ABSTRACT
Here, we prepared the novel combined adjuvants, CTB as intra-molecular adjuvant, CpG and aluminum hydroxide (Alum) to strengthen the immunogenicity of clumping factor A221-550 of Staphylococcus aureus (S. aureus). The protein-immunoactive results showed CTB-ClfA221-550 elicited the strong immune responses to serum from mice immunized with CTB and ClfA221-550, respectively. The mice immunized with CTB-ClfA221-550 plus CpG and Alum adjuvant exhibited significantly stronger CD4+ T cell responses for IFN-γ, IL-2, IL-4, and IL-17 and displayed the higher proliferation response of splenic lymphocytes than the control groups, in addition, these mice generated the strongest humoral immune response against ClfA221-550 among all groups. Our results also showed CTB-ClfA221-550 plus CpG and Alum adjuvant obviously increased the survival percentage of the mice challenged by S. aureus. These data suggested that the novel combined adjuvants, CTB, CpG, and Alum, significantly enhance the immune responses triggered with ClfA221-550, and could provide a new approach against infection of S. aureus.
Abbreviations
CTB: Cholera Toxin B; CpG: Cytosine preceding Guanosine; ODN: Oligodeoxynucleotides; Alum: Aluminum hydroxide; TRAP: Target of RNAIII-activating Protein; TLR9: Toll-like Receptor 9; TMB: 3, 3ʹ, 5, 5ʹ-tetramethylbenzidine; mAbs: Monoclonal Antibodies; OD: Optical Densities; S. aureus: Staphylococcus aureus; ClfA: Clumping factor A; FnBPA: Fibronection-binding protein A; IsdB: Iron-regulated surface determinant B; SasA: Staphylococcus aureus Surface Protein A; GapC: Glycer-aldehyde-3-phosphate dehydrogenase-C
Graphic abstract
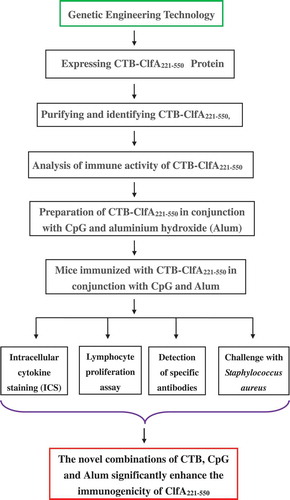
The research outline of the combined adjuvants boosted the immunogenicity of clumping factor A221-550.
Staphylococcus aureus (S. aureus) has become the major pathogen associated with human-related pneumonia, endocarditis, septicemia, medical devices, and artificial joint infections [Citation1–Citation3], and in animals, it also causes a variety of infections, such as mastitis in bovine and sheep, and canine pyoderma [Citation4–Citation6], etc. Over the years, antibiotic therapy has been regarded as the main method of preventing infection of S. aureus, but the excessive use of antibiotics has leaded to the increasing emergence of resistant strains in many countries and regions [Citation7–Citation9]. Therefore, developing the new effective vaccine approaches would be a promising strategy to treat infection of S. aureus.
S. aureus generates dozens of surface proteins that mediate the binding of bacteria to plasma proteins and extracellular matrix components, promoting its colonization and infection at different locations in vivo [Citation10]. These surface proteins, including ClfA, FnBPA, IsdB, SasA, GapC [Citation11–Citation14], perform the pivotal role during infection of S. aureus and have been deemed to be candidate targets for vaccines against S. aureus. In the stages of infection of S. aureus, one of the most important adhesives is the clumping factor A (ClfA) [Citation15], causing S. aureus enveloped by fibrinogen and fibrin to resist phagocytosis [Citation16,Citation17]. ClfA is a greatly conserved surface protein among S. aureus isolates, whose intrinsic characteristics make it an effective immunogen, additionally, mice immunized with adhesion zone A of ClfA were less likely to suffer from severe arthritis caused by S. aureus [Citation3,Citation18]. Our previous research has shown the immunodominant domain of ClfA221-550 could induce protective immune responses in mice, but the ClfA221-550 immunogenicity was still needed to be further enhanced to effectively defend infection of S. aureus.
