ABSTRACT
Capsaicinoids are responsible for the pungent flavor of peppers (Capsicum sp.). The cultivar CH-19 Sweet is a non-pungent pepper mutant that biosynthesizes the low-pungent capsaicinoid analogs, capsinoids. Capsinoids possess important pharmaceutical properties. However, capsinoid concentrations are very low in CH-19 Sweet, and Capsicum cultivars with high content capsinoids are desirable for industrial applications of capsinoids.
Habanero, Bhut Jolokia, and Infinity are species of Capsicum chinense, and have strong pungency and intense fruity flavors. In the present study, we report new cultivars with high concentrations of capsinoids (more than ten-fold higher than in CH-19 Sweet), and showed that these cultivars (Dieta0011-0301 and Dieta0011-0602 from Bhut Jolokia, Dieta0041-0401 and Dieta0041-0601 from Infinity) are of nutritional and medicinal value and have fruity aromas. We also obtained a vanilla bean flavor, vanillyl alcohol, and vanillyl ethyl ether from capsinoids in the fruit of these cultivars following the addition of ethanol at room temperature.
Abbreviations
p-AMT: putative aminotransferase; C. annuum: Capsicum annuum; C. chinense: Capsicum chinense; dCAPS: derived Cleaved Amplified Polymorphic Sequences.
GRAPHICAL ABTSRACT

New cultivars contained high capsinoid concentrations and a strong fruity aroma, and produced vanillyl ether following the addition of alcohol to fruit.
Fruit from cultivated forms of the genus Capsicum sp. are some of the most popular plant foods in the world, and peppers are an important ingredient in many dishes, pastes, sauces, drinks, and sweets [Citation1]. They possess chemical compounds that are beneficial for health, such as capsaicinoids, carotenoids (provitamin A), flavonoids, vitamins (Vitamins C and E), minerals, essential oils and fruit aromas [Citation2–Citation8]. These compounds have been shown to exhibit anticancer [Citation9–Citation12], anti-inflammatory [Citation13], antimicrobial [Citation14], and antioxidant activities [Citation15]. Capsaicinoids, which are pungent compounds composed of capsaicin and its analogues, have been extensively examined (e.g., food technology, chemistry, genetics, and breeding) [Citation16–Citation18]. Each capsaicinoid possesses an amide bond derived from a vanillylamine and a fatty acid catalyzed by capsaicinoid synthase (CS) in the placental tissues of pungent peppers [Citation19–Citation24]. Capsaicinoids exhibit a wide range of bioactivities, such as the promotion of thermogenesis and suppression of fat accumulation [Citation25,Citation26]. However, their strong pungency prevents their use as ingredients in foods and supplements. Although the fruity aroma of peppers is a desirable characteristic [Citation27–Citation29], the use of peppers as a food material is limited by their strong pungency.
Capsinoids are non-pungent capsaicinoid analogues that have an ester bond derived from a vanillyl alcohol and fatty acid, and are classified as capsiate, dihydrocapsiate, and nordihydrocapsiate [Citation30–Citation32]. Previous studies reported that non-pungent CH-19 Sweet, mildly pungent Capsicum lines, such as Ají Dulce, and SNU11-001 (Capsicum chinense), and S3212 (C. frutescens) contained capsinoids [Citation33–Citation36]. Pungent Capsicum lines were also found to contain trace amounts of capsinoids [Citation37]. Since Capsicum cultivars have p-amt/p-amt alleles, higher concentrations of capsinoids accumulate than capsaicinoids [Citation33–Citation36]. Similar to capsaicinoids, capsinoids have important pharmaceutical properties. They promote metabolism and suppress the accumulation of body fat [Citation38], increase the mRNA levels of uncoupling proteins [Citation39], and potentially exhibit anti-cancer and antioxidant activities [Citation40,Citation41]. The intake of capsinoids may surpass that of capsaicinoids because they are not pungent. Tanaka et al. [Citation42] previously reported that a new cultivar, Maru Salad was selected and developed from the cross of CH-19 Sweet and Murasaki by using a dCAPS marker (detecting p-AMT genotypes) and a SCAR marker (detecting Pun1genotpes), and the capsinoid content was higher than the capsaicinoid content in the fruits of Maru Salad. However, the purpose of breeding of “Maru Salad” was suitable to be consumed raw, because the fruits of CH-19 Sweet were unpalatable and small. The fruits of Maru Salad are larger than those of CH-19 Sweet and suitable to be consumed raw; however, the capsinoid content of the fruits of Maru Salad is lower than that of CH-19 Sweet.
C. chinense cultivars, such as Habanero, Bhut Jolokia, and Infinity, are highly pungent, have strong aromatic flavors [Citation27,Citation43,Citation44], and are used in many regions of the world, particularly Mexico, the Caribbean, and India [Citation1,Citation45]. However, some mildly pungent cultivars of C. chinense, including Ají Dulce, have low concentrations of capsinoids, which may be useful for the selection of a new line with other C. chinense cultivars. Highly aromatic and low-pungency C. chinense cultivars are rare [Citation46]. Increases and decreases in the concentrations of capsinoids and capsaicinoids, respectively, in Capsicum lines are important for the use of its fruit in the food and medical industries. Furthermore, due to their fruity aroma, they may be widely used as an ingredient in food preparation.
Sutoh et al. [Citation47] previously reported that capsinoids decomposed in water, methanol, or ethanol, and produced vanillyl alcohol, methyl vanillyl ether, or ethyl vanillyl ether, respectively. However, their study mainly focused on the stability of purified capsinoids. Vanilla beans and vanilla bean extracts are used in the preparation of ice creams, chocolates, cakes, soft drinks, pharmaceuticals, liquors, perfume, and nutraceuticals [Citation48], and natural vanilla flavors are expensive. Vanillyl alcohol and vanillyl ethyl ether contribute to the sweet flavor of vanilla beans [Citation49–Citation52] and are chemically synthesized and widely used in the food industry.
The main aims of the present study were to i) select Capsicum lines containing high concentrations of capsinoids by breeding C. chinense, ii) characterize the aromas and carotenoid concentrations of the selected lines, and iii) produce a vanilla bean-like sweet flavor in their fruit by the addition of alcohol.
