ABSTRACT
Amazake is a traditional Japanese health drink. Here, we examined the effects of amazake on skin in cells and humans. Treatment with sake cake or rice koji suppressed intracellular lipid accumulation in differentiated hamster sebocytes, likely through the reduced expression of peroxisome proliferator-activated receptor-gamma (PPARγ) mRNA. In double-blind, placebo-controlled trial, seventeen Japanese women ingested either amazake or placebo for 4 weeks. Ingestion of the amazake decreased the sebum content compared to the placebo. The questionnaires showed improvements in “face color,” “dark circles under the eyes,” “glossy hair,” and “waking up well”, only in the amazake. In accordance with the questionnaires, additional analysis revealed the change in the L* values under the eyes was statistically increased in the amazake compared to the placebo. These results indicate that amazake may decrease sebum content in cells and humans and increase the L* values under the eyes, with some additional beneficial effects in humans.
GRAPHICAL ABSTRACT
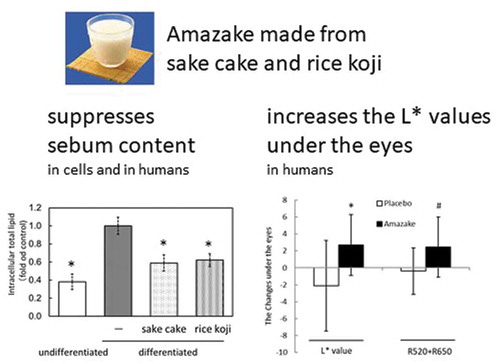
Amazake made from sake cake and rice koji suppresses sebum content and increases the L* values under the eyes.
Amazake is a traditional, Japanese beverage that originates from “Amanotamuzake,” which has been mentioned in Nihonshoki of 720. There are several methods for producing amazake. Rice koji (rice malt) amazake is made from glycosylated starch, yielded through the action of amylase present in rice malt. Sake cake amazake is made using sake cake, which contain fermented yeast, various nutrients, and carbohydrate. Amazake can be made using both rice koji and sake cake.
Generally, amazake contains healthy ingredients, such as glucoses, amino acids, and vitamin B. It also contains many bioactive substances, including the fermentation products of rice malt fungi and yeasts obtained from rice malt and sake cake. Amazake is sometimes called a “drinkable drip” for its high nutritional value. In Japan, amazake is thought to prevent summer weariness and winter colds [Citation1]. Using a silkworm muscle contraction assay, we previously reported that amazake has an innate immune-stimulating activity [Citation2]. Amazake is also reported to have antihypertensive effects [Citation3], cholesterol lowering effects [Citation4], and tyrosinase-inhibiting activity [Citation5]. Furthermore, nutritious amazake ingestion may have positive effects on the skin, yet there are only a few scientific reports on its effect on human skin.
In this study, we aimed to investigate the effects of amazake consumption on lipid metabolism, and the effects on human skin and physical conditions. First, we examined the lipid metabolizing effects of amazake on sebaceous glands using hamster sebocytes. Next, as sebaceous glands are present in the dermis and supplied by blood capillaries [Citation6], we investigated the effects of amazake consumption on human skin, in a placebo-controlled, double-blind trial using specialized equipment. In addition, changes in the physical condition caused by amazake consumption were evaluated using a visual analog scale (VAS) questionnaire and through analysis of the intestinal flora.
Materials and methods
Cell culture and treatment
Hamster sebocytes were obtained from Kurabo Industries Ltd. (Osaka, Japan). The cells were grown in Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F12 medium (1:1) (Thermo Fisher Scientific K.K.), containing 8% fetal bovine serum (FBS), 2% human serum (HS), and 10 ng/mL epidermal growth factor (EGF), in a humidified incubator at 37°C with 5% CO2. After seeding the cells, the cells were cultured in DMEM/Ham’s F12 medium (1:1) containing 8% FBS, 2% HS and 10 μg/mL insulin for 9 days, to promote differentiation into adipocytes. The freeze-dried sake cake and rice koji, obtained from Morinaga & Co., Ltd (Japan), were dissolved in culture medium and filtered through a pore size of 0.22 µm, and added to the cells 1 h prior to sampling (final concentration is 20 µg/mL each).