Cholera toxin B subunits (CTB), as intra-molecular adjuvant, can specifically bind to ganglioside GM1 on cell surface to provide target effects. CTB plays a variety of immunological activations. Lee’s study showed that CTB interacts with GM1 to induce T-helper type 17 (Th17) cells production [Citation19]. Meza-sanchez et al. demonstrated that CTB induced Th17 cell differentiation by increasing interleukin 6 (IL-6) and transforming growth factor-β (TGF-β) secretion [Citation20]. Later, Thiam et al. confirmed that CTB promoted antigen-specific Th17 cells differentiation and development [Citation21]. Therefore, CTB can improve Th17-cell-mediated immune response. Several studies have shown that the cytosine preceding the guanosine (CpG) oligodeoxynucleotides (ODN) adjuvant, as short synthetic cDNA consisting of unmethylated CG dinucleotides, which binds Toll-like receptor 9 (TLR9), activating B cells, dendritic cells and inducing Th1-cell-mediated immune responses [Citation22,Citation23]. Aluminum adjuvant can adsorb protein antigens in solution and forms immunogenic particles, and it induces a Th2-biased immune response by activating the NALP3 inflammasome, followed by the release of IL-1β, IL-18, IL-33 [Citation24,Citation25]. However, a single adjuvant often induces a weak immune response. Therefore, we postulated that the combinations of CTB, CpG, and aluminum adjuvants could form a synergistic function to enlarge immune response, which were responsible for driving the polarization of naïve CD4+ T cells toward Th1, Th2, or Th17 cells.
Here, we performed the preparation of CTB-ClfA221-550 proteins, CTB as an intra-molecular adjuvant, plus CpG and aluminum (Alum) hydroxide to strengthen the immunogenicity of ClfA221-550.
Materials and methods
Mice and bacterial strains
Specific-pathogen-free BALB/c mice (6–8 weeks of age, female) were purchased from the Changchun Institute of Biological Products (Changchun, China). Care of laboratory animals and animal experimentation were performed in accordance with animal ethics guidelines and approved by the Animal Ethics Committee of Heilongjiang BaYi Agricultural University. Staphylococcus aureus strain Newman and Escherichia coli strain BL21 were stored in our laboratory. The Staphylococcus aureus strain Newman and Escherichia coli strain BL21 were cultured in tryptic soy agar (TSA) and Luria-Bertani (LB) broth at 37°C overnight, respectively.
Generation of recombinant CTB-ClfA221-550 protein
The ctb gene, of which the signal peptide sequence was deleted, was amplified from pET-32a (+)-ctb plasmid by using PCR method with forward primer 5′- GTCGACAAACACCTCAAAATATTACTGATTTGT-3′ (Sal I site underlined) and reverse primer 5′- GCTACCTCCTCCACTTCCGCCTCCAATTTGCCATACTAATT-GCG-3′ (linker sequence underlined). The PCR products were confirmed through agarose gel electrophoresis, then purified and stored at −20°C. The clfa221-550 was acquired from pET-32 a (+)-clfa221-550 plasmid by PCR with forward primer 5′- GGAGGCGGAAGTGGAGGAGGTAGCGTAGCTGCAGATGCACCGG −3′(linker sequence underlined) and reverse primer 5′- GCGGCCGCCTACTCATCAGGTTGT-TCAGGAACAA-3′ (Not I site underlined). The clfa221-550 PCR products were purified and stored at −20°C. Then, ctb-clfa221-550 fusion gene was prepared by chimeric PCR with the forward primer 5′- GTCGACAAACACCTCAAAATATTACTGATTTGT-3′ and reverse primer 5′- GCGGCCGCCTACTCATCAGGTTGTTCAGGAACAA-3′ plus templates of ctb and clfa221-550 PCR products. The PCR conditions were as follows: denaturation at 98°C for 3 min; followed by 30 cycles of 95°C, 30 s; 56°C, 50 s; 72°C, 90 s; extension at 72°C for 10 min. The target fragments were cloned into pET-32a (+) vectors, and the recombinant plasmids were transformed into in E. coli BL21 (DE3) (Tiangen, Beijing, China) and were expressed the CTB-CLFA221-550 proteins with 0.1 mM isopropyl-β-D − 1-thiogalactopyranoside (IPTG, Biosharp, Hefei, China) induction at 37°C for 4 h, then the bacterial cells were collected by centrifugation and were ultrasonicated, and the suspension was acquired. The His-tagged CTB-CLFA221-550 proteins were purified by using His-Binding-resin (Novagen, Germany) according to the manufacturer’s instructions. The CTB-CLFA221-550 proteins were detected by SDS-PAGE and Western blotting, finally divided into small aliquots and stored at −70°C.