Materials and methods
Plant materials
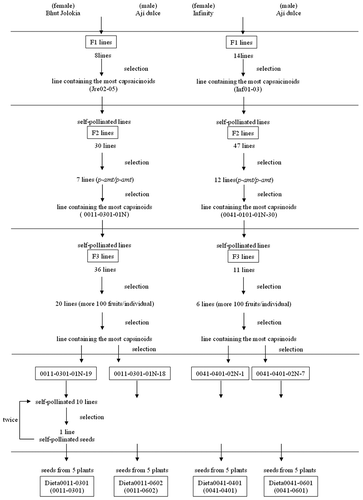
C. annuum, CH-19 Sweet was provided by the Department of Agronomy and Horticultural Science, Kyoto University (Yazawa et al.). C. chinense cultivars were used in the present study: one mildly pungent cultivar, the Ají Dulce strain produced capsinoids, was from Sunaoyasai (http://www.sunaoyasai.com). The other strongly pungent cultivars (Habanero Red, Habanero Red 2nd line, Habanero Red 3rd line, Bhut Jolokia, Jolokia Brown, Jolokia Yellow, Jolokia Purple, Jolokia Peach, Naga Viper, Naga Morich, Dorset Naga, Infinity, Butch Taylor, and Moruga Scorpion Choco) were from Togarashi Akutagawa (http://www.chili.jp/). The selection was performed as shown in . F1 populations were derived from crosses between Ají Dulce and strongly pungent cultivars (Habanero Red, Bhut Jolokia, and Infinity). F2 populations were self-pollinated from Jre02-05 (the line from Bhut Jolokia containing the highest concentrations of capsaicinoids) or Inf01-03 (the line from Infinity containing the highest concentrations of capsaicinoids). 0011–0301-01N (the F2 line from Jre02-05 containing the highest concentrations of capsinoids) and 0041–0101-01N (the F2 line from Inf01-03 containing the highest concentrations of capsinoids) were selected using the method of Lang et al. (a derived Cleaved Amplified Polymorphic Sequence (dCAPS) analysis) (p-amt/p-amt) [Citation33], followed by a quantitative HPLC analysis of capsinoids [Citation33]. 0011–0301-01N-18 and 0011–0301-01N-19 (capsinoid-rich F3 lines from 0011–0301-01N) were selected by fruit numbers (more than 100 fruits), followed by a quantitative HPLC analysis of capsinoids. Similarly, 0041–0401-02N-1 and 0041–0401-02N-7 (capsinoid-rich F3 lines from 0041–0101-01N) were selected by fruit numbers and the HPLC analysis. 0011–0301 was gathered from 5 plants after 0011–0301-01N-19 had self-pollinated, was selected, self-pollinated, and then reselected. Similarly, each line from 0011–0602, 0041–0401, and 0041–0601 was gathered from 5 plants after 0011–0301-01N-18, 0041–0401-02N-1, or 0041–0401-02N-7 had self-pollinated, been selected, self-pollinated, and then reselected. These plants were grown between 2013 and 2019 alongside other Capsicum strains bearing fruit of varying pungencies in an experimental greenhouse at Ajinomoto Co., Inc.
Chemical materials
We obtained standards for capsiate, dihydrocapsiate, nordihydrocapsiate, vanillyl methyl ether, vanillyl ethyl ether, vanillyl propyl ether, and vanillyl butyl ether from the Application & Development of Natural Products Group of the Research Institute for Health Fundamentals (Ajinomoto Co., Inc.) using the same method described by Lang et al. [Citation33] and Sutoh et al. [Citation47]. Carotenoid standards were purchased from commercial suppliers: capsorubin, capsanthin, anteraxanthin, beta-apo-carotene, and beta-carotene (Wako Pure Chemical Co.). Other standards were purchased from commercial suppliers: capsaicin (Wako Pure Chemical Co.), dihydrocapsaicin (Nacalai Tesque), norhydrocapsaicin (Nacalai Tesque), and vanillyl alcohol (Wako Pure Chemical Co.).
Alpha-cyclodextrin and beta-cyclodextrin were purchased from CycloChem Co., Ltd. Other chemicals (ethylacetate, methanol, acetonitrile, hydrochloric acid, sodium hydroxide, potassium hydroxide, and ammonium acetate) were from Nacalai Tesque.
Extraction of capsinoids and capsaicinoids for an HPLC analysis in breeding and selection
The immature or mature fruit of peppers was harvested approximately 4 or 7 weeks after flowering for the extraction of capsinoids and capsaicinoids. To minimize sample variations, at least five fruits were pooled from each plant. The leaves and stem ends were removed and discarded, and harvested fresh fruit was stored immediately at −80°C. Fruit was lyophilized for 3 days using an FDU-2100 lyophilizer (Tokyo Rikakikai Co., Ltd.), and then ground to a fine powder using a blender. To extract capsinoids and capsaicinoids, each powdered sample (approximately 0.05 g) was placed in a 15-mL tube and 3 mL of ethylacetate was added. The samples were vortexed for 1 min and then shaken at 50 rpm at 30°C for 24 h. The mixtures were centrifuged at 1000 × g at room temperature for 5 min. An aliquot of the supernatant (1 mL) was diluted 20-fold; then, filtered through a 0.45-μm chromatodisc filter (Kurabo Industries, Ltd.) before the quantitative analysis of capsinoids and capsaicinoids.
HPLC analysis of concentrations of capsinoids and capsaicinoids in breeding and selection
The concentrations of capsiate, dihydrocapsiate, nordihydrocapsiate, capsaicin, dihydrocapsaicin, nordihydrocapsaicin, vanillyl methyl ether, vanillyl ethyl ether, vanillyl propyl ether, and vanillyl butyl ether were measured in extracts by an HPLC analysis. The HPLC analysis was performed using a Waters Alliance 2695 HPLC system equipped with a 2487 dual λ fluorescence detector. The concentrations of capsinoid and capsaicinoid standards were 25, 12.5, 2.5, and 0.5 μM. All standard solutions were prepared by dissolving the appropriate quantity of each compound in ethylacetate.
Samples were chromatographed using a YMC Triart Phenyl plus HPLC column (particle size 3 μm, internal diameter 4.6 mm, length 250 mm; YMC Co., Ltd.). The mobile phase consisted of 70% methanol and 30% water (v/v) at a flow rate of 0.7 mL/min. The total analysis run time was 60 min, and excitation and emission wavelengths were 280 and 320 nm, respectively. The autosampler was maintained at 5°C and the column at 40°C, and the injection volume was 5 μL. Each analysis was performed twice. The capsinoid and capsaicinoid concentrations of pepper fruit extracts were expressed as mg/g dry weight (D.W.) of fruit. The total capsinoid concentration represented the sum of capsiate, dihydrocapsiate, and nordihydrocapsiate, and the total capsaicinoid concentration represented the sum of capsaicin, dihydrocapsaicin, and nordihydrocapsaicin.
Extraction and saponification of carotenoids for HPLC
Extraction and saponification were performed in the dark or subdued light. Mature pepper fruits were harvested approximately 7 weeks after flowering for the extraction of carotenoids. The other sampling, drying, and powdering methods are the same ways of “Extraction of capsinoids and capsaicinoids for a HPLC analysis in breeding and selection.” To extract carotenoids, each powdered sample (approximately 0.1 g) was placed in a 15-mL tube and 3-mL of ethylacetate was added. Samples were vortexed for 1 min and then shaken at 50 rpm at 30°C in a water bath for 3 h. The mixtures were centrifuged at 1000 × g at room temperature for 5 min. An aliquot of the supernatant was filtered through a 0.45-μm chromatodisc filter (Kurabo Industries, Ltd.).
Aliquots (1 mL) of the extracts were vacuum-dried in a tube for 1 h using an SPD2010 SpeedVac System vacuum evaporator (ThermoSavant), then treated with 5% methanolic potassium hydroxide solution (0.1 mL) and shaken at 50 rpm at room temperature for 24 h. Water (0.2 mL) was then added and the mixture was extracted with ethylacetate (0.3 mL × 3). Combined ethylacetate extracts were vacuum dried in a tube for 3 h using the SPD2010 SpeedVac System vacuum evaporator, and the residue was then dissolved in ethylacetate (0.1 mL) and filtered through a 0.45-μm chromatodisc filter for the HPLC analysis.
HPLC analysis of carotenoid contents
The concentrations of carotenoids were measured by the same system and column of “HPLC analysis of concentrations of capsinoids and capsaicinoids in breeding and selection.” The mobile phase consisted of methanol-water-ammonium acetate (9199:800:1, w/w) at a flow rate of 0.7 mL/min. The total analysis run time was 65 min, and the monitor wavelength was 475 nm. The autosampler was maintained at 5°C and the column at 40°C, and the injection volume was 10 μL. Each analysis was performed twice. The concentrations of carotenoid standards were 100, 50, 25, 5, and 1 μg/mL. All standard solutions were prepared by dissolving the appropriate quantity of each compound in ethylacetate. The carotenoid concentrations of pepper fruit extracts were expressed as mg/g D.W. of fruit. The total carotenoid concentration represented the sum of capsorubin, capsanthin, anteraxanthin, beta-apo-carotene, and beta-carotene7,8.
