ABSTRACT
The enhancing effects of yeasts on the viability of lactic acid bacteria (LAB) under acidic conditions were investigated. Meyerozyma guilliermondii, coaggregative with both LAB strains under acidic conditions, significantly enhanced the viability of Lactobacillus pentosus and L. paracasei in pH 3.0 lactic acid (LA) buffer at 10°C (p < 0.05). Non-coaggregative yeasts (Saccharomyces cerevisiae, Schizosaccharomyces pombe, and Cyberlindnera saturnus) also significantly enhanced the LAB viability (p < 0.05), and physical contact between LAB and yeasts was not essential for the viability-enhancing effect, indicating that the coaggregation had no relation to the enhancing mechanism. Although yeast metabolites and LA assimilation had no enhancing effect, hydrogen peroxide (H2O2) decreased after yeast coincubation, and H2O2 elimination improved L. pentosus viability. H2O2 elimination alone did not sufficiently improve L. paracasei viability, but the addition of antioxidants was effective. These results suggest that the antioxidant activity of yeast increased the LAB viability under acidic conditions.
GRAPHICAL ABSTRACT

Yeasts coincubation enhanced the LAB viability under acidic conditions and decreased the H2O2 accumulations. Yeast antioxidant activity might increase the LAB viability.
Acidic conditions owing to lactic acid (LA) fermentation are extremely harsh, primarily because of pH decrease and the antibiotic properties of LA molecules. These environments have been employed for the stable production of various fermented foods or for biopreservation to ensure long-term storage of foods [Citation1–Citation3]. The coexistence and interaction of LA bacteria (LAB) with yeast have been widely observed in various fermented foods and the ensuing microbial interaction plays an essential role in the quality of the final products, in which the microbial metabolic activities result in the desired changes [Citation4–Citation6]. Therefore, the elucidation of various microbial interactions that occur in the LA fermentation process is important for their application in manufacturing fermented foods. However, our knowledge regarding the microbial interactions that specifically occur under acidic conditions with LA is limited.
In their pioneering studies on microbial interactions between LAB and yeasts, Momose et al. [Citation7] observed coaggregation between Lactobacillus plantarum isolated from putrefied sake mash and sake yeast Kyokai No. 7 specifically under acidic conditions (pH 2–4) with hydrochloric acid. They showed that this coaggregation was induced by the electrostatic interaction between LAB and yeast cells and was reversible with respect to the change in pH, although the biological interpretations of the coaggregation were not described. Katakura et al. [Citation8] reported that cytosolic proteins, including DnaK, cause the coaggregation and adhesion of LAB and yeast under acidic conditions at approximately pH 4. DnaK recognized yeast mannan, and adhesion occurred even in the presence of 2 M NaCl, which was different from the electrostatic aggregation reported by Momose et al. [Citation7]. In the subsequent report, Yamasaki-Yashiki et al. [Citation9] showed that direct contact of L. paracasei ATCC 334 with heat-inactivated yeast cells induced the upregulated gene expression of proteins related to exopolysaccharide synthesis, surface protein modification, and transport systems. Regarding the physiological significance of coaggregation, Furukawa et al. [Citation10–Citation12] reported that coaggregation between LAB and yeast isolated from pot vinegar induced mixed-species biofilm formation. Although these reports provide important information regarding the physiological interpretation of coaggregation between LAB and yeasts, their coaggregation (pH 4.0–11.5) was not restricted under acidic conditions [Citation11].
Liu et al. [Citation13–Citation17] reported that coculture with different species of yeast prolonged the viability of LAB in milk culture. Viability-enhancing effects were influenced by various factors, including differences in the combination of LAB and yeast strains [Citation13], incubation temperature, total milk solids level, cocultured yeast viability, the addition of yeast extracts [Citation14], temperatures of storage and fermentation, inoculation time of yeast, yeast concentration, yeast viability, oxygen removal, yeast metabolites [Citation15], pH, direct cell-to-cell contact coaggregation [Citation16], yeast supernatant, viability of yeast, and physical contact between LAB and yeast [Citation17]. Besides the experiments using milk culture, Saccharomyces cerevisiae EC-1118 was elucidated to have viability-enhancing and coaggregation effects on the probiotic strain L. rhamnosus HN001 in McIlvaine’s buffer as a model system (non-growing cells) at pH 2.5–4.0 [Citation16]. Although the coaggregation mechanism was not clarified, the finding of the correlation of viability-enhancing effect and coaggregation was important for the understanding of the interaction between LAB and yeasts under acidic conditions.
