?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The recovery of algal biomass is one of the critical steps involved in the commercial production of beneficial metabolites from Arthrospira platensis. Efficient and safe harvesting methods that do not sacrifice quality of final product are important for commercial application. Phytic acid (PA) is a natural non-toxic phytochemical widely distributed in plant tissues. Effect of PA from rice bran on the growth, trichome morphology such as spiral number and algal filament length, and harvesting efficiency of A. platensis were investigated. Cells aggregated into large cell flocs after the addition of PA in the medium, and algal spiral number and filament length increased. UV-vis spectra indicated the interactions between PA and algal cells. Adding PA at stationary growth phase is a good strategy for harvesting, since no adverse effect to biomass growth and harvesting efficiency. Harvesting efficiency of 95.69% at 0.5% (v/v) PA was superior to other conventional harvesting methodologies.
Abbreviations
PA: Phytic acid; PUFAs: Polyunsaturated fatty acids; FAO: Food and Agriculture Organization; γ-PGA: Poly (γ-glutamic acid); CNF: Cellulose nanofibrils; NIES: National Institute for Environmental Studies; SOT: Spirulina–Ogawa–Terui; CG: Control group; pI: Isoelectric point.
Graphical abstract
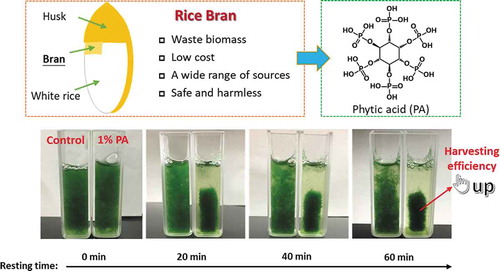
Phytic acid derived from rice bran is an effective flocculant for A. platensis harvesting.
Arthrospira platensis is a cyanobacterium whose cells are filamentous and spiral, and widely distributed in alkaline brackish and saline waters. A. platensis contains extremely high content of proteins, which account for 53–68% of the dry weight of cell biomass, including almost all the essential amino acids for the human body [Citation1]. Its absorption rate in the human body is high due to its single-cell form. Therefore, it is especially suitable for people with malnutrition, poor digestion, and absorption capacity. Polyunsaturated fatty acids (PUFAs) content in A. platensis was also high, and account for 4–6% of the total lipid content. Iron content (mostly combined with phycocyanin) in A. platensis was almost 6 times higher than that of whole grains and 4.5 times higher than that of spinach [Citation2]. As the nutrition in A. platensis is rich, balanced, and easy to absorb, it has been endorsed as a “super food” [Citation3] and recommended by the Food and Agriculture Organization (FAO) of the United Nations [Citation4]. Therefore, the demand of consumers for A. platensis has been surging in recent years.
Production of microalgae-derived metabolites requires screening of the algal species, cultivation, recovery of the biomass, and further downstream processing to purify the metabolite from the biomass. However, cost-effective harvesting of microalgae has always been a major bottleneck in the commercial process because of the low cell concentrations (0.5–5 g·L−1 dry biomass), range of small sizes (measured in micrometers) [Citation5], and strong ionic force of seawater [Citation6]. One or more solid-liquid separation steps such as centrifugation, filtration, and gravity sedimentation were necessary for the harvesting of biomass; hence, large volumes need to be handled for its recovery. However, without any pretreatment, these processes consume significant amounts of energy, resulting in high costs. Flocculation prior to these processes may increase the effective size of cell mass, and hence ease the sedimentation, centrifugal recovery, and filtration [Citation5,Citation7]. There are many different methods of flocculation that can be used to aggregate microalgal cells, such as auto-flocculation, bio-flocculation, electrolytic flocculation, and chemical flocculation [Citation8]. Auto-flocculation is generally caused by carbonate precipitation at a higher pH level, which is related to the consumption of carbon dioxide by photosynthesis of algal cells. This process has been known to be time-consuming and inefficient [Citation9]. Microalgae can also be bio-flocculated through the addition of microorganisms such as yeast and bacteria. For example, Ndikubwimana et al. [Citation10] used the broth of Bacillus licheniformis CGMCC 2876 to harvest Desmodesmus sp. and found poly (γ-glutamic acid) (γ-PGA) produced by the bacteria was the key bio-flocculant. Zhou et al. [Citation11] also developed an alternative bio-flocculation method for harvesting Chlorella vulgaris UMN235 using pellet-forming