Isolation of DNA, PCR, and dCAPS analysis
Total DNA was isolated from approximately 100 mg of young leaves using a DNeasy Plant Mini Kit (Qiagen) according to the manufacturer’s protocol. Total DNA (100 ng) was PCR‐amplified using the Gene Amp PCR system 9700 (Applied Biosystems). The PCR program was as follows: at 95°C for 30 sec, at 54°C for 30 sec, and 72°C for 2 min (30 cycles), and at 72°C for 7 min (one cycle). The p-AMT and p-amt Ají Dulce gene-specific primers for amplification were as follows: forward (5ʹATATGTAGGTGAAGATGGTGTGGTATTACAATAATGCCCTTGGGAGCCCA3ʹ) and reverse (5ʹATTTACCCAGAGAGACCAGC 3ʹ) [Citation33,Citation34]. The p-amt Ají Dulce allele contained an 8-bp insertion (GCCACACC) in the sixth exon, and the PCR-amplified production of the p-amt Ají Dulce allele was GCCCACACCGC, whereas that of p-AMT was not. The PCR-amplified production from the p-amt Ají Dulce allele was digested by MwoI in a derived cleaved amplified polymorphic sequence (dCAP) analysis, whereas the PCR-amplified production from p-AMT was not digested [Citation33,Citation34]. The PCR products were digested using Mwo1 (New England BioLabs https://www.nebj.jp/, digesting GCNNNNNNNGC) according to the manufacturer’s protocol, and the digested fragments were separated by agarose gel electrophoresis [Citation33,Citation34].
Synthesis of vanillyl alcohol and vanillyl ether from powders of selected lines and alcohols
Methyl alcohol, ethyl alcohol, 1-propanol, 1-butyl alcohol, and NaOH were purchased from Nacalai Tesque. Mature fruit was harvested approximately 7 weeks after flowering. To minimize sample variations, at least five fruits were pooled from each plant. The leaves and stem ends were removed and discarded, and harvested fresh fruit was stored immediately at −80°C. Fruits were lyophilized for 3 days using an FDU‐2100 lyophilizer (Tokyo Rikakikai Co., Ltd.) and then ground to a fine powder using a blender. The powder of each pepper (0.1 g) was dissolved in an appropriate alcohol (9.9 mL) and 1 M NaOH (0.1 mL) was added dropwise to the solution under 20°C. The mixture was stirred for 3 h under 20°C, followed by the addition of 1 M HCl (0.1 mL) to acidify the solution. The mixtures were centrifuged at 1000 × g at room temperature for 5 min. An aliquot of the supernatant (1 mL) was diluted 20-fold, then filtered through a 0.45-μm chromatodisc filter (Kurabo Industries, Ltd.) before the quantitative analysis of capsinoids, vanillyl alcohol, and vanillyl ethers.
HPLC analysis of concentrations of capsinoids, vanillyl alcohol, and vanillyl ether in the production from fruits
The concentrations of capsiate, dihydrocapsiate, nordihydrocapsiate, vanillyl methyl ether, vanillyl ethyl ether, vanillyl propyl ether, and vanillyl butyl ether were measured in extracts by the same method of “ HPLC analysis of concentrations of capsinoids and capsaicinoids in breeding and selection.” Each analysis was performed twice. The capsinoid, vanillyl alcohol, and vanillyl ether concentrations of pepper fruit extracts were expressed as mg/g dry weight (D.W.) of fruit.
Sensory studies
A panel of six experienced assessors (three males and three females) experienced in the sensory analysis of foods and spices was established [Citation28]. Tests were always performed before midday (between 10:00 and 11:30). To adapt the organoleptic expertise of our panel to capsicum tastings, assessors performed a preliminary study in which they judged the taste and aroma of cuttings or powders from the mature fruit of the four new lines and two original lines in different sessions. Harvested fresh fruit was stored immediately at −80°C. Fresh fruit (cut fruit) was cut immediately after thawing. Three types of powders were prepared. 1) Powders were lyophilized for 3 days using the FDU-2100 lyophilizer and ground into a fine powder using the blender (“fruit powder”). 2) Alpha-cyclodextrin or beta-cyclodextrin (1% w/w, vs. fresh fruit) was added to the powders, which were cut, mixed for 1 h under 4°C, and frozen. Powders were then lyophilized for 3 days using the FDU-2100 lyophilizer and ground into a fine powder using the blender (“fruit powders with cyclodextrin added”). 3) Ethanol (25% w/w, vs. fresh fruit) and 1 M NaOH solution (0.040% NaOH, w/w vs. fresh fruit) were added to the powders, which were cut and mixed for 2 h under 4°C, followed 1 M HCl solution (0.037% HCl, w/w, vs. fresh fruit), and mixed again for 1 min. Alpha-cyclodextrin or beta-cyclodextrin (1% w/w, vs. fresh fruit) was then added, and powders were mixed for 1 h under 4°C, frozen, lyophilized for 3 days using the FDU-2100 lyophilizer, and then ground into a fine powder using the blender (“fruit powders with ethanol and cyclodextrin added”).
After aromas had been profiled with free descriptor terms (free-choice profiling), 14 adequate descriptors were selected to profile the overall aroma of accessions: (1) vegetative/canned green bean, (2) vegetative/fresh-cut green glass, (3) vegetative/paprika, bell pepper, (4) earthy/dusty (5) floral/rose, (6) fruity/citrus, grapefruit, (7) fruity tree/peach, apple, (8) fruity-tropical/banana, melon, pineapple, (9) fruity-berry/strawberry, (10) fruity-dry strawberry jam, prune, fig, (11) woody-resinous/vanilla beans, (12) caramel-sweet/butterscotch, (13) pungent-hot/spicy, black pepper, alcohol, and (14) pungent hot 5 min after tasting. The intensity of each descriptor was also judged on a five-point scale (0 = very weak, 1 = weak, 2 = moderate, 3 = strong, 4 = very strong).
Results
Capsaicinoid and capsinoid concentrations and fruit weights of original lines
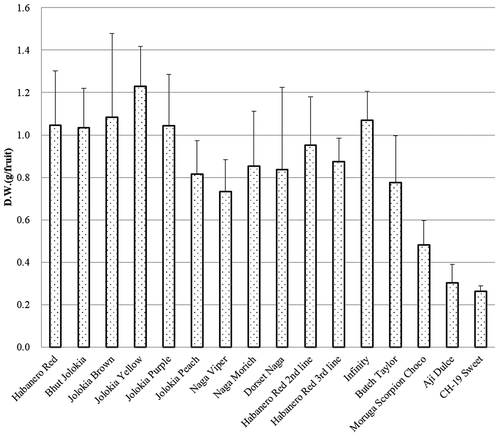
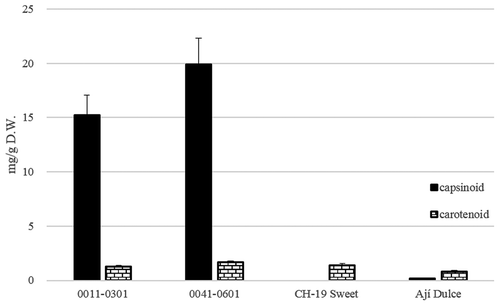
The pungent cultivar, Habanero Red (3 lines), Bhut Jolokia, Jolokia Brown, Jolokia Yellow, Jolokia Purple, Jolokia Peach, Naga Viper, Naga Morich, Dorset Naga, Infinity Butch Tayler, and Moruga Scorpion Choco mainly contained capsaicinoids and produced capsinoids in trace amounts. Bhut Jolokia, Nagaviper, Naga Morich, Habanero Red 2nd line, Infinity, Butch Taylor, and Moruga Scorpion Choco contained more than 30 mg capsaicinoids/g D.W. Among the mildly pungent cultivars, Ají dulce contained higher concentrations of capsinoids than capsaicinoids; however, the concentration of capsinoids was lower than 2 mg/g D.W. ().