Immune activity of recombinant protein
Firstly, endotoxin from CTB-CLFA221-550 solution was treated by Triton X-114 (Sigma-Aldrich (St. Louis, MO)) according to manufacturer’s recommendation, then the endotoxin activities of CTB-CLFA221-550 solution were evaluated with Limulus Amebocyte Lysate (LAL) assay as previously described [Citation26]. Finally, the immune activity of CTB-CLFA221-550 was assessed by ELISA. Briefly, BSA, CTB, CLFA221-550, and CTB-CLFA221-550 were coated at a concentration of 10 μg/mL, respectively. BSA was used as negative control group, CTB and CLFA221-550, as positive control group, and CTB-CLFA221-550 as experimental group. After blocking of 3% BSA, a twofold serial dilution of serum from mice immunized with CTB or CLFA221-550 was prepared and loaded into 96-well ELISA plate, then incubated for 2 h at room temperature. After washing, HRP-conjugated goat anti-mice IgG mAbs were loaded and reacted for 1 h at room temperature. After washing, TMB solution as substrate was decomposed and 2 M H2SO4 solution was used to terminate this reaction. Finally, optical densities (OD450 nm) were measured with an automated ELISA plate reader (BioRad, CA, USA).
Mice immunization and challenge
After acquired the desired proteins, we next confirmed immunogenicity of proteins. Specific-pathogen-free female BALB/c mice were immunized intramuscularly with the dosage of 100 μg ClfA221-550/CTB-CLFA221-550 alone or mixed with 10 μg of CpG ODN plus aluminum hydroxide (2%, (Sigma-Aldrich (St. Louis, MO))) to a final volume of 0.2 mL. The motif of CpG ODN contains an unmethylated cytosine preceding the guanosine (5′-TCCATGACGTTCCTGACGTT- 3′) was synthesized (Sangon Biotech (Shanghai) Co., Ltd, China). Immunization was performed on days 0, 14, and 21, respectively. All the animals were fed in a special pathogen-free environment.
To assess the immune-protective effect of CTB-CLFA221-550 plus the corresponding adjuvant, the challenge assay was performed. One week after the last immunization, the immunized BALB/c mice in the challenge group were infected by intraperitoneal injection with a lethal dose of 1 × 109 colony-forming units (CFU) of Staphylococcus aureus strain Newman. The mice were monitored for mortality and recorded every day after infection. Finally, the survival rate of mice from each group was determined after 14 days.
Intracellular cytokine staining
To detect intracellular cytokines of CD4+ T cells, intracellular cytokine staining (ICS) was carried out after vaccination. Briefly, splenocytes from immunized mice 2 weeks after the second immunization were prepared as previously described [Citation27]. Splenocytes were cultured for 4 h at 37°C in Roswell Park Memorial Institute 1640 medium plus 10% FBS, with or not ClfA221-550 proteins (10 μg/mL) in the presence of monensin (BD Biosciences (San Jose, CA)), which blocks cytokine secretion. Then, splenocytes were treated with an FITC-labeled anti-mouse CD4 monoclonal antibody (BD Biosciences (San Jose, CA)) for 30 min at 4°C, followed by permeabilization with Cytofix/Cytoperm (BD Biosciences (San Jose, CA)) for 20 min at 4°C. The cells were then incubated with a PE-labeled anti-mouse IFN-γ or Cy3-labeled anti-mouse IL-2, IL-4, and IL-17 antibody (BD Biosciences (San Jose, CA)). Finally, the labeled cells were detected by flow cytometry, the unstimulated T cells counts were regarded as background controls and were subtracted when processing the data. The results were considered positive when the percentage of total cytokine-producing cells was at least twice the background.