fungal strain (Aspergillus oryzae) isolated from municipal wastewater sludge, resulting in the harvesting efficiencies for heterotrophy and autotrophy to be 99.2% and 63%, respectively. The efficiency of bio-flocculation was high, but the application allowed to use microorganism are limited, and the purification of products was also difficult. Electrolytic flocculation requires both high energy consumption and equipment cost, although the process is considered to be highly efficient. Adding chemicals to microalgal culture to induce flocculation is the most widely used method as a pre-treatment stage at present, which is applicable to large quantities of numerous kinds of microalgal species [Citation12]. According to its chemical composition, it is divided into inorganic flocculants and organic flocculants (mainly polyelectrolyte flocculants) [Citation8]. Their principle is to reduce or neutralize the negative charges on the surface of microalgae cells to break the balance and promote flocculation. The flocculation efficiency was up to 90% while the flocculation process was sensitive to pH [Citation8]. In addition, the polymer flocculants can also bring particles together by physically linking one or more particles through a process called bridging [Citation13]. However, the high salinity within the marine environment can be inhibitory, as the polymers shrink to smaller dimensions, failing in bridging the cells [Citation5]. Moreover, these traditional chemical flocculants are expensive and some are known to be harmful to human health [Citation14], and cation modification of some polymer flocculants weights the environmental and harvesting process costs [Citation15].
In recent years, the method of utilizing some abandoned biomass to harvest microalgae has emerged. For example, Vandamme et al. [Citation16] introduced the possibility of using lime as a more cost-efficient approach to achieving high-pH-induced flocculation of C. vulgaris. Yu et al. [Citation15] demonstrated that cellulose nanofibrils (CNF) served as a flocculant for Chlamydomonas reinhardtii via its network geometry without cation modification. It gives us a hint that biomass with security and efficiency is a good option for microalgae harvesting. Phytic acid (inositol hexakisphosphate, PA) is the principal storage form of phosphorus in plant tissues, especially in legume seed, cereal bran, and germ. Simultaneously, it is also the major source of phosphate loading in aquatic ecosystems. Wise utilization of PA could contribute to alleviating the aquatic environment stress. The structure of PA is very special. One molecule of PA consists of an inositol ring and six phosphate groups, which results in its strong chelating ability to polyvalent metal elements. Furthermore, PA is an antioxidant with a variety of physiological functions such as anti-tumors [Citation17], enhancing immunity [Citation18], preventing Alzheimer’s disease [Citation19] and Parkinson’s disease [Citation20]. Its safety has also been proved before and its toxicity was even lower than that of sodium chloride [Citation21]. In our previous research, we found that PA from agro-waste rice bran has a strong promotion effect on the growth of Euglena gracilis [Citation22], but its application in cell flocculation has not yet been studied. In this study, the potential of PA from rice bran for the harvesting of A. platensis was evaluated. The effect of PA on the growth and trichome morphology of A. platensis in the early growth stages was investigated. The absorption spectra associated with culture filtrates and the harvesting efficiency of PA at the stationary phase were also studied and measured. The objective of this study is to provide an efficient and safe harvesting method for A. platensis using PA discarded in nature, alleviating the load on the aquatic environment.
Materials and methods
Microalga and cultivation conditions
Arthrospira platensis Gomont (NIES-39) was obtained from the National Institute for Environmental Studies (NIES) and incubated in axenic Spirulina–Ogawa–Terui (SOT) medium with the following composition (mg·L−1): NaHCO3, 16800; K2HPO4, 500; NaNO3, 2500; K2 SO4, 1000; NaCl, 1000; MgSO4 · 7H2O, 200; CaCl2 · 2H2O, 40; FeSO4 · 7H2O, 10; Na2EDTA·2H2O, 80; H3BO3, 2.86; MnSO4 · 5H2O, 2.5; ZnSO4 · 7H2O, 0.22; CuSO4 · 5H2O, 0.08; Na2MoO4 · 2H2O, 0.02. A. platensis cells were cultured at 25°C and illuminated under the light condition of 1500 lx by cool-white fluorescent lamps (12:12 h light-dark cycle).