Previously, we have investigated the interaction between LAB and yeasts specifically under acidic conditions (in an LA buffer system) using various strains of plant origin LAB and yeasts isolated from commercial pickles in Japan. In our preliminary studies, we found several coaggregative combinations of LAB and yeasts only at acidic pHs, and the viability-enhancing effects of yeast on LAB were observed in some of the coaggregative combinations.
In this paper, we aim to clarify the following points: (1) the relationship between the viability-enhancing effects of yeast on LAB specifically observed at acidic pHs and the coaggregation, and (2) the factors (physical contact, yeast metabolites, LA assimilation by yeasts, hydrogen peroxide (H2O2), and antioxidants) that affect this process.
Materials and methods
Strains and media
All mandatory laboratory health and safety procedures were complied with in the course of conducting the experimental work in this study. LAB and yeast isolated in our laboratory were mainly used in this study. The LAB strains were L. pentosus N1 from nukadoko (fermented rice bran) and L. paracasei N9 from Pacific saury narezushi (fermented sushi). The yeast strains used were Meyerozyma guilliermondii K25 isolated from cucumber nukazuke (pickles) and S. cerevisiae NBRC 10217, Schizosaccharomyces pombe NBRC 1628, and Cyberlindnera saturnus NBRC 10697 obtained from the National Institute of Technology and Evaluation Biological Resource Center (Chiba, Japan). M. guilliermondii was isolated most frequently from pickles in preliminary investigations, in which the strain K25 had high and broad coaggregation activity toward various LAB strains. The identification of L. pentosus (by 16S rRNA and recA gene sequencing), L. paracasei (by 16S rRNA gene sequencing), and M. guilliermondii (by 26S rRNA gene sequencing) was conducted at TechnoSuruga Laboratory Co., Ltd. (Shizuoka, Japan). LAB were grown in de Man, Rogosa, and Sharpe broth (Oxoid Ltd., Hampshire, UK) at 28°C for 24–48 h without shaking, and yeasts were grown in yeast malt (YM) broth at 28°C for 24–48 h with shaking (100 rpm). The YM broth comprised 1% (w/v) glucose (Wako Pure Chemical Industries, Ltd., Osaka, Japan), 0.5% (w/v) Bacto™ Peptone (BD Biosciences, NJ, USA), 0.3% (w/v) malt extract (Oxoid Ltd.), and 0.3% (w/v) yeast extract (Sigma-Aldrich Japan KK, Tokyo, Japan).
Coaggregation assay
A coaggregation assay between LAB and yeast was performed using a described previously method [Citation7] with some modifications. Briefly, cultured LAB and yeast cells were washed twice with sterile water. Then, the LAB cells (5 × 108) and yeast cells (5 × 107) were mixed in 1 mL of LA solution (diluted LA with sterile water) at various pHs (1.0–5.0) or sterile water in a test tube of 1 cm diameter. After incubation for 30 min at room temperature and 10°C under static conditions, 0.1 mL of the surface layer of the suspension was removed to determine the optical density at 600 nm (OD600) using a GloMax® Discover Microplate Reader (Promega KK, Tokyo, Japan) with a 96-well microplate. The coaggregation activity was calculated using the equation: (A0 − A30)/A0, where A0 and A30 are OD600 at 0 and 30 min, respectively. Coaggregation was defined as coaggregation activity that exceeded 0.25. The ratio of yeast cells for coaggregation assay was determined to maximize the coaggregation activity based on the prior experiments.
LAB viability assay
To evaluate the effect of yeast on LAB viability under acidic conditions without pH changes, the coaggregation assay was performed in 55 mM LA buffer (mixture of LA and sodium lactate) at pH 3.0, as a model system of LA fermentation, and stored at 10°C under static conditions. Samples were diluted appropriately with phosphate-buffered saline (pH 7.4) to completely release the aggregation and cultured on plate count agar with bromocresol purple (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) containing 0.5 g/mL of cycloheximide (Wako Pure Chemical Industries, Ltd.) to inhibit yeast growth. Duplicate plates were incubated at 30°C for 48 h. The viability of yeast without LAB was investigated using YM agar for culture.