Cell measurements
Cytoplasmic lipid droplets were stained with Oil Red O. Cultured sebocytes were stained with 0.3% (wt/vol) Oil Red O in isopropanol:distilled H2O (3:2, vol:vol) at 37°C for 15 min; the degree of lipid droplet staining was evaluated spectrometrically at 520 nm. Peroxisome proliferator-activated receptor-alpha (PPARα) and PPARγ mRNA expression were measured using real-time PCR. Total RNA was extracted from cells using an RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Expression of PPARα and PPARγ mRNA was measured using real-time PCR (Applied Biosystems 7900HT, Thermo Fisher Scientific Inc.) with a One Step SYBRR PrimeScript RT-PCR Kit II (TaKaRa Bio Inc., Shiga, Japan). PPARα and PPARγ mRNA were quantified. GAPDH was selected as the housekeeping gene. Primers were purchased from Qiagen GmbH. Experiments were performed in triplicate (n = 3). For analysis, the target threshold cycle (Ct) was determined using the difference in Ct values between the housekeeping gene and genes of interest (ΔCt: [Target gene Ct] – [Housekeeping gene Ct]). mRNA expression was compared between the genes of interest by calculating differences in ΔCt (ΔΔCt).
Clinical study design and subjects
This study was a randomized, placebo-controlled, double-blind trial on healthy Japanese women, aged 40–69 years, carried out in 2014. The study was approved by the ethics committee of the Hibiya Skin Clinic and conducted in accordance with the Declaration of Helsinki. Subjects provided written informed consent before the start of the study. This study was registered in the University Hospital Medical Information Network Clinical Trial Registry (Japan, registration no. UMIN000036579) in 2019. Details are available at https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000041670.
The exclusion criteria were as follows: 1) Subjects who have food allergies; 2) Subjects who are pregnant or lactating, or planned to become pregnant in the near future; 3) Subjects who have previously taken supplements and felt unwell; 4) Subjects who are under medical treatment for illness or injury (except subjects who are under stable hypertension treatment); 5) Subjects who have taken supplements in the past 1 month; 6) Subjects who have an abnormally weak tolerance for alcohol; 7) Subjects who cannot cooperate with examination and intake test food twice a day.
Twenty healthy women, who complained of rough skin and constipation, were recruited to participate, and three women were excluded because of not meeting the criteria (one subject in the placebo group had a poor physical condition not related to the examination food, and one subject in the placebo group and one in the amazake group had low ingestion rates). Subject background information is shown in .
Table 1. Subject background information.
Freeze-dried test food
Freeze-dried amazake-like placebo and commercial amazake drink were obtained from Morinaga & Co., Ltd (Japan). The amazake-like placebo was made indistinguishable from amazake using flavorants. The nutritional composition and alcohol content of both products are shown in . The detailed composition of the placebo is shown in Supplemental Table 1. Amazake contained sake cake, rice koji, sugar, dietary fiber, sodium chloride, and sweetener. The placebo primarily contained sugar, starch syrup, starch, fat, sodium chloride, and sweetener. The study subjects, randomly divided into the amazake group and the placebo group, consumed freeze-dried test foods dissolved in hot water (100 mL) twice a day for 4 weeks.
Table 2. Composition of the freeze-dried amazake-like placebo and amazake.
Questionnaires
At 0 and 4 weeks, the subjects answered the VAS questionnaires. In the VAS questionnaire, each condition was rated on a single-item 100 mm VAS, anchored at each end by the descriptions “very attractive” (0 mm) and “very unattractive” (100 mm). The distance of the point from the left-hand side (0 mm) was measured before and after treatment. The subjects rated 20 questionnaire items: skin wrinkles, skin fitness, dry skin, oily skin, skin roughness, makeup, porous skin, stretching after cleansing, acne, stains and freckles, dull skin, face color, dark circles under the eyes, glossy hair, glossy nails, constipation, perspiration, waking up well, sleeping well, and fatigue.