Effect of PA on A. platensis in the early growth stage
PA from rice bran was supplied by Tsuno Rice Fine Chemicals Co., Ltd. (Wakayama, Japan). The effect of different concentrations of PA on the growth of A. platensis cells in the early growth stage was evaluated. Ten milliliters of a late logarithmic growth phase culture was used as the inoculum, transferring to the Erlenmeyer flask (250 mL). Fresh PA stock solution was then added, with each flask finally containing 100 mL medium. The initial pH of each group was slowly adjusted to the same level (approximately 9) by 0.5 M sodium hydroxide solution. The final PA concentrations were 0, 0.01%, 0.1%, and 0.5% (v/v). All groups were cultured in triplicate for further analysis.
Cell growth evaluation
The growth of A. platensis was evaluated by periodically measuring the dry weight of cell biomass. Sample tube and filter paper (pore size, 0.45 μm) (ADVANTEC, GC-50) were pre-dried and weighed. Then, 5 mL of cell suspension was filtered using the pre-dried filter paper and the algal cells were harvested. The sample tube and the cells deposited on the filter paper were dried at 90°C overnight and weighed again. Dry weight of the cell biomass was calculated by comparing the difference between before and after drying. pH of the medium was periodically determined by portable pH meter (LAQUA-2103AL, Horiba, Japan).
Trichome morphology observation
The trichome morphology of A. platensis was observed by the optical microscope (Model BA210a, Motic, Japan) and recorded using the corresponding software Image Plus 2.2S. The cell photograph was introduced into image analysis software ImageJ (open source), and the spiral number and algal filament were measured.
Changes in absorption spectra of culture filtrates
Absorption spectra of the culture filtrates before and after the flocculation were measured by the spectrophotometer (Genesys 10 S UV-Vis, Thermo Scientific, USA). The wavelength from 200 nm to 600 nm was acquired and the interval of the scan was set at 0.5 nm. In addition, absorption spectra of filtered PA, sodium phytate solution, and fresh SOT medium were also measured.
Flocculation efficiency
Flocculation experiment of A. platensis with PA was carried out at the stationary phase. An aliquot of 30 mL A. platensis culture was placed into a 50 mL glass bottle and different concentrations of PA were added (0, 0.01%, 0.1%, 0.5%, and 1%; v/v). The algal cells were shaken evenly by hand at room temperature, with a 3 mL sample taken to measure the initial optical density at 750 nm. As the sample kept, the cells began to sink and the optical density of the upper layer began to decrease. The optical density at 750 nm was measured at regular intervals using the algal cultures taken from the clarified zone. Harvesting efficiency was evaluated by comparing the optical density at 750 nm before and after the flocculation. It was calculated according to the following formula.
where ODT0 is the initial optical density at 750 nm before starting the flocculation, and ODT1 is the optical density of the sample at a certain point of time during the process.