The importance of physical contact between the LAB and M. guilliermondii cells was investigated by incubating LAB with yeast under separation using a cellulose dialysis tube (12,000–14,000 molecular weight cutoff; Nihon Medical Science, Osaka, Japan). LAB (1.5 × 109 cells) were suspended in 10 mL of LA buffer, poured into a cellulose tube, sealed, and then placed in a 50-mL plastic tube filled with 20 mL of LA buffer in which M. guilliermondii (1.5 × 108 cells) were suspended. The plastic tube was statically incubated at 10°C, and the LAB viability was investigated.
To assess the effect of M. guilliermondii metabolites on LAB viability, LAB cells were incubated in yeast supernatant, which was obtained by statically incubating yeast (5 × 107 cells/mL) in LA buffer at 10°C for 15 days. The buffer was filtered through a 0.2-μm membrane prior to use. To ascertain whether yeast produces specific metabolites when coaggregating with LAB, the LAB viability in the yeast-LAB supernatant was also investigated. The yeast-LAB supernatant was obtained by coincubating yeast with LAB (5 × 108 cells/mL) as described above.
To determine the influence of H2O2 on LAB viability, 100 mg/mL of manganese (Ⅳ) oxide (MnO2; Sigma-Aldrich Japan KK) was added to the LAB suspension, and LAB viability was assessed. MnO2 was selected as an inorganic catalyst to eliminate H2O2 under acidic conditions because acid-tolerant catalases were not commercially available. Additionally, to clarify the effect of antioxidants on L. paracasei viability, L(+)-ascorbic acid sodium salt (AA; Fujifilm Wako Pure Chemical Corporation, Osaka, Japan) or (–)-epigallocatechin gallate (EGCG; Fujifilm Wako Pure Chemical Corporation) was added at various concentrations to LA buffer, and the LAB viability was investigated.
Quantitative analysis of LA concentration
To evaluate the effect of M. guilliermondii on the LA concentration in LA buffer in which LAB were incubated, quantitative analysis of LA in the supernatant was performed following a previously described method [Citation18] with some modifications. The LA concentration was determined with a Chromaster® high-performance liquid chromatography system comprising a Chromaster® 5110 pump, 5310 column oven, and 5430 diode array detector (Hitachi High-Technologies Corp., Tokyo, Japan). For the separation of LA, a 5-μm LaChrom C18-AQ column (Hitachi High-Technologies Corp.) was used. The sample was filtered through a 0.2-μm membrane before injection. A calibration curve was created with L-(+)-LA (Sigma-Aldrich Japan KK). The pH of the sample was also measured.
Quantitative analysis of H2O2
To investigate the effect of yeast on the H2O2 concentration, the amount of extracellular H2O2 present in the supernatant of the suspensions in which LAB and yeast were incubated as described above was determined. The H2O2 concentration was determined by ferrous oxidation in a xylenol orange assay, following the procedure described by Toh et al. [Citation19], with a calibration curve created using 27% H2O2 (Wako Pure Chemical Industries, Ltd.).
Statistical analysis
Statistical analysis was performed using SPSS version 24.0 (IBM Company, Tokyo, Japan). One-way analysis of variance followed by Scheffe’s test was used to compare more than two groups. A p-value of <0.05 was considered statistically significant. Values were presented as means ± standard deviation (SD).
Results
Coaggregation activity
shows the coaggregation activity between LAB and yeast in the LA solution at various pHs or sterile water at room temperature. L. pentosus and L. paracasei coaggregated with M. guilliermondii at pH 2.0–3.0 and pH 2.0–4.0, respectively, and no coaggregation was observed in sterile water. Both LAB also coaggregated at 10°C (data not shown). Autoaggregation did not occur, and both LAB coaggregated reversibly with yeast in response to changes in pH.
Table 1. Coaggregation activity of LAB and M. guilliermondii in LA solution or sterile water at room temperature.