Lymphocyte proliferation assay
For evaluating antigen-specific lymphocyte proliferation, lymphocyte proliferation assay was performed. Briefly, 1 week after the last immunization, splenocytes from immunized mice were acquired as previously described [Citation27]. One-hundred microliters splenocyte suspension (8 × 105 cells per well) was added into 96-well flat-bottom culture plates (Falcon, Montreal Canada) in complete medium containing 25 μg/mL of ClfA221-550 at 37°C in 5% CO2. ConA-stimulated cells were used as positive control, and PBS-stimulated cells were used as negative control. After 48 h of antigen stimulation, the cell proliferation was assessed by cell counting kit (CCK)-8 according to the manufacturer’s instruction. The absorbance was measured at 450 nm wavelength using an ELISA Reader.
Detection of humoral immune response
At 7 days after the last immunization, the blood collection from the eyeballs of mice was performed, then the humoral immune response against ClfA221-550 was evaluated. A twofold serial dilution of sera was prepared and used as the primary antibody for ELISA assay. ClfA221-550 proteins at a concentration of 10 μg/mL were used as the coating antigen. HRP-conjugated goat anti-mouse IgG mAbs (Promega, San Luis Obispo, CA, USA) were used as the secondary antibodies. After washing, TMB solution was added and incubated for 15 min, then 2 M H2SO4 solution was performed to stop this reaction. Finally, antibody production was measured at OD450 nm with an automated ELISA plate reader.
Statistical analysis
The data were analyzed using Student’s t-test of the software SPSS 13.0. The data were presented as the means plus standard deviations from three independent experiments. P values < 0.05 were considered as significant difference.
Results
Confirmation of CTB-ClfA221-550 expression and immunogenicity
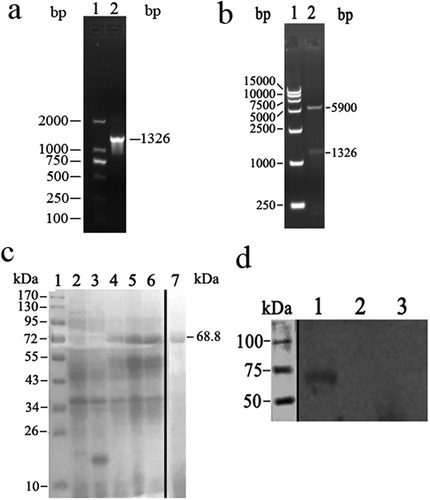
To construct recombinant ctb-clfa221-550 plasmids, the ctb and clfa221-550 fragments were acquired, respectively, and a nucleotide sequence encoding a GGGSGGGS linker was introduced between ctb and clfa221-550 fragments to keep the native conformation of the desired protein. About 1300 bp ctb-clfa221-550 fragments were obtained by chimeric PCR and cloned into pET-32a (+) vectors ()). The result of enzyme digestion showed that the recombinant plasmids pET-32a (+)-ctb-clfa221-550 were successfully constructed ()). Finally, SDS-PAGE results revealed that 68.8 kDa fusion proteins were expressed and subsequently purified ()), and Western blotting analysis also showed that CTB-ClfA221-550 proteins were correctly expressed ()).
Figure 1. The recombinant CTB-ClfA221-550 protein was prepared. (a) The target genes were amplified by PCR. The DNA Mark 15,000 is in lane 1; the PCR products of about 1300 bp are shown in lane 2. (b) The pET-32a (+)-ctb-clfa vectors were identified with the restriction endonucleases Sal I and Not I. The digested results in lane 1 showed the DNA Mark 15,000 and two fragments, about 1300 bp and 5900 bp, is in lane 2. (c) Confirmation of CTB-ClfA221-550 proteins. Lane 1: Protein marker. Lanes 2–7 are detected in SDS-PAGE gel with coomassie blue dye. Lane 2, 3: E. coli BL21 (DE3) and E. coli BL21 (DE3) with pET-32a (+) induced by IPTG as control, respectively. Lane 4: E. coli BL21 with pET-32a (+)-ctb-clfa without induced by IPTG. Lane 5, 6: E. coli BL21 with pET-32a (+)-ctb-clfa induced by IPTG; Lane 7: Purified CTB-ClfA221-550 protein. (d) The CTB-ClfA221-550 protein was detected with Western blotting. Lane 1: E. coli BL21 with pET-32a (+)-ctb-clfa; Lane 2: E. coli BL21 with pET-32a (+); Lane 3: E. coli BL21.