Figure 2. Capsaicinoid and capsinoid contents of Capsicum cultivated species (original species). The bar indicates the standard deviation (n = 5).
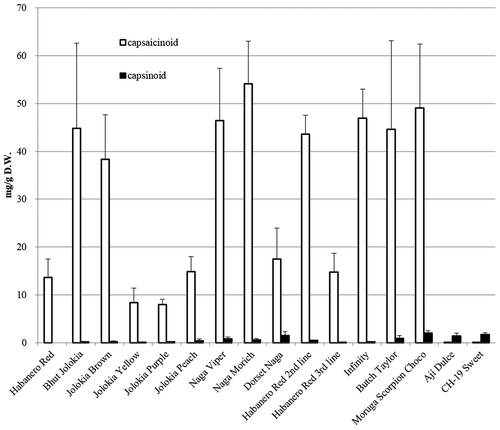
The D.W. of these lines ranged between 0.4 and 1.3 g. Habanero Red, Bhut Jolokia, Jolokia Brown, Jolokia Yellow, Jolokia Purple, and Infinity were more than 1 g (). The D.W. of the Bhut Jolokia and Infinity lines was higher than 1 g in and contained more than 30 mg capsaicinoids/g.
Capsaicinoid and capsinoid concentrations in F1 lines and selection from F1 lines
We selected the F1 generation from a hybrid cross between pungent cultivators (Habanero Red, Bhut Jolokia, or Infinity) and Ají dulce. We analyzed 43 F1 lines between Habanero Red and Ají dulce using HPLC, and found that all plants mainly produced capsaicinoids. The CH-19 Sweet and Ají dulce lines had mutations in putative aminotransferase (p-amt), and these mutations may suppress the formation of vanillylamine from vanillin, instead of which vanillyl alcohol may be produced, in turn, they mainly accumulated capsinoids in the biosynthetic pathway of capsaicinoids and capsinoids in Capsicum. Since the p-amt gene is recessive, F1 lines mainly produced capsaicinoids. We also analyzed 8 F1 lines between Bhut Jolokia and Ají dulce and 14 F1 lines between Infinity and Ají dulce using HPLC, and the results obtained showed that all plants mainly produced capsaicinoids. The F1 lines between Habanero Red and Ají dulce, the F1 lines between Bhut Jolokia and Ají dulce, and the F1 lines between Infinity and Ají dulce contained capsaicinoid concentrations of 8.82, 12.03, and 12.94 mg/g D.W., respectively, and capsinoid concentrations of 0.30, 0.43, and 0.38 mg/g D.W., respectively. Capsaicinoid concentrations in F1 line fruit depended on those in the original line. We selected the F1 line (Jre02-05) of the F2 parent (from Bhut Jolokia and Ají dulce) with the largest concentration of capsaicinoids (13.72 mg/g D.W.), and also selected the F1 line (Inf01-03) of the F2 parent (from Infinity and Ají dulce) with the largest concentration of capsaicinoids (20.09 mg/g D.W.).
Capsaicinoid and capsinoid concentrations in F2 lines and selection from F2 lines
We selected the F2 generation from the F1 lines (Jre02-05 from Bhut Jolokia and Ají dulce) using the dCAPS marker method of loss-of-function p-AMT alleles (p-amt). We found that 7 out of 30 plants had the p-amt/p-amt gene. We analyzed 7 F2 lines (p-amt/p-amt) between Bhut Jolokia and Ají dulce using HPLC, and these plants mainly produced capsinoids. Similarly, we selected the F2 generation from the F1 lines (Inf01-03 from Infinity and Ají dulce) using the dCAPS marker method of loss-of-function p-AMT alleles (p-amt). We found that 12 out of 47 plants had the p-amt/p-amt gene (p-amt). We analyzed 12 F2 lines (p-amt/p-amt) between Infinity and Ají dulce using HPLC, and all plants mainly produced capsinoids. Therefore, the loss-of-function alleles of p-amt contributed to the generation of mild pungency and capsinoid biosynthesis in C. chinense.
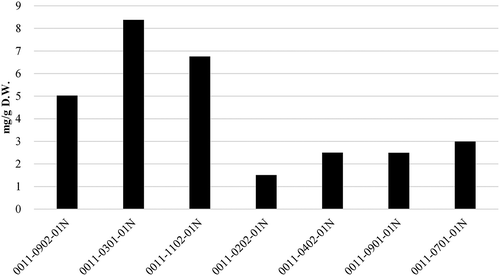
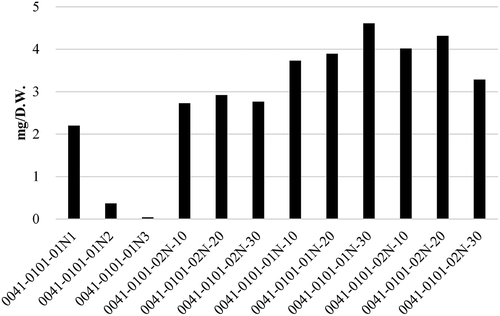
As shown in , 0011–0301-01N was selected from 7 F2 lines (p-amt/p-amt) between Bhut Jolokia and Ají dulce with the highest concentration of capsinoids (8.38 mg/g D.W.), and as shown in , 0041–0101-01N-30 was selected from 12 F2 lines (p-amt/p-amt) between Infinity and Ají dulce with the highest concentration of capsinoids (4.61 mg/g D.W.).
Fruit numbers and capsaicinoid and capsinoid concentrations in F3 lines and selection from F3 lines
We selected the F3 generation from F2 lines (0011–0301-01N crossed from Bhut Jolokia and Ají dulce or 0041–0101-01N-30 crossed from Infinity and Ají dulce).
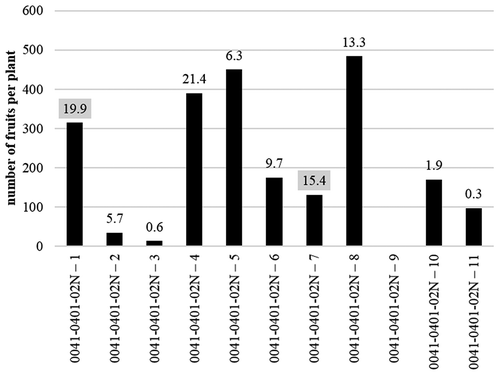
We transplanted 36 F3 lines self-pollinated from 0011–0301-01N and measured how many fruits each line had after 20 weeks. We selected lines with more than 100 fruits, and measured capsinoid concentrations in the fruits of these lines. As shown in , we selected 0011–0301-01N-18 and 0011–0301-01N-19, which had high concentrations of capsinoids (15.2 mg/g D.W. and 9.6 mg/g D.W., respectively). Similarly, we transplanted 11 F3 lines self-pollinated from 0041–0101-01N-30, and measured how many fruits each line had after 20 weeks. We selected lines with more than 100 fruits, and measured capsinoid concentrations in the fruits of these lines. As shown in , 001–0401-02N-4 had many fruits and high capsinoid concentration (21.9 mg/g D.W.). However, the fruits of 0041–0401-02N-4 had no seeds, resulting in a self-sterile plant. We selected 0041–0401-02N-1 and 0041–0401-02N-7, which had high capsinoid concentrations (19.9 mg/g D.W. and 15.4 mg/g D.W., respectively).