Effect of coaggregative yeast and their metabolites on LAB viability
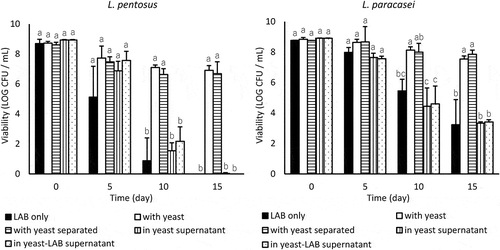
L. pentosus and L. paracasei died within 15 days, and the coincubation with M. guilliermondii significantly enhanced the viability of L. pentosus and L. paracasei after 10 days (p < 0.05) (). At day 15, L. pentosus and L. paracasei with yeast maintained log 6.9 colony-forming units (CFU)/mL and log 7.5 CFU/mL, respectively. Even when the yeast was separated by a cellulose membrane, the viability of L. pentosus and L. paracasei increased and no significant differences in the LAB viability owing to physical contact with yeast cells were observed. The viability of M. guilliermondii was stable at log 7–8 CFU/mL throughout the experiment (data not shown). L. pentosus and L. paracasei died in yeast supernatant and yeast-LAB supernatant (). Metabolites of M. guilliermondii exerted almost no influence on the viability of L. pentosus and L. paracasei.
Figure 1. Effect of coincubating M. guilliermondii and their metabolites on the viability of L. pentosus and L. paracasei in LA buffer (pH 3.0) at 10°C. Values are expressed as the means of triplicate experiments (n = 3), with error bars representing the SD. Different letters indicate significant differences (p < 0.05, by Scheffe’s test) within the same day.
Quantitative analysis of LA concentration
shows the effect of M. guilliermondii on the LA concentration in the supernatant of LA buffer in which LAB were suspended. No notable change in the LA concentration was observed in any of the samples. The maximum change in the LA concentration was a decrease of 5 mM in the sample of L. pentosus with yeast on days 10 and 15. The pHs of all samples were stable at pH 3.0–3.1 throughout the experiment (data not shown).
Table 2. The effect of M. guilliermondii on the LA concentration in the supernatant of LA buffer in which LAB cells were incubated at 10°C.
Effect of non-coaggregative yeasts on LAB viability
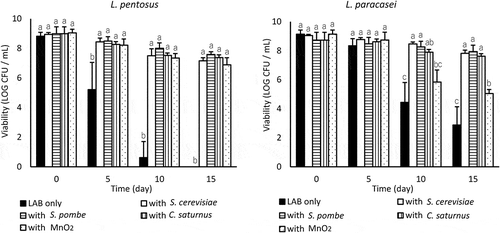
Every non-coaggregative yeast strain (S. cerevisiae, S. pombe, and C. saturnus) significantly enhanced the viability of L. pentosus and L. paracasei (p < 0.05) (). The viability of S. pombe and C. saturnus was stable at log 7–8 CFU/mL throughout the experiment. S. cerevisiae gradually died at approximately log 5 CFU/mL on day 15 (data not shown).
Figure 2. Effect of coincubating non-coaggregative yeast and MnO2 on the viability of L. pentosus and L. paracasei in LA buffer (pH 3.0) at 10°C. Values are expressed as means of triplicate experiments (n = 3), with error bars representing the SD. Different letters indicate significant differences (p < 0.05, by Scheffe’s test) within the same day.
Quantitative analysis of H2O2 and its effect on LAB viability
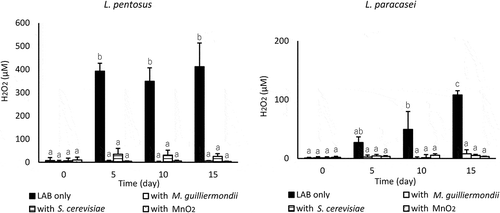
L. pentosus incubated alone produced a large amount of H2O2 under acidic conditions; H2O2 was stable at 349–411 µM from day 5–15, and coincubation with M. guilliermondii or S. cerevisiae and the addition of MnO2 significantly reduced H2O2 from day 5–15 (p < 0.001) (). L. paracasei incubated alone also produced H2O2, and the H2O2 concentration gradually increased to approximately 100 µM. When coincubated with yeast, the H2O2 concentration remained at a remarkably low level throughout the experiment, and the addition of MnO2 also reduced the levels of H2O2. The viability of L. pentosus was significantly enhanced by the addition of MnO2 (p < 0.05), which was similar to the effect of yeast coincubation (). Moreover, the addition of MnO2 enhanced the viability of L. paracasei; however, MnO2 was insufficiently potentiated compared with the results obtained with yeasts.