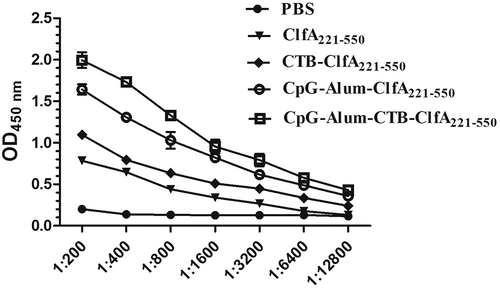
As shown in , CTB-ClfA221-550 solution was vastly decontaminated to have removed the endotoxin with Triton X-114. To determine if CTB can impair ClfA221-550 immunogenicity, ELISA assay was performed, BSA was used as negative group, CTB and CLFA221-550 as positive group, respectively, and CTB-CLFA221-550 as experimental group. After coating antigens, BSA, CTB, and CTB-CLFA221-550, incubated with serum from mice immunized with CTB, statistical analysis showed that there was significant difference between CTB and BSA groups, more importantly, obvious difference between CTB-CLFA221-550 and BSA groups was also exhibited, and there was no significant difference between CTB and CTB-CLFA221-550 groups by measuring optical densities (OD450nm) ()). In addition, after coating antigens, BSA, CLFA221-550, and CTB-CLFA221-550, reacted with serum from mice immunized with CLFA221-550, ELISA results revealed that the significant difference was observed between CLFA221-550 and BSA groups, moreover, remarkable difference between CTB-CLFA221-550 and BSA groups was exhibited, and the difference was not obvious between CLFA221-550 and CTB-CLFA221-550 groups by analyzing optical densities (OD450nm) ()). These results displayed that CTB-ClfA221-550 can react with serum of mice immunized with CTB and ClfA221-550, respectively. Therefore, these data indicated that CTB-ClfA221-550 can exhibit immune response in vitro, and also revealed that CTB did not destroy ClfA221-550 immunogenicity. Thus, CTB might be a suitable intra-molecular adjuvant for ClfA221-550.
Figure 2. Assessment of endotoxin activity. The endotoxin activities of CTB-ClfA221-550 preparation before and after treatment with Triton X-114 were determined by LAL assay. Significant differences are shown by *** P < 0.001. The values are the mean ± SD (n = 3).
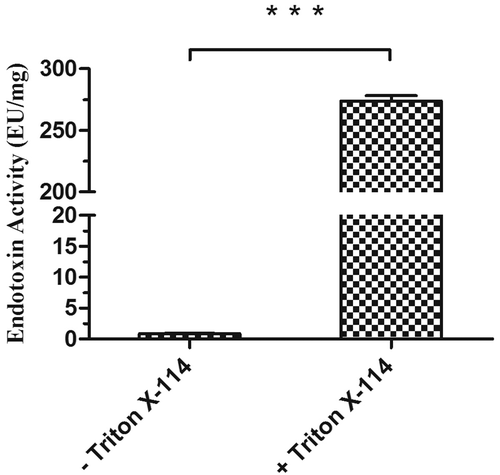
Figure 3. Analysis of CTB-ClfA221-550 immune activity. ELISA was performed by using BSA, CTB, ClfA221-550, and CTB-ClfA221-550 as coat antigens, respectively. Coat antigens incubated with serum from mice immunized with CTB (a) and ClfA221-550 (b), respectively. Significant differences were indicated by ** P < 0.01. The values are the mean ± SD (n = 3).