Figure 6. The fruit numbers of each F3 lines bred from Bhut Jolokia crossed with Ají Dulce. The heights of the columns are the fruit numbers of each line. The numbers on the columns are the capsinoid concentrations of each line.
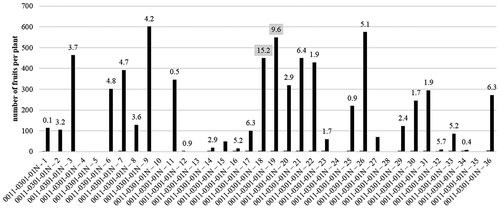
Figure 7. The fruit numbers of each F3 lines bred from Infinity crossed with Ají Dulce. The heights of the columns are the fruit numbers of each line. The numbers on the columns are the capsinoid concentrations of each line.
We reselected each line with more fruits and a stable color in mature fruit twice. The following four new lines in C. chinense were stable by repeating selection and self-pollination, 0011–0301-01N-19 was developed and named ‘Dieta0011-0301ʹ (0011–0301) ( and ). In the same ways, 0011–0301-01N-18, 0041–0401-02N-1 and 0041–0401-02N-7 were developed and named Dieta0011-0602 (0011–0602), Dieta 0041–0401 (0041-0401), and Dieta0041-0601 (0041–0601), respectively. 0011–0301 is a lineage from Bhut Jolokia and has red fruit in the maturing stage. 0011–0602 is a lineage from Bhut Jolokia and has orange fruit in the maturing stage. 0041–0401 is a lineage from Infinity and has orange fruit in the maturing stage. 0041–0601 is a lineage from Infinity and has orange fruit in the maturing stage.
Figure 8. Capsaicinoid and capsinoid concentrations of new bred lines (Dieta0011-0301, Dieta 0011–0602, Dieta 0041–0401, and Dieta 0041–0601), CH-19 Sweet, and Ají Dulce.
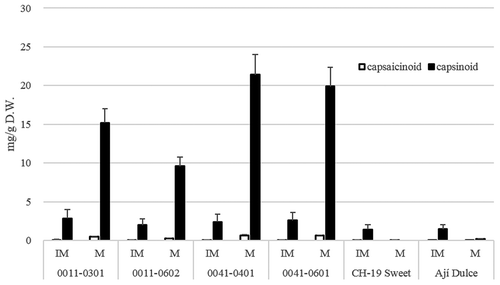
Capsaicinoid and capsinoid concentrations in selected lines
The initially discovered capsinoid-containing cultivar, C. annuum strain, CH-19 Sweet, mainly contained capsinoids in immature fruit (after 4 weeks of flowering). However, we did not detect capsinoids in mature fruit (after 7 weeks of flowering). In contrast, C. chinense mildly pungent cultivars contained high capsinoid concentrations in mature fruit. 0011–0301 descended from Bhut Jolokia and 0041–0601 descended from Infinity had red mature fruit that contained both carotenoids and capsinoids ().
Sensorial evaluation of fresh fruit and powders from selected lines
The sniffing test revealed few differences in aroma between 0011–0301 () and 0011–0602 () descended from Bhut Jolokia. However, the pungency of them was significantly different from that of Bhut Jolokia original. In this case, 0011–0301 () exhibited weaker and more short-term pungency than the Bhut Jolokia original. The loss of pungency may have been due to the hydrolysis of capsinoids in the mouth. Fresh fruit from 0011–0301 had strong aromas of (fruity-tree/peach, apple) and (fruity-tropical/banana, melon, pineapple). These aromas were similar to fruity aromas. Fresh fruit from 0011–0301 had aromas of (fruity/citrus, grapefruit) and (earthy/dusty). The fruity aromas of 0011–0301 were lost in the lyophilized powder of fruit within 6 months at room temperature. However, they were not lost following the addition of alpha-cyclodextrin or beta-cyclodextrin before lyophilization. The aromas of the fruit and alpha-cyclodextrin-added freeze-dried powders of 0011–0301 were similar to that of fresh fruit. When fruit from 0011–0301 was mixed with ethanol and NaOH, it produced caramel-sweet and woody-resinous aromas, and showed more pungent-hot, and lost its vegetative aroma. These aromas were not lost following the addition of alpha-cyclodextrin or beta-cyclodextrin before lyophilization.
Figure 10. Odor profiles of different processed materials in Delta0011-0301. (1) Fresh fruit, (2) freeze-dried fruit powder, (3) freeze-dried fruit powder with alpha-cyclodextrin added, (4) freeze-dried fruit powder with beta-cyclodextrin added, (5) freeze-dried fruit powder with EtOH and alpha-cyclodextrin added, (6) freeze-dried fruit powder with EtOH and beta-cyclodextrin added, and (7) fresh Bhut Jolokia original fruit. n = 6.
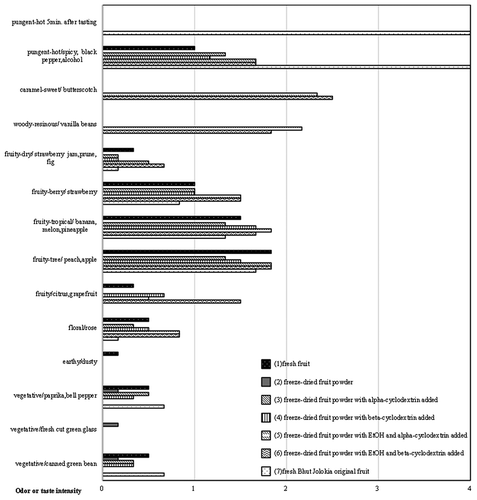
Figure 11. Odor profiles of different processed materials in Delta0011-0602. (1) Fresh fruit, (2) freeze-dried fruit powder, (3) freeze-dried fruit powder with alpha-cyclodextrin added, (4) freeze-dried fruit powder with beta-cyclodextrin added, (5) freeze-dried fruit powder with EtOH and alpha-cyclodextrin added, (6) freeze-dried fruit powder with EtOH and beta-cyclodextrin added, and (7) fresh Bhut Jolokia original fruit. n = 6.
0011–0602 () showed similar results to those obtained for 0011–0301 without its color. It showed weak and short-term pungency. The aroma of 0011–0602 for (fruity-tropical/banana, melon, pineapple) was slightly stronger than those of 0011–0301. The other aromas and flavors of the line 0011–0602 were similar to those of the line 0011–0301.
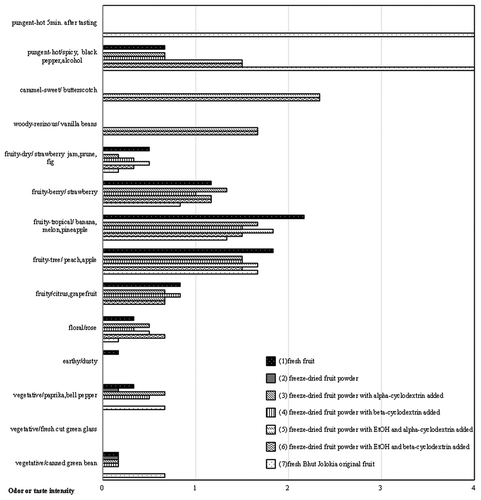
The pungency of 0041–0401 () was weaker and more short term than that from the Infinity original. Fresh fruit from 0041–0401 had strong aromas, such as (fruity-citrus, grapefruit). These aromas were exotic and fruity aromas and similar to those of the Infinity original. The stability of the aromas in 0041–0401 was consistent with those in 0011–0301 and 0011–0602, and the aromas of powders made from fruit to which alpha-cyclodextrin or beta-cyclodextrin had been added were not lost.
