ABSTRACT
The volatile components emitted from two scale insects, Ceroplastes japonicus and Ceroplastes rubens, were identified using GC–MS analysis. The major volatile components of the solvent extract from C. japonicus were α-humulene (35.8%) and δ-cadinene (17.0%), while those of C. rubens were β-selinene (10.3%) and β-elemene (5.1%). In GC/olfactometry, linalool, butyric acid, 3-methylbutyric acid, 2-methylbutyric acid, and vanillin were identified as the odor-active components of the extract from C. japonicus, in addition to trace amounts of trans-4,5-epoxy-(2E)-decenal, 4-methyl-(3E)-hexenoic acid, and phenylacetic acid. With regard to C. rubens, trans-4,5-epoxy-(2E)-decenal, 3-methylbutyric acid, and phenylacetic acid were identified as the odor-active components. Besides, decan-1,4-olide (γ-decalactone) with milky cherry-like note and 3-hydroxy-4,5-dimethylfuran-2(5H)-one (sotolone) with brown sugar-like note were also detected as the characteristic cherry-like sweet-and-sour note of these two scale insects.
Abbreviations
GC: Gas chromatography; GC/O: gas chromatography/olfactometry
GRAPHICAL ABSTRACT
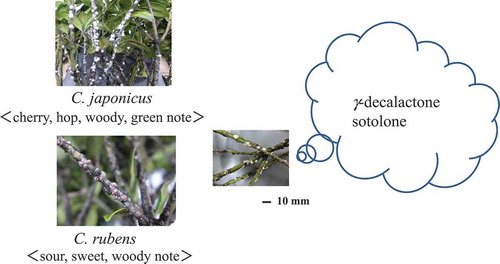
The characteristic smell emitted from two scale insects, Ceroplastes japonicus and Ceroplastes rubens
It is known that 220 species of scale insects inhabit in Japan. These small insects are generally covered with thick wax and epiphytically live on the twigs of deciduous and evergreen trees. Most of them damage Citrus, Camellia, Persimmon, Laurus, and Fatsia species. Ceroplastes japonicus and Ceroplastes rubens belong to the family Coccidae, which are widely distributed in Japan, Southeast Asia, Australia, and so on. They are known as an insect pest which infest various fruit trees and trees including Citrus families. The female body bulges round and is covered with wax-like or cotton-like secretion in about 3–5 mm of diameter. These scale insects attract of Planococcus kuraunhiae [Citation1].
When the former insect C. japonicus is crushed, cherry-like sweet, hop, woody, green, and sour scent is emitted. In order to confirm the chemical components of the odor of this insect, 500 g of C. japonicus was collected from the twigs of Podocarpus nagi in Kyoto in 1974 and we knew that C. japonicus produced a large amount of sesquiterpenoids, particularly β-caryophyllene and α-humulene in good yield from the wax of the insect [Citation2]. Then, 4 years later, in 1978, Naya et al. analyzed our samples and reported that C. japonicus produced enantiomeric sesquiterpenoids, such as β-bourbonene, α-humulene, and β-caryophyllene [Citation3]. While C. rubens is smaller than C. japonicus, it lives epiphytically on the twigs of Ilex integra and Laurus nobilis. C. rubens also emits similar sour, sweet, and woody odor to those identified in C. japonicus. However, the host plant, Ilex contains neither enantio-sesquiterpenoids nor the other secondary metabolites found in the two scale insects on the basis of GC/MS data of the ether extract of the fresh Ilex samples [Citation2].
C. rubens produces stemodan-type diterpenes (1–4) shown in [Citation4]. A similar compound, aphidicolin (5) having antitumor activity, was isolated from the fungus Cephalospolium aphidicola [Citation5]; however, they have almost no smell.
Figure 1. Aphidicolane (Stemodane) diterpenoides (1–4) isolated from Ceroplastes japonicas [Citation4] and 5 from fungus, Cephalosporium aphidicola [Citation5].
![Figure 1. Aphidicolane (Stemodane) diterpenoides (1–4) isolated from Ceroplastes japonicas [Citation4] and 5 from fungus, Cephalosporium aphidicola [Citation5].](/cms/asset/7f9a36ed-c47a-4137-b3fd-c3d338514c50/tbbb_a_1763156_f0001_b.gif)
In this way, from two scale insects, C. japonicus and C. rubens, many sesquiterpen hydrocarbons and diterpenes have been identified. And as the sex pheromones of scale insects, a lot of components which was derived from caryophyllene or humulene and monoterpene esters were reported [Citation6]. However, there are no components which showed the sweet-and-sour note. Moreover, there is no example in which whether or not the sweet substance is a pheromone or how it works has been examined. And then, the unique sweet odor produced by crushing scale insects has not clarified at all.
Therefore, we reinvestigated the chemical components of the female C. japonicus and C. rubens from the point of view of identification of their characteristic sweet odors.