Results and discussion
PA added in the early growth stage of A. platensis
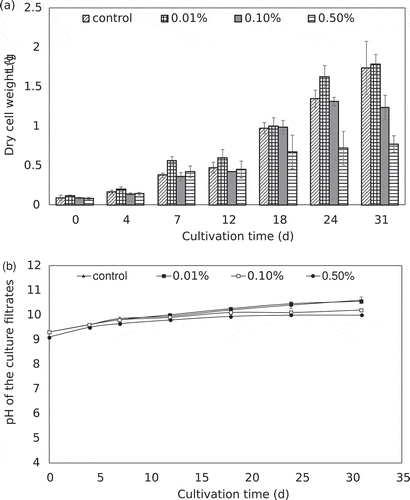
To evaluate the effect of PA on the biomass accumulation of A. platensis, PA was added to the medium at the onset of the culture. Dry cell weight and pH variation of A. platensis cultures under different concentrations of PA were assessed periodically, with the results shown in . Growth of A. platensis was comparable to that of the control group at a lower dosage of PA, but the inhibitory effect was observed at higher concentrations. On day 31, compared with the dry weight of 1.74 g·L−1 in the control group, the dry weight of the cells decreased to 0.78 g·L−1 in the 0.5% PA treatment group. The initial pH of the culture filtrate was at 9.3. During the growth process, the pH kept rising and finally stabilized, making the pH of each concentration treatment groups within the optimal range for A. platensis. Cell flocculation was discovered in the PA treatment group, and the medium became clarified and the flocculated cell mass became compact. The addition of PA in the early growth stage was unfavorable to the growth of A. platensis, mainly due to the cell proliferation required sufficient light. When the inoculated young cells were in compact form by flocculation, the available light to the unit cells was greatly reduced, especially those in the center of the cell mass. The results suggested that the addition of phyto-flocculant PA at the onset of growth would adversely affect biomass yield. Therefore, it is important to determine the correct time to apply the flocculant. The addition of PA at the log exponential growth phase seems to be a more feasible approach, since it can balance the maximum biomass yield and harvesting efficiency.
Trichome morphology
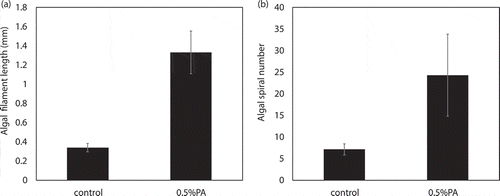
The addition of PA in the early growth stage resulted in the flocculation of algal cells. As trichome morphologies of A. platensis could reflect the physiology states of the cells to a certain extent, cell spiral number and filament length were measured at the end of the culture. As reported previously [Citation23], according to the cell spiral number and filament length, morphotypes of A. platensis could be divided into 3 types: H-type, S-type, and C-type, which represent tightly coiled cells, spiral or loosely coiled cells, and intermediately coiled cells, respectively, for which S-type cells are the most common type. When both light intensity and nutrient concentration were high, the S-type cells would change to C-type cells. For when the light intensity is high and the nutrient content is low, the S-type cells would change to the H-type [Citation24]. The trichome morphology of the control group and the 0.5% treatment group was compared (), and algal filament length and spiral number are shown in . In the control group, the cells were in S-type and their algal filament length and spiral number were within normal ranges [Citation25]. Trichome (filament) length was 0.339 mm and the number of coils per trichome was 7.1. However, in the 0.5% PA treatment group, longer algal cells with more coils were observed. The spiral number and trichome length increased by 3.93 and 3.42 fold, respectively.
Figure 2. Micrographs of A. platensis cells under different conditions. (a): control group (low magnification); (b): 0.5% PA treatment group (low magnification); (c): control group (high magnification); (d): 0.5% PA treatment group (high magnification).
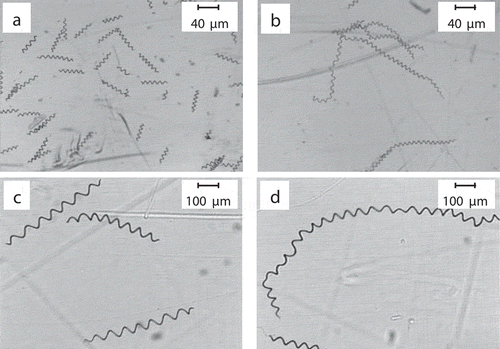
Figure 3. Algal spiral number and filament length of A. platensis in the control and 0.5% PA treatment groups. (a): algal filament length; (b) algal spiral number.
According to the classification described above, cells in the 0.5% PA treatment group were still classified as S-type, but there was a certain degree of variation. These individual cells could be obtained from the cell mass by adding buffer solution and shaking evenly. We speculated that the flocculated cell mass became tighter and tighter during the culture process, resulting in the internal cells not easily detaching. The cell division was inhibited by external conditions, leading to an increase in the helix number and the cell filament length. On the other hand, longer cells were more prone to bending and twisting, resulting in cells being more likely to clump together and difficult to separate.