Figure 3. Effect of coincubating yeast or MnO2 on the extracellular H2O2 concentration in LA buffer in which LAB cells were incubated at 10°C. Values are expressed as means of triplicate experiments (n = 3), with error bars representing the SD. Different letters indicate significant differences (p < 0.05, by Scheffe’s test) throughout the 15-day experiment.
Effect of antioxidants on L. paracasei viability
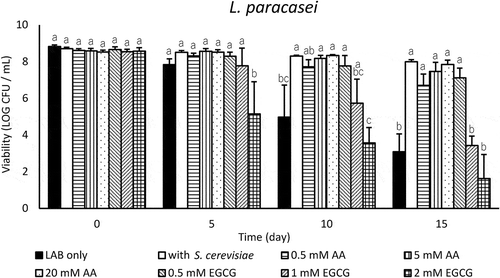
The addition of 0.5–20 mM AA significantly enhanced the viability of L. paracasei (p < 0.05), and higher concentrations tended to be more effective in enhancing ability (). The effects of AA were comparable to that of yeast coincubation. EGCG increased L. paracasei viability when added at 0.5 mM, but accelerated the death of L. paracasei when added at more than 1 mM.
Figure 4. Effect of antioxidants on the viability of L. paracasei in LA buffer (pH 3.0) at 10°C. Values are expressed as means of triplicate experiments (n = 3), with error bars representing the SD. Different letters indicate significant differences (p < 0.05, by Scheffe’s test) within the same day.
Discussion
In the current study, we used an LA buffer system to assure coaggregation and viability-enhancing effects and to obtain reproducible and clear data by minimizing factors involved in the interactions. For the LAB and yeast strains, we selected two LAB strains (L. pentosus and L. paracasei) and one yeast strain (M. guilliermondii) because their combinations have both high coaggregation and high viability-enhancing activities in our experimental system. We did not stick to the combination of strains from the same pickle but aimed to glean more general information from the basic microbiological point of view. The survival of LAB stored at 10°C was examined in our experiment. Several fermented foods are stored under cool conditions following the fermentation acidification process. Our condition setting was assumed to be close to the storage conditions below ambient temperature, although the temperature effect of the yeast viability-enhancing effect on LAB should be examined in the next step of our investigations.
With respect to the significance of adherence of LAB to yeast, Katakura et al. [Citation8] suggested that yeast decreases stress-inducing compounds such as LA or H2O2 by assimilation or degradation, which relieves the acid stress or oxidative stress. The coaggregation of LAB and yeast might be a survival strategy to obtain a mutual advantage, and we speculated that the coaggregation might contribute to cell-to-cell interaction and strengthen the tolerance of LAB to acid stress. Contrary to our hypothesis, yeast coincubation without physical contact enhanced the LAB viability to the same extent as that of coaggregation with physical contact (). The coincubation with the non-coaggregative yeasts S. cerevisiae, S. pombe, and C. saturnus also enhanced the viability of L. pentosus and L. paracasei (). Thus, in our study, the increase in the LAB viability by yeast coincubation was not owing to their coaggregation ability. The viability of yeasts used in this study was stable at log 7–8 CFU/mL throughout the 15-day experiment, except for S. cerevisiae. Although S. cerevisiae died to approximately 1% of the initial CFU on day 15, all four yeasts enhanced the viability of L. pentosus and L. paracasei to the same level regardless of the yeast species and viable cell count. Further investigations are necessary to determine the association between the strain of yeast or the number of viable yeast cells in the coincubating medium and the degree of improvement in LAB viability.
The viability of L. pentosus and L. paracasei was unaffected by M. guilliermondii supernatant, which contained yeast metabolites, and yeast-LAB supernatant, which contained yeast metabolites when yeast coaggregated with LAB (). This indicates that a supply of yeast metabolites is not the main factor required for an improvement in the viability of L. pentosus and L. paracasei. Conversely, Suharja et al. [Citation15] and Lim et al. [Citation16] noted that yeast metabolites that could protect the probiotics in the acidic environment might be present in the yeast supernatant. They reported that the cell-free supernatant of a S. cerevisiae culture enhanced the survival of L. rhamnosus in fermented milk [Citation15] and in McIlvaine’s buffer (pH 3.0) [Citation16]. The yeast supernatants used in these reports [Citation15,Citation16] were obtained from a nutrient-rich environment, and the addition of YM broth components [Citation15] or yeast derivatives [Citation20] has been revealed to enhance the survival of L. rhamnosus. The supernatants in our experiment were from an acidic and starved environment, and even if yeast metabolites are involved in improving LAB viability, the limited supply might have restricted the effects.