Analysis of intestinal flora
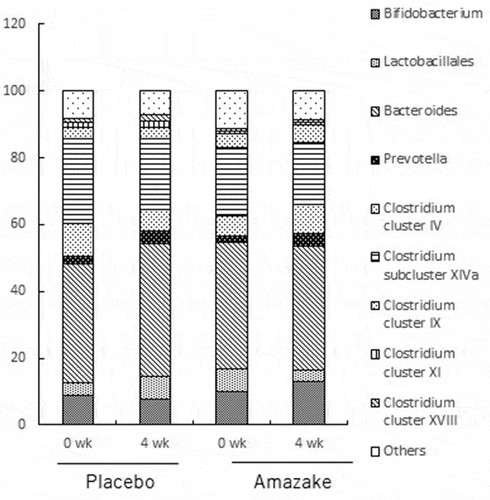
All subjects provided fecal samples during the week before the start of treatment (week 0) and at week 4 of treatment, which were tested using a feces sampling kit (TechnoSuruga Laboratory Co. Ltd., Shizuoka, Japan). The samples were analyzed with terminal restriction fragment length polymorphism, performed by Techno Suruga Laboratory Co., Ltd. (Shizuoka, Japan).
Skin measurements
The skin of the subjects was evaluated at 0 and 4 weeks; the cheeks were the site of measurement. After washing and rinsing the face, the subjects were acclimatized for 15 min in a temperature- and humidity-controlled room (temperature 22 ± 2°C, humidity 40 ± 10%) before starting the measurements. Sebum content was evaluated using a Sebumeter (Courage+Khazaka Electronic GmbH, Cologne, Germany). The measurement was conducted five times, and the mean value was taken to be the sebum content at the site. Transepidermal water loss was measured using a Tewameter TM300® (Courage+Khazaka Electronic GmbH, Cologne, Germany). The measurement was carried out for 90 s at least twice, and the mean value was used. Skin viscoelasticity was measured with a Cutometer MPA850® (Courage+Khazaka Electronic GmbH, Cologne, Germany). As indices of elasticity, R2 was calculated as Ua/Uf and R7 as Ur/Uf. Uf (final distension) is the maximum height of the skin drawn into the probe by negative pressure, Ur (immediate retraction) is the height retracted from maximum distension at 0.1 s after releasing the negative pressure, and Ua (final retraction) is the height retracted from maximum distension after releasing the negative pressure. Uf (R0) represents the plasticity. Ua/Uf (R2) is the ratio of total retraction to total distension, and Ur/Uf (R7) is the ratio of immediate retraction to total distension. R2 and R7 represent gross elasticity. The measurement was performed at least three times, and the mean value was used. Skin color was measured using a Spectrophotometer NF344 (Nippon denshoku Industries Co., Ltd., Tokyo, Japan) and Chroma Meter CR-200 (Konica Minolta Inc., Tokyo, Japan). Skin color of the area under the eye was also measured. The former skin color parameters were R520 + R650, which represents oxyhemoglobin and melanin levels, a measure of skin brightness. The measurement was carried out at least five times, and the mean value was used. The latter skin color parameters were L*, a*, and b*, which is defined by Commission Internationale d’Eclairage (CIE) in 1976. This measurement was carried out once. Skin temperatures were measured by thermography using a Thermo Shot F30 (Nippon Avionics Co., Ltd. (Tokyo, Japan). The temperatures of the right and left cheeks were averaged.
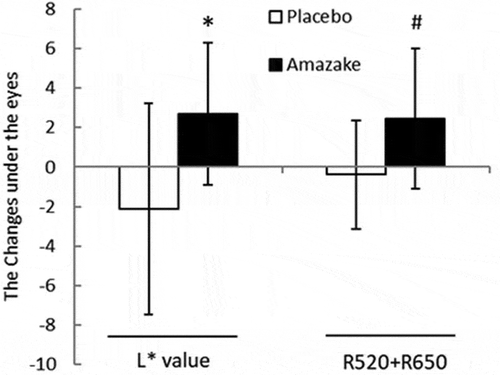
After disclosing the groups, we found the “dark circles under the eyes” of the VAS questionnaire were changed only in the amazake group, leading to additional analyzes; the L* value and R520+ R650 under the eye area were analyzed by calculating the amount of change in those four weeks.
Statistical analysis
The results were expressed as mean ± standard deviation for each group. Statistical analysis was carried out using IBM SPSS Statistics 26 software (SPSS IBM Japan Inc., Tokyo, Japan). For the in vitro study, the student’s unpaired t-test was used to compare the two groups (undifferentiated and differentiated control, control and sake cake or rice koji). For the clinical study, the student’s unpaired t-test was used to compare the two groups (the placebo group and the amazake group), and the student’s paired t-test was used to compare between 0 and 4 weeks of each group. Changes in parameters from week 0 were calculated and compared between groups using the student’s unpaired t-test. Changes in responses to the VAS questionnaire from week 0 were calculated and compared between groups using the Mann-Whitney U test. P < 0.05 was considered to indicate a statistically significant change.