UV-vis absorption spectra of culture filtrates
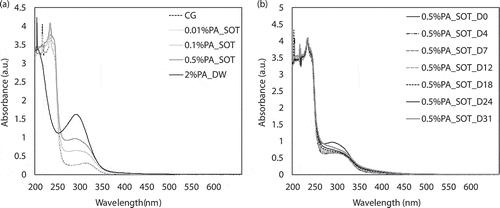
The UV-vis absorption spectra of the culture filtrates with different concentrations of PA were detected periodically. As seen from ), fresh SOT medium without PA (control group, CG) showed a weak peak at 300 nm, and the peaks at 200 to 250 nm were complex, which were common for various inorganic salts. The spectra showed that the addition of 0.01% PA had little effect on the spectrum, which basically coincided with that of SOT. However, with the increase of PA concentration, the peak at 300 nm gradually shifted to 280 nm, and the absorbance at 280 nm increased significantly with the increase of PA, which was in a concentration-dependent manner. In addition, the UV-vis absorption spectrum of sodium phytate in deionized water also had a significant absorption peak at 280 nm, so the peak at 280 nm could be attributed to the salt of PA in the medium. Surprisingly, the absorption peak at 280 nm decreased as the culture progressed ()). By day 31, there were almost no signs of peaks at 280 nm, which indicated that there was a certain interaction between phytate and algal cells, such as absorption or adsorption, so PA was removed at the same time as the algal cell biomass was recovered.
Figure 4. UV-vis absorption spectra of culture filtrates with different concentrations of PA at different time. (a): spectra of different concentrations; (b): spectra of 1% PA at different time. CG: control group (fresh SOT medium); PA_SOT: different concentrations of PA dissolved in SOT medium; PA_DW: PA dissolved in deionized water; D0-D31 denote day 0–31.
Flocculation experiment
The harvesting efficiency of A. platensis under different concentrations of PA was evaluated when the cell biomass reached its maximum. In order to show the harvesting efficiency more intuitively, small-scale simulation experiments in test tubes (10 mL) and cuvettes (3 mL) were conducted. As shown in , distinct stratified phases were clearly evident after 20 min from the addition of 1% PA, while natural sedimentation in the control group was very slow. As the resting time increased, the cell mass continued to concentrate and the enrichment factor of 1% PA treatment group increased.
Figure 5. Examples of a flocculation experiment of A. platensis using the phytochemical flocculant PA (1%). (a–c): control group resting for 0 min; (d–f): addition of 1% PA group resting for 30 min; g, i, k, and m: control resting for 0, 20, 40, and 60 min, respectively; h, j, l, and n: addition of 1% PA group resting for 0, 20, 40, and 60 min, respectively.

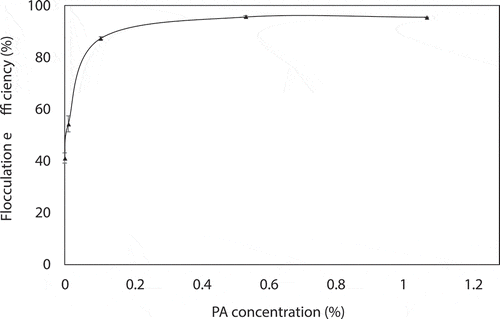
Flocculation efficiency of A. platensis after 24 h with different concentrations of PA is shown in . The harvesting efficiency of natural sedimentation within 24 h was 41.15%. When the concentration of PA increased to 0.1%, the harvesting efficiency greatly increased to 87.43%. As the PA concentration continued to rise, there was little change in harvesting efficiency. The harvesting efficiency at 0.5% and 1% PA was 95.69% and 95.60%, respectively. Based on economic considerations and efficiency issues, 0.1% and 0.5% PA used for harvesting seemed to be a more viable option.