In fermented foods, LAB often coexist with yeast which can assimilate LA to reduce the acid concentration and relieve the acid stress [Citation8,Citation9]. We hypothesized that M. guilliermondii assimilated LA, and the resultant decrease in LA improved LAB viability. We found a maximum decrease in LA of only 5 mM in the coaggregated samples, i.e. a reduction of <10% of the original content (). To investigate whether a 10% reduction of LA content could affect the LAB viability, a LAB viability assay was performed in 50 mM LA buffer (pH 3.0), and the result was similar to that in 55 mM LA buffer (data not shown). This suggests that the reduction of LA content caused by M. guilliermondii coincubation did not significantly improve the viability of L. pentosus and L. paracasei.
Toh et al. [Citation19] showed that the amount of extracellular H2O2 in fermented milk monocultured with Bifidobacterium lactis (approximately 16 µM) was reduced to 23–60% by coculturing with yeasts, and yeast coculture stimulated the growth of bifidobacteria after 12 h of fermentation. They proposed that the reduction in the reactive oxygen species (ROS) in the medium that was caused by yeast contributed to growth-stimulating activities of bifidobacteria; however, the presence of yeasts did not affect the survival of the bifidobacteria within the storage period of 8 weeks at 10°C. In our experiment, the extracellular accumulation of H2O2 in LA buffer incubated with L. pentosus and L. paracasei was approximately 400 µM and 100 µM, respectively (). In the presence of M. guilliermondii or S. cerevisiae, H2O2 decreased to 1–6% for L. pentosus and 5–7% for L. paracasei on day 15. The addition of MnO2 for H2O2 elimination under pH 3.0 decomposed the extracellular H2O2, and the H2O2 concentration was extremely low throughout the 15-day incubation of L. pentosus and L. paracasei (). L. pentosus viability was significantly improved by MnO2 similar to that observed when coincubated with yeast, but MnO2 exerted a smaller effect in improving L. paracasei viability than yeast (). When L. pentosus were incubated in water solution containing 400 µM H2O2 at 10°C, they died similar to the LAB only shown in (data not shown). These results for L. pentosus indicate that H2O2 was decomposed by the activity of yeast catalase, resulting in the notable alleviation of stress that induces L. pentosus to enhance their viability. We might provide evidence supporting the relation between yeast H2O2 decomposing ability and the viability of L. pentosus by using catalase deficient mutants of yeast. In contrast, H2O2 elimination alone did not sufficiently improve L. paracasei viability, but the addition of antioxidants, AA and EGCG, was effective. AA (0.5–20 mM) and EGCG (0.5 mM) significantly enhanced L. paracasei viability (p < 0.05) (). These results suggest that removal of ROS not only H2O2 induced the remarkable improvement of L. paracasei survival. Effectiveness of AA as a ROS scavenger for the survival of Lactobacillus has been reported by Dave et al. [Citation21], and our results coincide with them. Additionally, 0.5 mM EGCG increased L. paracasei viability, and more than 1 mM EGCG accelerate the death of them (). In several reports [Citation22–Citation24], green tea extracts rich in EGCG improved the growth of LAB, but EGCG inhibited their growth depending on its concentration [Citation23]. EGCG appears to adversely affect the LAB viability.
LAB are catalase-negative, and many strains of LAB which can decompose ROS have been identified in earlier studies [Citation25,Citation26]; for example, NADH peroxidase in Streptococcus [Citation27,Citation28] and Lactobacillus [Citation29,Citation30] and manganese catalase in Lactobacillus [Citation31]. Furthermore, L. acidophilus, L. bulgaricus, and S. thermophilus have demonstrated antioxidative activity, including the scavenging of ROS [Citation32]. The exopolysaccharide from L. plantarum has been identified to possess antioxidant activities [Citation33]. The antioxidant activity for ROS varies according to the bacterial species [Citation26,Citation34], and the benefits that LAB receive from yeast coexistence may depend on these factors.