The combined adjuvants increased antigen-specific CD4+ T cell immune response
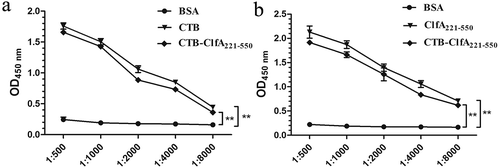
CD4+ T cell response may effectively mediate cellular and humoral immunity by secreting cytokines, so we detected CD4+ T cell response induced by ClfA221-550 plus different adjuvant. IFN-γ, IL-2, IL-4, and IL-17 production of antigen-specific CD4+ T cells from the immunized mice are displayed in . These results showed the mice immunized with CTB-ClfA221-550 or ClfA221-550 plus different adjuvant generated significantly higher antigen-specific CD4+ T cell responses for IFN-γ, IL-2, IL-4, and IL-17 than the group of ClfA221-550 and PBS. Obviously, the mice vaccinated with ClfA221-550 plus the combined adjuvants (CTB, CpG, and Alum adjuvant) exhibited the strongest antigen-specific CD4+ T cell responses for IFN-γ, IL-2, IL-4, and IL-17 among all groups (). However, the mice vaccinated with ClfA221-550 plus CpG and Alum adjuvant displayed lower antigen-specific CD4+ T cell responses for IL-17 than groups with ClfA221-550 plus CTB, indicating CTB adjuvant could increase the Th17-cell-mediated immune reaction ()). These results revealed that the combined adjuvants could markedly strengthen the antigen-specific CD4+ T cell responses and more significantly increased the immune responses to ClfA221-550 than single adjuvant in mice.
Figure 4. Detection of antigen-specific CD4+ T cells producing IFN-γ, IL-2, IL-4, and IL-17. Splenocytes from three mice per group were isolated 2 weeks after the secondary immunization. After the splenocytes were treated with the ClfA221-550 proteins, the percentage of CD4+ T cells producing IFN-γ, IL-2, IL-4, and IL-17 was analyzed by flow cytometric method. The CD4+ T cells response for IFN-γ (a), IL-2 (b), IL-4 (c) and IL-17 (d). The statistical differences between the groups are shown as **P < 0.01, *P < 0.05. The data are shown as the mean ± SD (n = 3).
In general, IL-4 is characteristic of the Th2 immune response and mediates immune activity of B cell. By contrast, Th1 response is characterized by elevated the levels of IL-2 and IFN-γ. IL-17 is mostly produced by Th17 cells and promotes neutrophil recruitment, enhances inflammation. In consequence, from these above results, the combined adjuvants of CTB, CpG, and Alum adjuvant can exert a coaction to induce the immune activation of Th1, Th2, and Th17 cells, as a result, to further enlarge T-cell-mediated immune response.
The combined adjuvants significantly improved antigen-specific lymphocyte proliferation response
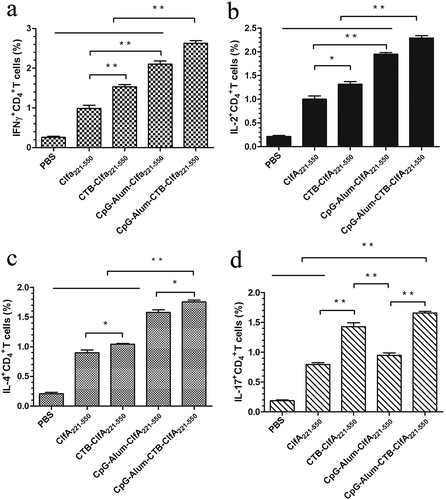
The immune cell activity was determined by using proliferation assays. As shown in , after ClfA221-550 stimulating, except the PBS group, splenic lymphocytes from all immunized mice generate significant proliferation response, by comparison, CTB-ClfA221-550 group induced higher cell proliferation response than ClfA221-550 group, and ClfA221-550 group plus CpG and Alum adjuvant generated higher cell proliferation response than ClfA221-550 and CTB-ClfA221-550 groups, especially, ClfA221-550 plus the combined adjuvants of CTB, CpG, and Alum group exhibited the highest cell proliferation response among all groups (). These results showed that combined adjuvants could obviously increase lymphocyte activation and proliferation induced with ClfA221-550 in mice.
Figure 5. Proliferative responses of lymphocytes in mice. One week after the last immunization, spleen lymphocytes were isolated from BALB/c mice (n = 5), and were incubated with 25 μg/mL ClfA221-550 for 48 h. Significant differences are indicated by * P < 0.05 and ** P < 0.01. The data are shown as the mean ± SD (n = 5).