The decline in pH value induced by PA was not a key factor in cell flocculation of A. platensis, as we found that cell flocculation still occurred in the pH-adjusted PA treatment group ()). In addition, SOT medium contained a large amount of sodium bicarbonate and potassium hydrogen phosphate, which gave the medium a wide buffer range for pH changes. We had also found that when a certain amount of cell flocs were inoculated in fresh medium, the cell mass can be restored to the state of single cells and continue to proliferate, indicating that the cells from the floc were alive.
Previous studies had suggested that the flocculation effect of PA was species-specific. PA could significantly promote the growth of E. gracilis and increase its cell aspect ratio [Citation26]. However, the flocculation phenomenon by PA was not found in other species (as shown in Figures S1, S2, and S3), such as E. gracilis, Haematococcus lacustris, and Botryococcus braunii, and indicated that flocculation effect of PA was cell-dependent. A. platensis is a type of cyanobacteria with extremely high levels of protein (around 70% of the dry weight) [Citation1,Citation27] and iron elements [Citation2], and its soft cell wall consists of complex sugars and proteins [Citation28]. However, protein content in E. gracilis, H. lacustris, and B. braunii was all less than 35% [Citation29–Citation31]. Based on the above characteristics, we speculated that the specific flocculation of PA on A. platensis was mainly due to its higher content of protein and iron, because PA can chelate divalent metal ions, and form complexes with abundant proteins on the surface of algal cells [Citation32]. It has been reported that when the external pH is lower than the isoelectric point (pI) of the protein, the protein is positively charged (mainly the ε-amino group of lysine, and the guanidino group of arginine and histidine) [Citation33], and is prone to form insoluble complexes with negatively charged PA due to the strong electrostatic effect. When the external pH is higher than the pI of the protein, the protein is negatively charged, and the protein will form a ternary complex with PA, by using a polyvalent cation as a bridge [Citation34]. Here the pH of the medium was higher than the pI of the protein, and PA might form a ternary complex to flocculate algae cells. This was consistent with the results of UV-vis spectroscopy, where there were interactions between PA and algal cells such as adsorption, chelation, or bridging. PA might play a “bridging” role that is why the absorption peak at 280 nm in the medium disappeared.
Comparison with other common chemical flocculants
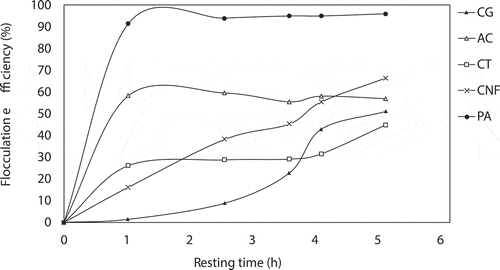
To further verify the superiority of PA as the flocculant for A. platensis, we compared PA (0.5%) with three other common chemical flocculants (0.5 g·L−1 aluminum chloride, 0.15 g·L−1 chitosan, and 0.5 g·L−1 CNF; the optimal concentration was referred to previous reports) [Citation15,Citation35,Citation36]. The recovery efficiency of A. platensis using different flocculants is shown in . Without any flocculant, the natural sedimentation of A. platensis is very slow. After resting for 1 h, an obvious separation occurred in the two treatment groups with PA and aluminum chloride. Among them, PA has the best flocculation effect on cells within a predefined period of time. Although the final recovery efficiency of both aluminum chloride and CNF was higher than those of the control group, they were still relatively low in reality and not suitable for commercial harvesting of A. platensis. Among them, chitosan has the lowest harvesting efficiency, and the final recovery rate after resting for 5 h was only 44.86%. Given that these results are slightly different from the previous reports, this may be due to differences in algae species. In previous studies [Citation15,Citation35,Citation36], these chemical flocculants were mainly used for the harvest of green algae, but their effects on cyanobacteria are still unknown. Our results demonstrated the superior harvesting efficiency of PA as a chemical flocculant for cyanobacteria A. platensis. Because PA is very cheap (0.156 USD·g−1), PA-based flocculation is highly promising in improving the harvesting process in commercial algal biomass production.