To our knowledge, this is the first report that shows the clear association between enhanced LAB viability and the yeast coincubation providing antioxidant activity. Although the results obtained in this study did not resolve the LAB viability-enhancing mechanisms by some yeasts that occur in certain fermented foods, we believe that information obtained here regarding the interaction between LAB and yeast will be beneficial in expanding the availability of LAB and the stable production and distribution of fermented foods containing live LAB. Further investigation into the other combinations of LAB and yeast, verification of our results in specific fermented foods, and the application of findings for better utilization of LAB are necessary.
In conclusion, coincubation with yeast significantly improved the viability of L. pentosus and L. paracasei under acidic conditions regardless of their coaggregation ability, physical contact of cells, yeast metabolites, and LA assimilation. Yeast coincubation decreased the H2O2 and possibly other ROS accumulation in the LAB incubation, suggesting that the yeast antioxidant activity might be the causes of the improvement of LAB viability with yeast coincubation.
Author contribution
Toshiyuki Kawasumi conceived and designed the experiments. Satomi Hirai performed the experiments and statistical analysis. Toshiyuki Kawasumi and Satomi Hirai wrote and edited the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors would like to thank Enago (www.enago.jp) for the English language review.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Settanni L, Corsetti A. Application of bacteriocins in vegetable food biopreservation. Int J Food Microbiol. 2008;121(2):123–138.
- Stiles ME. Biopreservation by lactic acid bacteria. Antonie Van Leeuwenhoek. 1996;70(2–4):331–345.
- Hayek SA, Ibrahim SA. Current limitations and challenges with lactic acid bacteria: a review. Food Nutr Sci. 2013;4(11):73–87.
- Viljoen BC. Yeast ecological interactions. Yeast-yeast, yeast-bacteria, yeast-fungi interactions and yeast as biocontrol agents. In: Querol A, Fleet GH, editors. Yeasts in food and beverages. Berlin: Springer-Verlag; 2006. p. 83–110.
- Viljoen BC. The interaction between yeasts and bacteria in dairy environments. Int J Food Microbiol. 2001;69(1–2):37–44.
- Peng X, Sun J, Iserentant D, et al. Flocculation and coflocculation of bacteria by yeasts. Appl Microbiol Biotechnol. 2001;55(6):777–781.
- Momose H, Iwano K, Tonoike R. Studies on the aggregation of yeast caused by lactobacilli IV. Force responsible for aggregation. J Gen Appl Microbiol. 1969;15(1):19–26.
- Katakura Y, Sano R, Hashimoto T, et al. Lactic acid bacteria display on the cell surface cytosolic proteins that recognize yeast mannan. Appl Microbiol Biotechnol. 2010;86(1):319–326.
- Yamasaki-Yashiki S, Sawada H, Kino-Oka M, et al. Analysis of gene expression profiles of Lactobacillus paracasei induced by direct contact with Saccharomyces cerevisiae through recognition of yeast mannan. Biosci Microbiota Food Health. 2017;36(1):17–25.
- Furukawa S, Nojima N, Yoshida K, et al. The importance of inter-species cell-cell co-aggregation between Lactobacillus plantarum ML11-11 and Saccharomyces cerevisiae BY4741 in mixed-species biofilm formation. Biosci Biotechnol Biochem. 2011;75(8):1430–1434.
- Hirayama S, Furukawa S, Ogihara H, et al. Yeast mannan structure necessary for co-aggregation with Lactobacillus plantarum ML11-11. Biochem Biophys Res Commun. 2012;419(4):652–655.
- Abe A, Furukawa S, Watanabe S, et al. Yeasts and lactic acid bacteria mixed-specie biofilm formation is a promising cell immobilization technology for ethanol fermentation. Appl Biochem Biotechnol. 2013;171(1):72–79.
- Liu SQ, Tsao M. Enhancement of survival of probiotic and non-probiotic lactic acid bacteria by yeasts in fermented milk under non-refrigerated conditions. Int J Food Microbiol. 2009;135(1):34–38.