Figure 12. Odor profiles of different processed materials in Delta0041-0401. (1) Fresh fruit, (2) freeze-dried fruit powder, (3) freeze-dried fruit powder with alpha-cyclodextrin added, (4) freeze-dried fruit powder with beta-cyclodextrin added, (5) freeze-dried fruit powder with EtOH and alpha-cyclodextrin added, (6) freeze-dried fruit powder with EtOH and beta-cyclodextrin added, and (7) fresh Infinity original fruit. n = 6.
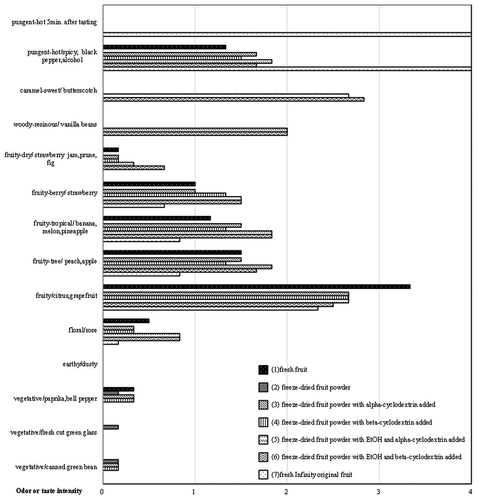
The aromas of 0041–0601 () were similar to those of 0041–0401 and Infinity, and it was not pungent. The fruity-citrus aromas of 0041–0601 were slightly weaker than those of 0041–0401. The other aromas, tastes, and stability of 0041–0601 were similar to those of 0041–0401.
Figure 13. Odor profiles of different processed materials in Delta0041-0601. (1) Fresh fruit, (2) freeze-dried fruit powder, (3) freeze-dried fruit powder with alpha-cyclodextrin added, (4) freeze-dried fruit powder with beta-cyclodextrin added, (5) freeze-dried fruit powder with EtOH and alpha-cyclodextrin added, (6) freeze-dried fruit powder with EtOH and beta-cyclodextrin added, and (7) fresh Infinity original fruit. n = 6.
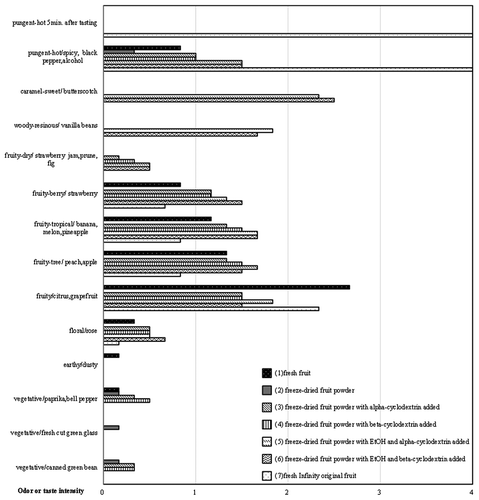
Each of the following lines: 0011–0301, 0011–0602, 0041–0401, 0041–0601, the Bhut Jolokia original, and Infinity original, had a weak earthy/dusty aroma.
Synthesis of vanillyl alcohol and vanillyl ether from powders of selected lines and alcohols
Powders made from 0011–0301 or 0041–0401 by mixing with an appropriate alcohol and NaOH solution followed by lyophilization produced vanillyl alcohol and vanillyl ethers (). The products of the fruit powders (probably contained a little water) induced by water, MeOH, EtOH, 1-PrOH, or 1-BuOH were vanillyl alcohol, a vanillyl alcohol/vanillyl methyl ether mixture, vanillyl alcohol/vanillyl ethyl ether mixture, vanillyl alcohol/vanillyl propyl ether mixture, or vanillyl alcohol/vanillyl butyl ether mixture, respectively. On the other hand, the powders made from the Bhut Jolokia or Infinity original did not produce vanillyl alcohol or vanillyl ethers. These results suggested that the capsinoids of the fruit powders of the selected lines were converted into both vanillyl alcohol and vanillyl ethers.
Table 1. Amounts of vanillyl alcohol and vanillyl ethers produced from Capsicum line Powders with solvents and NaOH.
Discussion
Three main results were obtained in the present study. Capsicum lines containing high concentrations of capsinoids were presumably bred from original lines containing high capsaicinoid concentrations by crossing lines with p-AMT alleles (p-amt). Furthermore, lines bred with p-amt/p-amt from C. chinense origins had a strong aroma and very low pungency. In addition, Capsicum lines containing high capsinoid concentrations produced vanillyl ether following the addition of alcohol to their fruits.
We bred and fixed Capsicum lines containing high capsinoid concentrations, namely, Dieta0011-0301 (the cross-breeding line from Bhut Jolokia with red mature fruit), Dieta0011-0602 (the cross-breeding line from Bhut Jolokia with orange mature fruit), Dieta0041-0401 (the cross-breeding line from Infinity with orange mature fruit), and Dieta0041-0601 (the cross-breeding line from Infinity with red mature fruit), from Bhut Jolokia or Infinity containing high capsaicinoid concentrations by crossing Ají Dulce with p-AMT alleles (p-amt/p-amt). In previous studies by Lang et al. [Citation33]., Tanaka et al. [Citation34,Citation42]., Park et al. [Citation35]., and Siyoung [Citation36], the loss-of-function of pAMT resulted in the production of capsinoids, which demonstrated that pAMT is a target for the molecular breeding of capsinoid-producing plants. However, these lines did not contain a higher capsinoid concentration than their original (p-amt/p-amt) [Citation36,Citation42]. The capsinoid contents in these lines were suitable to be consumed raw; however, Capsicum cultivars with a higher content capsinoids are feasible and desirable for industrial applications of capsinoids. The present results support the hypothesis that the creation of loss-of-function mutants of pAMT using Capsicum cultivars with high capsaicinoid concentrations may lead to Capsicum lines producing high concentrations of capsinoids. The results of the present study showed that new Bhut Jolokia and Infinity lines with high capsinoid concentrations may be developed by introducing a dysfunctional pAMT allele. The capsinoid concentrations of these lines were higher than those of Ají Dulce (the p-amt/amt parent), suggesting that cross-breeding between lines containing high concentrations of capsinoids and a dysfunctional pAMT allele is useful for the selection of lines containing high concentrations of capsinoids. Among C. chinense mildly pungent cultivars, 0011–0301 descended from Bhut Jolokia and 0041–0601 descended from Infinity had red mature fruit that contained both carotenoids and capsinoids. Since carotenoids have many health benefits [Citation7,Citation8,Citation53,Citation54], the presence of carotenoids and capsinoids in fruit is advantageous.
Previous studies reported that the concentrations of functional components in the fruit of Capsicum cultivars, such as carotenoids, ascorbic acid, tocopherol, sugars, and organic acids, increased during ripening [Citation55,Citation56]. Thus, high capsinoid concentrations in the mature fruit of the bred lines are useful in the food industry.
C. chinense cultivars, such as Habanero or Bhut Jolokia, are very pungent and have a fruity aroma [Citation27,Citation43,Citation44]. 0011–0301-01 × 01N, 0011–0602-01 × 01N, 0041–0401-02N01N, and 0041–0601-02x01x03N had a strong aroma and very low pungency even though their originals, Bhut Jolokia and Infinity, had intense pungency. Capsaicinoids and ester flavors may be biosynthesized by common pathways [Citation46], and, thus, difficulties have been associated with separating the aroma of esters from strong pungency. The present results indicated that the new lines containing a strong aroma and low pungency were bred from Capsicum cultivars by crossing origins with p-AMT alleles (p-amt/p-amt).
