Zhao Y, Yang L, et al. Anti-proliferative effect of clitocine from the mushroom Leucopaxillus giganteus on human cervical cancer HeLa cells by inducing apoptosis. Cancer Lett. 2008;262:190–200.
- Sun J, Yeung CA, Co NN, et al. Clitocine reversal of P-Glycoprotein associated multi-drug resistance through down-regulation of transcription factor NF-kB in R-HepG2 cell line. PLoS One. 2012;7:e40720.
- Sun J, Li H, Li X, et al. Clitocine targets Mcl-1 to induce drug-resistant human cancer cell apoptosis in vitro tumor growth inhibition in vivo. Apoptosis. 2014;19:871–882.
- Wu F, Li J, Jang C, et al. The Role of Axl in drug resistance and epithelial-to-mesenchymal transition of non-small cell lung carcinoma. Int J Clin Exp Pathol. 2014;7:6653–6661.
- Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25:1935–1940.
- Uchikawa O, Okukado N, Sakata T, et al. Synthesis of (S)- and (R)-3-hydroxy-4-butanolide and (2S,4S)-, (2R,4S)-, (2S,4R)-, and (2R,4R)-2-hydroxy-4-hydroxymethyl-4-butanolide and their satiety and hunger modulating activities. Bull Chem Soc Jpn. 1988;61:2025–2029.
- Abdel-Kader MS, Al-Taweel AM, El-Deeb KS. Bioactivity guided phytochemical study of Clematis hirsuta growing in Saudi Arabia. Nat Prod Sci. 2008;14:56–61.
- Cohen SG, Neuwirth Z, Winstein SY. Association of substrates with α-chymotrypsin, diethyl α-acetoxysuccinate, and diethyl malate. J Am Chem Soc. 1966;88:5306–5314.
- Yang X, Jiang M, Hsieh K, et al. Chemical constituents from the seeds of Morinda citrifolia. Chin J Nat Med. 2009;7:119–122.
- Yang S, Shen T, Zhao L, et al. Chemical constituents of Lobelia chinensis. Fitoterapia. 2014;93:168–174.
- Xiong H, Mi J, Le J, et al. Chemical constituents of Ampelopsis japonica. Chem Nat Comp. 2017;53:791–793.
- Chang Y, Zhi C, Wang X. Synthesis of ethyl (2-oxo-1-pyrrolidinyl)acetate. J TYUT. 2005;36:186–189.
- Wu J, Tsujimori M, Hirai H, et al. Novel Compounds from the mycelia and fruiting bodies of Climacodon septentrionalis. Biosci Biotech Biochem. 2011;15:783–785.
- Zhang L, Shen Y, Zhu H, et al. Pentanol derivatives from basidiomycete Catathelasma imperial and their 11β-Hydroxysteroid dehydrogenases inhibitory activity. J Antibiot. 2009;62:239–242.
- Arpha K, Phosri C, Suwannasai N, et al. Astraodoric acids A–D: new lanostane triterpenes from edible mushroom Astreus odoratus and their anti-mycobacterium tuberculosis H37Ra and cytotoxic activity. J Agric Food Chem. 2012;60:9834–9841.
- Zou J, Dai J. Chemical constituents in marine fungus of Cladosporium cladoporioides. Chin Pharm J. 2009;44:418–421.
- Komoto N, Ikegami S, Tamura S. Isolation of acidic growth inhibitors in dwarf peas. Agric Biol Chem. 1972;36:2547–2553.