- Liu SQ, Tsao M. Enhancing stability of lactic acid bacteria and probiotics by Wiliopsis saturnus var. saturnus in fermented milks. Nutr Food Sci. 2010;40(3):314–322.
- Suharja AAS, Henriksson A, Liu SQ. Impact of Saccharomyces cerevisiae on viability of probiotic Lactobacillus rhamnosus in fermented milk under ambient conditions. J Food Process Preserv. 2014;38(1):326–337.
- Lim PL, Toh M, Liu SQ. Saccharomyces cerevisiae EC-1118 enhances the survivability of probiotic Lactobacillus rhamnosus HN001 in an acidic environment. Appl Microbiol Biotechnol. 2015;99(16):6803–6811.
- Yeo AYY, Toh MZ, Liu SQ. Enhancement of bifidobacteria survival by Williopsis saturnus var. saturnus in milk. Benef Microbes. 2015;7(1):135–144.
- Li X, Liu YH, Zhang X, et al. Evaluation of biogas production performance and dynamics of the microbial community in different straws. J Microbiol Biotechnol. 2017;27(3):524–534.
- Toh M, Liu SQ. Impact of coculturing Bifidobacterium animalis subsp. lactis HN019 with yeasts on microbial viability and metabolite formation. J Appl Microbiol. 2017;123(4):956–968.
- Toh M, Liu SQ. Influence of commercial inactivated yeast derivatives on the survival of probiotic bacterium Lactobacillus rhamnosus HN001 in an acidic environment. AMB Express. 2017;7(1):156.
- Dave RI, Shah NP. Effectiveness of ascorbic acid as an oxygen scavenger in improving viability of probiotic bacteria in yoghurts made with commercial starter cultures. Int Dairy J. 1997;7(6–7):435–443.
- Gaudreau H, Champagne CP, Remondetto GE, et al. Effect of catechins on the growth of oxygen-sensitive probiotic bacteria. Food Res Int. 2013;53(2):751–757.
- Theobald S, Pfeiffer P, Zuber U, et al. Influence of epigallocatechin gallate and phenolic compounds from green tea on the growth of Oenococcus oeni. J Appl Microbiol. 2008;104:566–572.
- Muniandy P, Shori AB, Baba AS. Influence of green, white and black tea addition on the antioxidant activity of probiotic yogurt during refrigerated storage. Food Packag Shelf Life. 2016;8:1–8.
- Condon S. Responses of lactic acid bacteria to oxygen. FEMS Microbiol Rev. 1987;46(3):269–280.
- Whittenbury R. Hydrogen peroxide formation and catalase activity in the lactic acid bacteria. J Gen Microbiol. 1964;35(1):13–26.
- Dolin MI. The DPNH-oxidizing enzymes of Streptococcus faecalis. II. The enzymes utilizing oxygen, cytochrome c, peroxide and 2,6-dichlorophenol-indophenol or ferricyanide as oxidants. Arch Biochem Biophys. 1955;55(2):415–435.
- Poole LB, Claiborne AI. Interactions of pyridine nucleotides with redox forms of the flavin-containing NADH peroxidase from Streptococcus faecalis. J Biol Chem. 1986;261(31):14525–14533.
- Strittmatter CF. Flavin-linked oxidative enzymes of Lactobacillus casei. J Biol Chem. 1959;234(10):2794–2800.
- Gotz F, Elstner EF, Sedewitz B, et al. Oxygen utilization by Lactobacillus plantarum. Ⅱ. Superoxide and superoxide dismutation. Arch Microbiol. 1980;125(3):215–220.
- Kono Y, Fridovich I. Isolation and characterization of the pseudocatalase of Lactobacillus plantarum. J Biol Chem. 1983;258(10):6015–6019.
- Lin MY, Yen CL. Antioxidative ability of lactic acid bacteria. J Agric Food Chem. 1999;47(4):1460–1466.
- Li S, Huang R, Shah NP, et al. Antioxidant and antibacterial activities of exopolysaccharides from Bifidobacterium bifidum WBIN03 and Lactobacillus plantarum R315. J Dairy Sci. 2014;97(12):7334–7343.
- Ito A, Sato Y, Kudo S, et al. The screening of hydrogen peroxide-producing lactic acid bacteria and their application to inactivating psychrotrophic food-borne pathogens. Curr Microbiol. 2003;47(3):231–236.