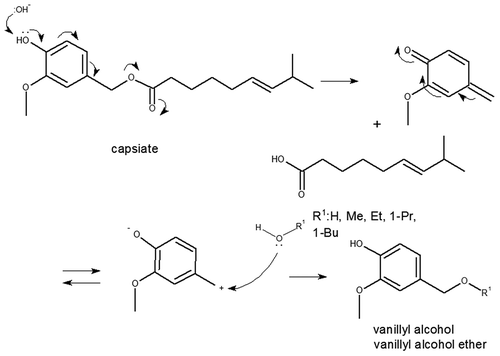
Our bred lines containing high capsinoid concentrations produced vanillyl ether following the addition of alcohol to fruit. Sutoh et al. [Citation47] reported that capsinoids were decomposed in water, methanol, or ethanol, and produced vanillyl alcohol, methyl vanillyl ether, or ethyl vanillyl ether, respectively. They showed that alcohol as a solvent attacked the benzyl cation formed by the cleavage of the benzyl moiety in capsinoids, leading to the formation of the corresponding vanillyl ether. We suggest that Capsicum fruit containing capsinoids produce vanillyl alcohol and vanillyl ethers in situ with the addition of alcohols, which add valuable flavors to fruit (, ). Vanillyl alcohol and vanillyl ethyl ether are famous flavors in vanilla beans [Citation49–Citation52]. The aroma of vanillyl alcohol is sweet, creamy, vanilla, milky, and powdery [Citation49], while that of vanillyl ethyl ether is sweet, rummy, chocolate, creamy, and vanilla [Citation50]. They differ from the flavors of the Capsicum originals. Capsicum lines containing high capsinoid concentrations are useful for the production of a vanilla-like fragrance and add sweet aromas to the original fruit aromas. These aromas may be lost with drying. However, the addition of alpha-cyclodextrin or beta-cyclodextrin to fruit may maintain fruity and sweet aromas. This system will provide new fruity and sweet flavors, and reduce the pungency of C. chinense original species.
Figure 14. Production of vanillyl alcohol or vanillyl alcohol ethers between capsinoids (an illustrated example is a capsiate) and water (or some alcohols). The process of capsinoids reaction is as follows: first, the polarization between the alpha-methylene of the benzyl moiety and the neighboring oxygen atom in capsinoid is increased by the presence of protic and/or polar compounds (water or some alcohols); then, the polarized portion is easily cleaved by means of heterolytic cleavage to give a benzyl cation and a carboxylate anion; finally, the compound attacks the benzyl cation as a nucleophilic reagent, and vanillyl alcohol or vanillyl alcohol ethers are formed.
Author contributions
T.S. and K.N. conceived and designed this study. T.S., M.O. and H.H. participated in the design and execution of all experiments, analyzed the data, and wrote the manuscript. T.S., M.O. and K.N. finally approved the version to be published.
Acknowledgments
The authors are grateful to Dr. Toshihisa Kato, Dr. Yoshio Kawahara, Dr. Ryuji Sugiyama, Dr. Yaqin Lang, Dr. Susumu Yazawa, Masaaki Komatsu, and Keiko Hashima for plant materials, their helpful discussions, and technical assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- DeWitt D, Bosland PW. Peppers of the world: an identification guide. Berkeley: Ten Speed Press; 1996.
- Gbolade AA, Omobuwajo OR, Soremekun RO. Evaluation of the quality of Nigerian chilies for pharmaceutical formulations. J Pharm Biomed Anal. 1997;15(4):545–548.
- Kumar OA, Tata SS. Ascorbic acid contents in chili peppers (Capsicum L.). Not Sci Biol. 2009;1:50–52.
- Howard LR. Antioxidant vitamin and phytochemical content of fresh and processed prepper fruit (Capsicum annuum). In: Wildman REC, editor. Handbook of nutraceuticals and functional foods. London: CRC Press; 2000. p. 165–192.
- Forero MD, Quijano CE, Pino JA. Volatile compounds of chile pepper (Capsicum annuum L. var. glabriusculum) at two ripening stages. Flavour Fragr J. 2009;24(1):25–30.
- Purkayastha J, Alam SI, Gogoi HK, et al. Molecular characterization of Bhut Jolokia the hottest chilli. J Biosci. 2012;37:757–768.
- Ranjith A, Ramesh BN, Ramakrishna KM, et al. Red pepper (Capsicum annuum) carotenoids as a source of natural food colors: analysis and stability—a review. J Food Sci Technol. 2015;52(3):1258–1271.
- Nagata M, Yoshida C, Matsunaga H. Changes in the expression of carotenoid biosynthetic genes during light irradiation of sweet pepper (Capsicum annuum L.) fruit after harvest. Hort Res. 2015;14(4):391–396. Japanese.
- Surh Y, Lee RC, Park K, et al. Chemoprotective effects of capsaicin and diallyl sulfide against mutagenesis or tumorigenesis by vinyl carbamate and N-nitrosodimethylamine. Carcinogenesis. 1995;16(10):2467–2471.
- Chanda S, Erexson G, Riach C, et al. Genotoxicity studies with pure trans-capsaicin. Mutat Res. 2004;557:85–97.
- Anandakumar P, Kamaraj S, Jagan S, et al. Capsaicin provokes apoptosis and restricts benzo(a)pyrene induced lung tumorigenesis in Swiss albino mice. Int Immunopharmacol. Elsevier B.V. 2013;17:254–259.
- Oyagbemi AA, Saba AB, Azeez OL. Capsaicin: a novel chemopreventive molecule and its underlying mechanism of action. Indian J Cancer. 2010;47(1):53–58.
- Spiller F, Alves MK, Vieira SM, et al. Anti-inflammatory effects of red pepper (Capsicum baccatum) on carrageenan- and antigen-induced inflammation. J Pharm Pharmacol. 2008;60(4):473–478.
- Careaga M, Fernandez E, Dorantes L, et al. Antibacterial activity of Capsicum extract against Salmonella typhimurium and Pseudomonas aeruginosa inoculated in raw beef meat. Int J Food Microbiol. 2003;83(3):331–335.
- Alvarez-Parrilla E, De La Rosa LA, Amarowicz R, et al. Antioxidant activity of fresh and processed Jalapeño and Serrano peppers. J Agric Food Chem. 2011;59(1):163–173.
- Bosland PW, Votava EJ. Peppers: vegetable and spice Capsicums. New York: CABI Publishing; 2000.
- Bennett DJ, Kirby GW. Constitution and biosynthesis of capsaicin. J Chem Soc C. 1968;4:442–446.
- Iwai K, Watanabe T. Red peppers. 2nd ed. Japan: SAIWAI SHOBO; 2008. Japanese.
- Fujiwake H, Suzuki T, Oka S, et al. Enzymatic formation of capsaicinoid from vanillylamine and iso-type fatty acids by cell-free extracts of Capsicum annuum var. annuum cv. karayatsubusa. Agric Biol Chem. 1980;44:2907–2912.
- Fujiwake H, Suzuki T, Iwai K. Capsaicinoid formation in the protoplast from the placenta of Capsicum fruits. Agric Biol Chem. 1982;46:2591–2592.
- Suzuki T, Kawada T, Iwai K. Biosynthesis of acyl moieties of capsaicin and its analogues from valine and leucine in Capsicum fruits. Plant Cell Physiol. 1981;22:23–32.
- Curry J, Aluru M, Mendoza M, et al. Transcripts for possible capsaicinoid biosynthetic genes are differentially accumulated in pungent and non-pungent Capsicum spp. Plant Sci. 1999;148(1):47–57.