Materials and methods
Materials
Preparation of the aroma extracts from C. japonicus and C. rubens.
C. japonicus and C. rubens were collected in Komatsushima at Route 55 in Tokushima Prefecture on February 8, 2014, by Mr. Hashizume M. and Asakawa Y. Each sample (0.80 g) was smashed and immersed into 10 mL of pentane for 1 h and the extract was dried over anhydrous sodium sulfate, followed by evaporation in vacuo at 80°C for 1 h to remove nonvolatile components, and followed by concentration using Kuderna-Danish at 42°C to give each sweet–sour crude extract. The amounts of volatile components from C. japonicus and C. rubens were 7 and 1 mg, respectively.
Methods
GC–MS analysis
GC–MS analyses were performed using a GCMS-QP2010 Ultra (Shimadzu Co., Kyoto, Japan) equipped with a BC-WAX column (50 m × 0.25 mm i.d., film thickness of 0.15 μm). The electron impact ionization was employed for the mass spectrometry at the ionization energy of 70 eV in scan mode. The injector and the ion source temperature were set at 230 and 200°C, respectively. The carrier gas was helium with a constant pressure held at 180 kPa. The oven temperature program was set from 70 to 220°C at a rate of 4°C/min. The retention indices were calculated relative to C8–C27 n-alkanes. Compounds were identified using Mass Finder 2.3 program [Citation7], NIST library [Citation8], Flavor and Fragrance Natural and Synthetic Compounds library Ver. 2, literature [Citation9], and mass spectra from the literature and our own library databases.
GC/Olfactometry
Each volatile of C. japonicus and C. rubens was introduced into an injector of a GC-2010 (Shimadzu) equipped with a BC-WAX column (50 m × 0.25 mm i.d., film thickness of 0.15 μm). The injector and the sniffing port temperatures were set at 250°C. The carrier gas was helium with a constant pressure held at 180 kPa. The oven temperature program was set from 70 to 220°C at a rate of 4°C/min. The detector part was split to Flame Ionization Detector (FID) and olfactory system. The retention time of each component was technically controlled to be the same as that of the GC–MS described above.
Results and discussion
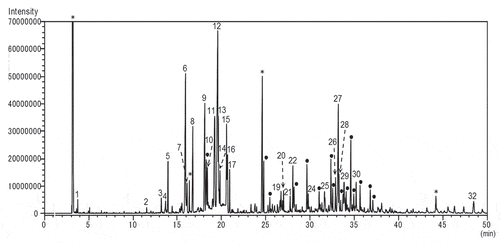
Both scale insects that had been growing on I. integra were carefully peeled off with tweezers, crushed separately in a mortar, and then extracted with pentane, and the presence 80 kinds of sesquiterpenes were confirmed from each crude extract. The odor when crushing the two kinds of scales with tweezers was just as the scent of C. japonicus. It was a woody with a cherry-like, hop-like, and then slightly green scent. On the other hand, the smell of C. rubens showed a weak woody scent with a slightly sweet-and-sour note. However, in comparison with C. rubens, the former C. japonicus showed stronger scent than C. rubens. The GC–MS profile of the pentane extract of C. japonicus was less complex than that of C. rubens as shown in and . And the identified compounds from n-pentane extracts of both C. japonicus and C. rubens were summarized in [Citation10].
Table 1. Comparison of the major volatiles Ceroplastes japonicus and C. rubens.
Figure 2. GC–MS TIC chromatogram of volatile components of Ceroplastes japonicus.
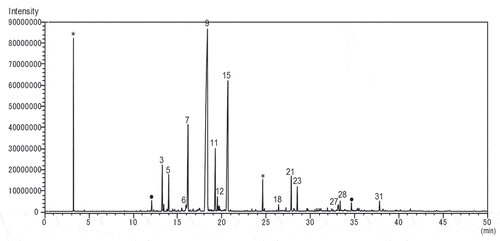
Figure 3. GC–MS TIC chromatogram of volatile components of Ceroplastes rubens.
The major components of C. japonicus were α-humulene (35.8%) and δ-cadinene (17.0%). The pentane extract of the former species contained α-copaene, β-bourbonene, β-elemene, β-caryophyllene, germacrene D, β-selinene, α-selinene, bicyclogermacrene, germacrene A, 1,2-epoxyhumulene, 2,7-germacradien-1-ol, epicubenol, and pimaradiene. The major volatile components of C. japonicus were sesquiterpenes such as α-humulene, and the presence of a lot unidentified diterpenes was confirmed.
The pentane extract of C. rubens produced β-selinene (10.3%) and β-elemene (5.1%) as the predominant components, together with the same components as those found in C. japonicus. In addition, ethanol, acetic acid, n-pentadecane, trans-caryophyllene oxide, salvial-4(14)-en-1-one, torreyol, selin-11-en-4-α-ol, decanoic acid, 14-hydroxy-α-muurolene, and sandaracopimarinal were detected as the minor components. This extract also showed the presence of β-bourbonene, selina-5,11-diene, α-curcumene, α-selinene, and pimaradiene as shown in .