Results
Lipid metabolism assay using differentiated hamster sebocytes
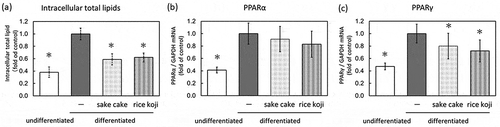
The effects of sake cake or rice koji (20 µg/mL each) on lipid metabolism were investigated using differentiated hamster sebocytes, as shown in . The cytoplasmic lipid droplets of the sebocytes were increased 2.6-fold in the differentiated cells compared to the undifferentiated cells. The cytoplasmic lipid droplets of the differentiated cells were reduced 0.59- and 0.62-fold by treatment with sake cake or rice koji, respectively. To explore the mechanism of lipid suppression, the expressions of PPARα and PPARγ, which are the transcription regulators of lipid metabolism, were examined. While PPARα expression was not affected by sake cake or rice koji treatment, PPARγ was suppressed by both treatments.
Figure 1. Lipid metabolism assay using hamster sebocytes. Quantification of intracellular total lipids (a), PPARα mRNA (b), and PPARγ mRNA (c). Data are shown as mean ± standard deviation. Intracellular total lipids of undifferentiated or differentiated hamster sebocytes without treatment or treated with sake cake or rice koji were measured using Oil Red O. Relative gene expression of PPARα and PPARγ of undifferentiated or differentiated hamster sebocytes without treatment or treated with sake cake or rice koji were investigated using qRT-PCR. *; P < 0.05, significant difference compared to control differentiated cells (untreated).
Skin measurements
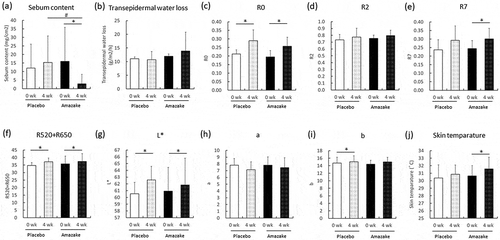
To explore the lipid suppression effect of sake cake and rice koji in humans, freeze-dried amazake containing sake cake and rice koji (the amazake group) and freeze-dried amazake-like placebo (the placebo group) were consumed twice a day for 4 weeks. Sebum content and other parameters are summarized in . Significant decreases in sebum content were seen in the amazake group compared to the placebo group after 4 weeks of consumption. R7 and skin temperature in the amazake group was also significantly increased at 4 weeks compared to 0 weeks. The R520+ R650 and L* values, which shows skin brightness, were statistically increased in both the placebo and amazake group at 4 weeks compared to 0 weeks.
Figure 2. Skin measurements of the placebo (Placebo) and amazake groups (Amazake). Sebum content (a), skin barrier: transepidermal water loss (b), skin viscoelasticity: R0 (c), R2 (d), R7 (e), skin color: R520+ R650 (f), L* (g), a (h), b (i), and skin temperature (j). Data are shown as mean ± standard deviation. Skin conditions of subjects who ingested placebo or amazake were measured using specialized equipment at 0 and 4 weeks. *; intra-group P < 0.05, significant difference between 0 and 4 weeks. #; inter-group P < 0.05, significant difference between Placebo and Amazake.
Questionnaires
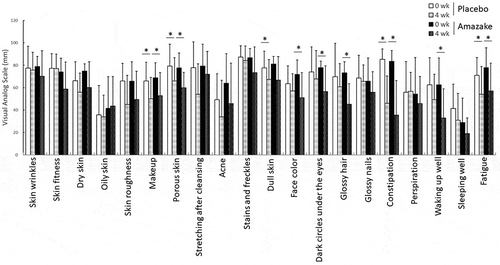
The results from the VAS questionnaire are summarized in . Only “glossy hair” changes (placebo – 0.91 ± 2.59, amazake −2.82 ± 2.11; P = 0.036, using the Mann-Whitney U test) was significantly improved between the placebo group and the amazake group. Statistically difference in “dark circles under the eyes”, “glossy hair”, and “waking up well” were only seen in the amazake group at 4 weeks compared to 0 weeks.