- Aluru MR, Mazourek M, Landry LG, et al. Differential expression of fatty acid synthase genes, Acl, Fat and Kas, in Capsicum fruit. J Exp Bot. 2003;54(388):1655–1664.
- Blum E, Mazourek M, O’Connell M, et al. Molecular mapping of capsaicinoid biosynthesis genes and quantitative trait loci analysis for capsaicinoid content in Capsicum. Theor Appl Genet. 2003;108(1):79–86.
- Kawada T, Hagihara K, Iwai K. Effects of capsaicin on lipid metabolism in rats fed a high fat diet. J Nutr. 1986;116:1272–1278.
- Kawada T, Sakabe S, Aoki N, et al. Intake of sweeteners and pungent ingredients increases the thermogenin content in brown adipose tissue of rat. J Agric Food Chem. 1991;39(4):651–654.
- Sarpras M, Rashmi G, Vineet S, et al. Comparative analysis of fruit metabolites and pungency candidate genes expression between Bhut Jolokia and other Capsicum species. PLoS One. 2016 Dec 9. DOI:10.1371
- Adrian RB, Hubert K, Carmen GM, et al. HS-SPME comparative analysis of genotypic diversity in the volatile fraction and aroma-contributing compounds of Capsicum fruits from the annuum-chinense-frutescens complex. J Agric Food Chem. 2010;58(7):4388–4400.
- Hubert K, Adrian RB, Siegfried N, et al. Volatile and capsaicinoid composition of ajı(Capsicum baccatum) and rocoto (Capsicum pubescens), two Andean species of chili peppers. J Sci Food Agric. 2011;91(9):1598–1611.
- Yazawa S, Suetome N, Okamoto K, et al. Content of capsaicinoids and capsaicinoid-like substance in fruit of pepper (Capsicum annuum L.) hybrids made with CH-19 sweet as a parent. J Japan Soc Hort Sci. 1989;58:601–607.
- Kobata K, Todo T, Yazawa S, et al. Novel capsaicinoid-like substances, capsiate and dihydrocapsiate, from the fruits of nonpungent cultivar, CH-19 sweet, of pepper (Capsicum annuum L.). J Agric Food Chem. 1998;46(5):1695–1697.
- Kobata K, Sutoh K, Todo T, et al. Nordihydrocapsiate, a new capsinoid from the fruit of a nonpungent pepper, Capsicum annuum. J Nat Prod. 1999;62(2):335–336.
- Lang Y, Kisaka H, Sugiyama R, et al. Functional loss of pAMT results in biosynthesis of capsinoids, capsaicinoid analogs, in Capsicum annuum cv. CH-19 Sweet. Plant J. 2009;59(6):953–961.
- Tanaka Y, Hosokawa M, Miwa T, et al. Novel loss-of-function putative aminotransferase Alleles cause biosynthesis of capsinoids, nonpungent capsaicinoid analogues, in mildly pungent chili peppers (Capsicum chinense). J Agric Food Chem. 2010;58(22):11762–11767.
- Park YJ, Nishikawa T, Minami M, et al. A low‑pungency S3212 genotype of Capsicum frutescens caused by a mutation in the putative aminotransferase (p‑AMT) gene. Mol Genet Genomics. 2015;290(6):2217–2224.
- Siyoung J, Koeun H, Yeoung DJ, et al. Substitution of a dysfunctional pAMT Allele results in low-pungency but high levels of capsinoid in Capsicum chinense ‘Habanero’. Plant Breed Biotech. 2015;3(2):119~128.
- Tanaka Y. Research on capsiconinoid contents, nonpungent capsaicinoid analogues, in Capsicum cultivars. Course Appl Plant Sci Okayama Univ Scientific Achievement Repository. 2014;103:37–43. Japanese.
- Ohnuki K, Niwa S, Maeda S, et al. CH-19 Sweet, a non-pungent cultivar of pepper, increases body temperature and oxygen consumption in humans. Biosci Biotechnol Biochem. 2001;65(9):2033–2036.
- Masuda Y, Haramizu S, Oki K, et al. Upregulation of uncoupling proteins by oral administration of capsiate, a nonpungent capsaicin analog. J Appl Physiol. 2003;95(6):2408–2415.
- Rosa A, Deiana M, Casu V, et al. Antioxidant activity of capsinoids. J Agric Food Chem. 2002;50:7396–7401.
- Macho A, Lucena C, Sancho R, et al. Non-pungent capsaicinoids from sweet pepper: synthesis and evaluation of the chemopreventive and anticancer potential. Eur J Nutr. 2003;42(1):2–9.
- Tanaka Y, Yoneda H, Hosokawa M, et al. Application of marker-assisted selection in breeding of a new fresh pepper cultivar (Capsicum annuum) containing capsinoids, low-pungent capsaicinoid analogs. Sci Hortic. 2014;165:242–245.
- Murakami Y, Iwabuchi H, Ohba Y, et al. Analysis of volatile compounds from chili peppers and characterization of Habanero (Capsicum chinense) volatiles. J Oleo Sci. 2019;68(12):1251–1260.
- Henderson N. Record-breaking’ chilli is hot news. BBC [Internet]. 2011 Feb 19. Available from: https://www.bbc.co.uk/news/uk-12505344
- Nuez F, Gil R, Costa J. El Cultivo de Pimientos, Chiles y Ajı´es [The crop of peppers, chilies, and Ajı´es]. Madrid (Spain): Mundi Prensa; 2003. Spanish.
- Koeda S, Sato K, Tomi K, et al. Analysis of non-pungency, aroma, and origin of a Capsicum chinense cultivar from a Caribbean Island. J Jpn Soc Hort Sci. 2014;83(3):244–251.
- Sutoh K, Kobata K, Watanabe T. Stability of capsinoid in various solvents. J Agric Food Chem. 2001;49:4026–4030.
- Sinha AK, Sharma UK, Sharma N. A comprehensive review on vanilla flavor: extraction, isolation and quantification of vanillin and others constituents. Int J Food Sci Nutr. 2008;59(4):299–326.
- The good scents company information system: para-vanillyl alcohol [Internet].USA: The Good Scents Company. 1994. [cited 2020 Feb 20]. Available from: http://www.thegoodscentscompany.com/data/rw1037781.html
- The good scents company information system: vanillyl ethyl ether [Internet].USA: The Good Scents Company. 1994 [cited 2020 Feb 20]. Available from: http://www.thegoodscentscompany.com/data/rw1434631.html
- Attokaran M. Natural food flavors and colorants. 2nd ed. USA: John Wiley & Sons; 2017. p. 368–374.
- Khan IA, Abourashed EA. Leung’s Encyclopedia of common natural ingredients used in food, drugs and cosmetics. USA: John Wiley & Sons; 2009. p. 616–617.
- Inakuma T. Study of carotenoid activity in vegetables: application to food development. Nippon Shokuhin Kagaku Kogaku Kaishi. 2015;62(6):263–273. Japanese.
- Saga K, Nakamura H. Changes in the contents of some constituents in a maturing sweet pepper(Capsicum annuum L.)fruits of some cultivars. Bull Faculty Agric Life Sci Hirosaki Univ. 2005;7(1):21–25. Japanese.
- Rahman FMM, Buckle KA. Pigment changes in capsicum cultivars during maturation and ripening. J Food Technol. 1980;15:241–249.
- Yahlia EM, Padilla MC, Aguilar GC. Ascorbic acid content in relation to ascorbic acid oxidase activity and polyamine content in tomato and bell pepper fruits during development, maturation and senescence. LWT - Food Sc Technol. 2001;34(7):452–457.