From both of two samples, 3-methylbutyric acid, 2-methylbutyric acid, trans-4,5-epoxy-(2E)-decenal, and phenylacetic acid were detected as the high contributing compounds for the characteristic odor shown in . Then, the components with sweaty note, waxy note, dusty and woody note, and herbal medicine-like note were detected; however, these components were not corresponding to the smell from both Ceroplastes species.
Table 2. GC/O analysis of the volatiles from extracts of C. japonicus and C. rubens (GC/O odor intensity: middle to strong).
In order to clarify the characteristic odor of two scale insects, C. japonicas and C. rubens, the GC/olfactometry (GC/O) analysis was carried out. And, from former one, linalool with green floral note, butyric acid with cheesy and sweaty note, 4-methyl-(3E)-hexenoic acid with strong sweaty note, and vanillin with vanilla-like sweet note were identified beside of 3-methylbutyric acid, 2-methylbutyric acid, trans-4,5-epoxy-(2E)-decenal, and phenylacetic acid. In addition, decan-1,4-olide (γ-decalactone) with milky coconut-like note and 3-hydroxy-4,5-dimethylfuran-2(5H)-one (sotolone) with brown sugar-like note were identified from both extracts of scale insects as the characteristic cherry-like sweet-and-sour note.
Each of γ-decalactone and sotolone was detected at 30.5 and 31.3 min on GC-Rt in our GC analytical condition. However, it was very difficult to clarify the chirality of both of two components, because their contents were too small to determine. Probably the (R)-enantiomer would be predominant on γ-decalactone if it was one of the insect pheromones.
The odor-contributing components of C. japonicus and C. rubens detected by GC/O analysis were shown in . The odor of C. japonicus was over 10 times stronger than that of C. rubens when both insects were crushed by fingers. The odor of the former insect emits more sweaty, cheesy odor, and sweet note which might be due to the presence of butyric acid, its derivatives in along with 4-methyl-(3E)-hexenoic acid, and vanillin. This fact is corresponding to the difference of volatile components of both two insects.
Conclusion
The characteristic volatiles from two Japanese scale insects, C. japonicus and C. rubens having sour cherry-like odor, were consisted of lower saturated fatty acids (C4 and C5) and phenylacetic acid, γ-decalactone, and sotolone. Especially, two components such as γ-decalactone and sotolone were shown to have the very high contribution to the characteristic odors of these insects. Then, the production of enantiomers of sesquiterpenoides by insects to those found in higher plants and γ-decalactone and sotolone are very rare in Nature.
Authors’ contribution
All authors designed the experiments and discussed the data. SK, TK, and YY performed experiments. AY gave SK and TK the important advice. SK and AY wrote the manuscript.
Acknowledgments
The authors thank Mr. Masaru Hashizume (Tokushima Bunri University) for his collection of two scale insects C. japonicus and C. rubens in the very cold winter season, twice.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ben-Dov Y, Hodgson CJ, Editors. Soft scale insects - their biology, Natural enemies and control (7B). Elsevier Science B. V.; 1997.C .japonicus: 135, 265, 268; C. rubens: 141, 211, 212.
- Asakawa Y. Unpublished results; 1975.
- Naya Y, Miyamoto F, Takemoto T. Formation of enatiomeric sesquiterpenes in the secretions of scale insects. Experientia. 1978;34(8):984–986.
- Wada H. Diterpenoids in Ceroplastes japonicus (Abstract of the diploma thesis). Tokushima Bunri University; 1999.
- Brundret KM, Dalziel W, Hesp B, et al. X-ray crystallographic determination of the structure of the antibiotic aphidicolin: a tetracycline diterpenoid containing a new ring system. J. Chem. Soc. D. Chemical Commun. 1972;(18):1027–1028. DOI:10.1039/c39720001027.
- Zou Y, Millar JG. Chemistry of the pheromones of mealybug and scale insects. Nat Prod Rep. 2015;32(7):1067–1113, Review.
- Web book of chemical data from NIST. Available from: http://www/cjemweb.com/
- Konig WA, Joulain D, Hoschmuth DH. 226–228; 2013. Available from: http:// www. massfinder.com/
- Joulain D, Konig WA. The atlas of spectral data of sesquiterpene hydrocarbons. Hamburg: E.B.-Verlag; 1998.
- Asakawa Y, Toyota M, Sakurai K, et al. Terpenoids of two scale insects, Ceroplastes japonicus and Ceroplastes rubens. In Proceedings of the 58th Symposium on the Chemistry of Terpenes, Essential oils, and Aromatics (in English); 367–369; 2014.