Figure 3. Differences in visual analog scale indices for specified conditions. Data are shown as mean ± standard deviation. Questionnaires were undertaken by subjects who ingested placebo (Placebo) or amazake (Amazake) at 0 and 4 weeks. *; Intra-group P < 0.05, significant difference between 0 and 4 weeks.
Fecal analysis
No significant difference in the intestinal flora was found in both the placebo and amazake groups as shown in .
Additional skin analysis
The questionnaires revealed that “dark circles under the eyes” was changed only in the amazake group. Then we performed an additional analysis of the acquired data, and treated this result as a single blind trial. The increase in the L* value and R520 + R650 under the eyes are shown in . Increases in L* values under the eyes are only observed in the amazake group compared to the placebo group.
Discussion
In this study, we demonstrated that amazake, containing sake cake and rice koji, suppressed sebum production in cells and in humans. Excess sebum triggers acne and causes skin disorders [Citation7]; continuous ingestion of amazake may suppress such skin problems. Moreover, amazake ingestion improved the glossiness of hair and increased L* values under the eyes. These results suggest that continuous ingestion of amazake could be a safe, skin-enhancing supplement. Meanwhile, amazake ingestion twice a day did not affect body weight (.) and no notable adverse events were observed in this study after 4 weeks of amazake consumption twice a day.
The suppression of sebum is thought to be due to decreased expression of PPARγ. PPARs are a family of ligand-activated transcription factors and consist of three isoforms: PPARα, PPARβ/δ, and PPARγ [Citation8]. PPARs are very important in the regulation of lipid metabolism [Citation9], and PPARγ in particular plays an important role in lipid metabolism in adipocytes [Citation10,Citation11]. A previous study reported that rice koji directly activated PPARα and suppressed triglyceride (TG) accumulation in the liver [Citation12]. In this study, sake cake and rice koji increased PPARγ mRNA; this may be related to differences between organs. Linoleic acid, oleic acid, and 9-(E,E)-HODE are reported to be active components of rice koji [Citation12], however, the active components in this study remain unknown. In the human study, the lipid and protein content of each ingestion was slightly different, though this is not considered to affect the results, as dairy fat and protein intake is reportedly not related to sebum content [Citation13].
The questionnaire was performed double-blind; some questions were statistically improved only in the amazake group. There was an improvement in “dark circles under the eyes” in the amazake group at 4 weeks compared to 0 weeks, and the changes in L* values under the eyes was also statistically decreased in the amazake group compared to the placebo group. This could be due to skin regeneration processes being promoted by the increase in skin temperature [Citation14]; skin temperature was significantly increased only in the amazake group at 4 weeks compared to 0 weeks, with no difference observed between the placebo and amazake groups. The small number of subjects would have limited the study.
Amazake contains abundant nutrients, including amino acids. Actually protein content of amazake is higher than that of placebo because sake cake and rice koji contains high protein. There is a report that sake cake ingestion increases branched-chain amino acids in the plasma [Citation15]. Furthermore, arginine and adenosine, which are contained in amazake, are reported to have a vasodilatation effect [Citation16,Citation17]. These nutrients may affect skin temperature through blood flow. Here, the amazake used contained less alcohol, so the observed effects were likely not caused by alcohol. Regarding the glossiness of hair, hair is reported to reflect amino acid ingestion [Citation18]. The consumption of rich nutrients including amino acids may be related to hair gloss. The question of “waking up well” was also improved at 4 weeks compared to 0 weeks in the amazake group, with no difference observed between the placebo and amazake groups. Adenosine and sake yeast are reported to improve the quality of sleep [Citation19]. These nutrients may improve physical conditions with a real feeling.
The microflora, which remained unchanged in this study, are known to have large individual differences [Citation20]. The microflora changes by sake cake and rice koji ingestion is reported in mice [Citation21]. A larger study cohort is required to better explore the effects of amazake on microflora.
In this study, we have clarified the beauty and wellness effects of amazake, which contains sake cake and rice koji, which are known as traditional Japanese health foods. However, there are limitations, such as the small number of study participants. Further studies are required to better explore the wellness effects of amazake.
Author contributions
H.M-U., M.S. and K.M. designed the study. H.M-U., S.Y. and K.M performed the experiments. H.M-U and K.M. analyzed the data. H.M-U wrote the manuscript, and was supported by M.M. and K.M. All authors reviewed and approved this paper.