The combined adjuvants increased humoral immune response
Humoral immune response is mainly estimated by antibody production. For evaluating the humoral immune response induced by ClfA221-550, we detected the production of antibodies against ClfA221-550 in mice. At 1 week after the last immunization, except PBS group, all groups generated strong humoral immune response against ClfA221-550, by contrast, CTB-ClfA221-550 group exhibited stronger humoral immune response against ClfA221-550 than ClfA221-550 group, and the group with ClfA221-550 plus CpG and Alum adjuvant generated stronger humoral immune response against ClfA221-550 than ClfA221-550 or CTB-ClfA221-550 group, but the group with ClfA221-550 plus the combined adjuvant of CTB, CpG, and Alum displayed the strongest humoral immune response against ClfA221-550 among all groups (). These results demonstrated that the combinations of CTB, CpG, and Alum adjuvants obviously enhanced the humoral immune response induced by ClfA221-550 in mice.
The combined adjuvants boosted protective immune response
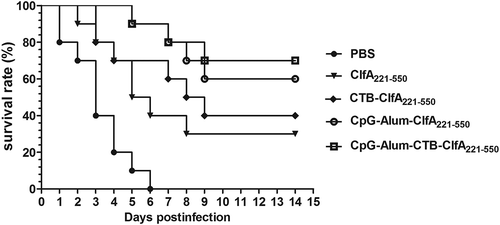
Because protective immune response was regarded as the most important measure for evaluating vaccine efficiency, we performed challenge assay by using S. aureus strain Newman. As shown in , on day 14 following S. aureus strain Newman challenge, the survival rate of groups immunized with ClfA221-550, CTB-ClfA221-550, ClfA221-550 plus CpG, and Alum adjuvant, ClfA221-550 plus the combined adjuvants of CTB, CpG, and Alum reached a protection of 30%, 40% 60%, and 70%, respectively, but the mice of PBS group were all dead within a week. These data indicated that the combined adjuvant containing CTB, CpG and Alum adjuvant exhibited the stronger enhancement of immune response and obviously increases the immuno-protection effect of ClfA221-550.
Discussion
In this study, CTB-ClfA221-550 proteins were prepared, the ELISA results showed that CTB-ClfA221-550 possessed strong immune activity, which indicated CTB as intra-molecular adjuvant, did not weaken the immunoactivity of ClfA221-550. Furthermore, the collaborations of CTB, CpG, and aluminum hydroxide adjuvants could significantly promote the immunogenicity of ClfA221-550 in mice.
S. aureus, as a ubiquitous pathogen, has been deemed as a major etiological factor of nosocomial infections worldwide, which was associated with high mortality both human and animals [Citation28,Citation29]. Therefore, an effective vaccine candidate preventing infection of S. aureus will urgently be developed. Although the surface proteins of S. aureus, such as ClfA, FnBPA, IsdB, etc., have exhibited strong immunogenicity, it is very necessary for the assistance of appropriate adjuvant to achieve their desired immune protection against infection of S. aureus. In addition, recent studies support that the combinations of different adjuvant can stimulate the broad and robust protective immune responses to fight chronic infectious diseases in vaccine design [Citation30]. Therefore, the combined use of adjuvant is a new trend in vaccine development. At present, combinations of different adjuvant have been developed, for example, the combinations of streptavidin-4-1BBL (SA-4-1BBL) and (MPL) monophosphoryl lipid A, CpG ODN and Poly(I:C), aluminum salts and CpG ODN plus innate defense regulator peptide HH2, which may effectively induce the activation of immune cells and produce a variety of cytokines, enhancing the magnitude, breadth, quality, and longevity of the immune response to a given antigen [Citation31–Citation33]. More importantly, with the exploration of S. aureus vaccine, it has been found that cellular immunity mediated by Th1, Th2, and Th17 cells plays a key role in the resistance to infection of S. aureus. Thus, it is very important for activating Th1, Th2, and Th17 cells to prevent infection of S. aureus. Therefore, in this study, three kinds of adjuvants, CTB, CpG, and aluminum hydroxide, were selected and formed a combined adjuvant to boost the immunogenicity of ClfA221-550 by activating Th1, Th2, and Th17 cells.