Figure 7. Comparison of phytic acid and other chemical flocculants (aluminum chloride, chitosan, and CNF) on the harvesting efficiency of A. platensis in the predefined period of time. CG: control group; AC: 0.5 g·L−1 aluminum chloride; CT: 0.15 g·L−1 chitosan; CNF: 0.5 g·L−1 cellulose nanofibrils; PA: 0.5% phytic acid. Phytic acid derived from rice bran is an effective flocculant for A. platensis harvesting.
Concluding remarks
Phytic acid from rice bran was able to serve as a nontoxic phytochemical flocculant for A. platensis, by which a highest harvesting efficiency of 95.69% was achieved at 0.5% PA treatment. Significant growth promotion effect of PA was not obtained as similar to our previous study with E. gracilis, but changes in trichome morphology such as the algal cell length and spiral numbers of A. platensis were observed. Addition of PA at stationary growth phase would be efficient to obtain maximum biomass. The detailed mechanism of PA needs to be determined further, but the interactions (adsorption, chelation, or bridging) between PA and algal cells might occur since PA decreased from the medium as indicated from UV-vis spectra.
Author contributions
M.W. and J.Z. designed this study and J.Z. conducted the experiments. M.W. contributed to the investigation, supervision, software, validation, writing – review & editing. J.Z. contributed to methodology, software, writing – original draft, data curation, visualization of data.
Supplementary_material.pdf
Download PDF (217.6 KB)Acknowledgments
The authors would like to thank Tsuno Rice Fine Chemicals Co., Ltd., Japan for providing the phytic acid derived from rice bran. Jiangyu Zhu would like to thank Ellis Ying Jiang and Marco Tomassi for the English proofreading.
Disclosure statement
The authors acknowledge they have no conflict of financial interest or benefit.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Clement G, Giddey C, Menzi R. Amino acid composition and nutritive value of the alga Spirulina maxima. J Sci Food Agric. 1967;18:497–501.
- Hoseini SM, Khosravi-Darani K, Mozafari MR. Nutritional and medical applications of spirulina microalgae. Mini-Rev Med Chem. 2013;13:1231–1237.
- Soni RA, Sudhakar K, Rana RS. Spirulina–From growth to nutritional product: A review. Trends Food Sci Technol. 2017;69:157–171.
- Pelizer LH, Danesi EDG, de O, et al. Influence of inoculum age and concentration in Spirulina platensis cultivation. J Food Eng. 2003;56:371–375.
- Grima EM, Belarbi E-H, Fernández FG, et al. Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol Adv. 2003;20(7–8):491–515. .
- Lee AK, Lewis DM, Ashman PJ. Microbial flocculation, a potentially low-cost harvesting technique for marine microalgae for the production of biodiesel. J Appl Phycol. 2009;21:559–567.
- Elmaleh S, Coma J, Grasmick A, et al. Magnesium induced algal flocculation in a fluidized bed. Water Sci Technol. 1991;23:1695–1702.
- Chen CY, Yeh KL, Aisyah R, et al. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol. 2011;102:71–81.
- Sukenik A, Shelef G. Algal autoflocculation—verification and proposed mechanism. Biotechnol Bioeng. 1984;26:142–147.
- Ndikubwimana T, Zeng X, Liu Y, et al. Harvesting of microalgae Desmodesmus sp. F51 by bioflocculation with bacterial bioflocculant. Algal Res. 2014;6:186–193.
- Zhou W, Min M, Hu B, et al. Filamentous fungi assisted bio-flocculation: a novel alternative technique for harvesting heterotrophic and autotrophic microalgal cells. Sep Purif Technol. 2013;107:158–165.
- Lee SJ, Kim SB, Kim JE, et al. Effects of harvesting method and growth stage on the flocculation of the green alga Botryococcus braunii. Lett Appl Microbiol. 1998;27:14–18.
- Tenney MW, Echelberger WF, Schuessler RG, et al. Algal flocculation with synthetic organic polyelectrolytes. Appl Environ Microbiol. 1969;18:965–971.
- Barros AI, Gonçalves AL, Simões M, et al. Harvesting techniques applied to microalgae: a review. Renewable Sustainable Energy Rev. 2015;41:1489–1500.