Supplemental_Table1_200079_YT.docx
Download MS Word (23.8 KB)Acknowledgments
We thank Toshihiko Kajino and Yasuhiko Katsumata from Morinaga & Co., Ltd for placebo manufacturing. We also thank Editage (www.editage.jp) for English language editing.
Disclosure statement
No potential conflict of interest were reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Koizumi T. Food and Japanese Wisdom. J Jpn Assoc Rural Med. 2013;61(6):839–854.
- Maruki-Uchida H, Sai M, Sekimizu K. Evaluation of the innate immune-stimulating activity of amazake using a silkworm muscle contraction assay. Drug Discov Ther. 2017;11(5):288–290.
- Saito Y, Wanezaki K, Kawato A, et al. Antihypertensive effects of peptide in sake and its by-products on spontaneously hypertensive rats. Biosci Biotechnol Biochem. 1994;58(5):812–816. .
- Hamajima H, Tanaka M, Miyagawa M, et al. Koji glycosylceramide commonly contained in Japanese traditional fermented foods alters cholesterol metabolism in obese mice. Biosci Biotechnol Biochem. 2019;83(8):1514–1522. .
- Jeon HJ, Noda M, Maruyama M, et al. Identification and kinetic study of tyrosinase inhibitors found in sake lees. J Agric Food Chem. 2006;54(26):9827–9833. .
- Ellis RA, Montagna W, Fanger H. Histology and cytochemistry of human skin. XIV. The blood supply of the cutaneous glands. J Invest Dermatol. 1958;30(3):137–145.
- Li X, He C, Chen Z, et al. A review of the role of sebum in the mechanism of acne pathogenesis. J Cosmet Dermatol. 2017;16(2):168–173. .
- Takeyama K, Kodera Y, Suzawa M, et al. Peroxisome proliferator-activated receptor(PPAR)–structure, function, tissue distribution, gene expression. Nihon Rinsho. 2000;58(2):357–363.
- Poulsen L, Siersbaek M, Mandrup S. PPARs: fatty acid sensors controlling metabolism. Semin Cell Dev Biol. 2012;23(6):631–639.
- Rosen ED, Sarraf P, Troy AE, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4(4):611–617. .
- Tamori Y, Masugi J, Nishino N, et al. Role of peroxisome proliferator-activated receptor-gamma in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes. 2002;51(7):2045–2055. .
- Takahashi H, Chi HY, Mohri S, et al. Rice koji extract enhances lipid metabolism through proliferator-activated receptor alpha (PPARalpha) activation in mouse liver. J Agric Food Chem. 2016;64(46):8848–8856. .
- Boelsma E, van de Vijver LP, Goldbohm RA, et al. Human skin condition and its associations with nutrient concentrations in serum and diet. Am J Clin Nutr. 2003;77(2):348–355. .
- Wilczynski S, Stolecka-Warzecha A, Deda A, et al. In vivo dynamic thermal imaging of skin radiofrequency treatment. J Cosmet Dermatol. 2018.
- Izu H, Shibata S, Fujii T, et al. Sake cake (sake-kasu) ingestion increases branched-chain amino acids in the plasma, muscles, and brains of senescence-accelerated mice prone 8. Biosci Biotechnol Biochem. 2019;83(8):1490–1497. .
- Costa F, Biaggioni I. Role of nitric oxide in adenosine-induced vasodilation in humans. Hypertension. 1998;31(5):1061–1064.
- Zhu Q, Yue X, Tian QY, et al. Effect of L-arginine supplementation on blood pressure in pregnant women: a meta-analysis of placebo-controlled trials. Hypertens Pregnancy. 2013;32(1):32–41. .
- Petzke KJ, Boeing H, Klaus S, et al. Carbon and nitrogen stable isotopic composition of hair protein and amino acids can be used as biomarkers for animal-derived dietary protein intake in humans. J Nutr. 2005;135(6):1515–1520. .
- Monoi N, Matsuno A, Nagamori Y, et al. Japanese sake yeast supplementation improves the quality of sleep: a double-blind randomised controlled clinical trial. J Sleep Res. 2016;25(1):116–123. .
- Vandeputte D, Kathagen G, D’Hoe K, et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature. 2017;551(7681):507–511. .
- Kawakami S, Ito R, Maruki-Uchida H, et al. Intake of a mixture of sake cake and rice malt increases mucin levels and changes in intestinal microbiota in mice. Nutrients. 2020;12(2):pii:E449. .