- Yu SI, Min SK, Shin HS. Nanocellulose size regulates microalgal flocculation and lipid metabolism. Sci Rep. 2016;6:35684.
- Vandamme D, Foubert I, Fraeye I, et al. Flocculation of Chlorella vulgaris induced by high pH: role of magnesium and calcium and practical implications. Bioresour Technol. 2012;105:114–119.
- Vucenik I, Tomazic VJ, Fabian D, et al. Antitumor activity of phytic acid (inositol hexaphosphate) in murine transplanted and metastatic fibrosarcoma, a pilot study. Cancer Lett. 1992;65:9–13.
- Fox CH, Eberl M. Phytic acid (IP6), novel broad spectrum anti-neoplastic agent: a systematic review. Complement Ther Med. 2002;10:229–234.
- Anekonda TS, Wadsworth TL, Sabin R, et al. Phytic acid as a potential treatment for alzheimer’s pathology: evidence from animal and in vitro models. J Alzheimer’s Dis. 2011;23:21–35.
- Xu Q, Kanthasamy AG, Reddy MB. Neuroprotective effect of the natural iron chelator, phytic acid in a cell culture model of Parkinson’s disease. Toxicology. 2008;245:101–108.
- Wu M, Yuan J. Toxicological evaluation and food safety of phytic acid. Food Sci. 1997;18:46–49. in Chinese.
- Zhu J, Hong DD, Wakisaka M. Phytic acid extracted from rice bran as a growth promoter for Euglena gracilis. Open Chem. 2018;17:57–63.
- Van Eykelenburg C. The ultrastructure of Spirulina platensis in relation to temperature and light intensity. Antonie Van Leeuwenhoek. 1979;45(3):369–390.
- Jeeji Bai N, Seshadri CV. On coiling and uncoiling of trichomes in the genus Spirulina. Arch Hydrobiol Suppl. 1980;60:32–47.
- Ogato T, Kifle D. Morphological variability of Arthrospira (Spirulina) fusiformis (Cyanophyta) in relation to environmental variables in the tropical soda lake Chitu. Ethiop Hydrobiologia. 2014;738:21–33. .
- Zhu J, Wakisaka M. Finding of phytase: understanding growth promotion mechanism of phytic acid to freshwater microalga Euglena gracilis. Bioresour Technol. 2019;296:122343.
- Khan Z, Bhadouria P, Bisen PS. Nutritional and therapeutic potential of Spirulina. Curr Pharm Biotechnol. 2005;6:373–379.
- Van Eykelenburg C. A glucan from the cell wall of the cyanobacterium Spirulina platensis. Antonie Van Leeuwenhoek. 1978;44:321–327.
- Wang Y, Seppänen-Laakso T, Rischer H, et al. Euglena gracilis growth and cell composition under different temperature, light and trophic conditions. PLoS One. 2018;13:e0195329.
- Ba F, Ursu AV, Laroche C, et al. Haematococcus pluvialis soluble proteins: extraction, characterization, concentration/fractionation and emulsifying properties. Bioresour Technol. 2016;200:147–152.
- Blifernez-Klassen O, Chaudhari S, Klassen V, et al. Metabolic survey of Botryococcus braunii: impact of the physiological state on product formation. PLoS One. 2018;13(6):e0198976.
- Erdman JW Jr. Effects of soya protein on mineral availability. J Am Oil Chem Soc. 1981;58:489.
- Schoch RB, Bertsch A, Renaud P. pH-controlled diffusion of proteins with different pI values across a nanochannel on a chip. Nano Lett. 2006;6:543–547.
- Serraino MR, Thompson LU, Savoie L, et al. Effect of phytic acid on the in‐vitro rate of digestibility of rapeseed protein and amino acids. J Food Sci. 1985;50:1689–1692.
- Papazi A, Makridis P, Divanach P. Harvesting Chlorella minutissima using cell coagulants. J Appl Phycol. 2010;22:349–355.
- Heasman M, Diemar J, O’connor W, et al. Development of extended shelf‐life microalgae concentrate diets harvested by centrifugation for Bivalve molluscs–a summary. Aquacult Res. 2000;31:637–659.