Here, the ICS results showed that the combinations of CTB, CpG, and Alum adjuvants can more significantly increased the ClfA221-550-specific CD4+ T cell responses for IFN-γ, IL-2, IL-4, and IL-17 than CTB alone or CpG plus Alum adjuvant did. In addition, our experimental design manifested that the combined adjuvants were also able to induce a stronger humoral immune response to ClfA221-550. Importantly, the humoral immune response against ClfA221-550 of CTB-ClfA221-550 group was obviously stronger than ClfA221-550 group, which suggested that it was probable that CTB could increase the efficiency of B cells activated by ClfA221-550 via targeting effect of GM1. Most importantly, the challenge data showed that ClfA221-550 plus the combined adjuvants obviously promoted the protective immune responses induced with ClfA221-550 against infection of S. aureus in mice.
Taken together, our results verified that the combinations of CTB, CpG, and Alum adjuvants could significantly boost T- and B- cell-mediated immune responses against ClfA221-550. However, because the pathogenesis of S. aureus is more complex, it is difficult to achieve the desired immune effect by a single antigen as vaccine. The previous data indicated that combinations of multiple antigens from S. aureus could induce the stronger immune protection [Citation34,Citation35], so ClfA221-550 plus other surface proteins of S. aureus could further enhance the immunoprotective effect against S. aureus with the combinations of CTB, CpG, and Alum adjuvants, which would be considered in further research. In addition, in this study, we just selected S. aureus strain Newman as challenge strains, other S. aureus strains should be used to further investigate the immuno-protection effects induced by ClfA221-550 plus the combined adjuvants in the future.
Conclusions
Our results demonstrated that the combinations of CTB, CpG, and aluminum hydroxide played a synergistic effect to enhance ClfA221-550-induced immune responses, which possessed the potential to develop a novel vaccine to prevent infection of S. aureus.
Author contributions
J. M. designed the study, interpreted the data, drafted and revised the article. B.W., L.Y., B.S., Y.Y., SH.W., Y.D., ZH.Z. contributed to data collection.
Ethical statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of Heilongjiang Bayi Agricultural University. The experimental protocols were approved by the Institutional Animal Care and Use Committee at Heilongjiang Bayi Agricultural University. All animal experiments were performed under sodium pentobarbital anesthesia to minimize mice suffering.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Torres VJ, Stauff DL, Pishchany G, et al. A Staphylococcus aureus regulatory system that responds to host heme and modulates virulence. Cell Host Microbe. 2007 Apr 19;1(2):109–119.
- Althaqafi AO, Matar MJ, Moghnieh R, et al. Burden of methicillin-resistant Staphylococcus aureus pneumonia among hospitalized patients in Lebanon and Saudi Arabia. Infect Drug Resist. 2017;10:49–55.
- Josefsson E, Hartford O, O’Brien L, et al. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J Infect Dis. 2001 Dec 15;184(12):1572–1580.
- Pu W, Su Y, Li J, et al. High incidence of oxacillin-susceptible mecA-positive Staphylococcus aureus (OS-MRSA) associated with bovine mastitis in China. PloS One. 2014;9(2):e88134.
- Vasileiou NGC, Chatzopoulos DC, Sarrou S, et al. Role of staphylococci in mastitis in sheep. J Dairy Res. 2019 Aug;86(3):254–266.
- Loeffler A, Lloyd DH. What has changed in canine pyoderma? A narrative review. Vet J. 2018 May;235:73–82.
- Durai R, Ng PC, Hoque H. Methicillin-resistant Staphylococcus aureus: an update. Aorn J. 2010 May;91(5):599–606; quiz 607–9.
- Howden BP, Davies JK, Johnson PD, et al. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev. 2010 Jan;23(1):99–139.
- Mehraj J, Akmatov MK, Strompl J, et al. Methicillin-sensitive and methicillin-resistant Staphylococcus aureus nasal carriage in a random sample of non-hospitalized adult population in northern Germany. PloS One. 2014;9(9):e107937.
- Broughan J, Anderson R, Anderson AS. Strategies for and advances in the development of Staphylococcus aureus prophylactic vaccines. Expert Rev Vaccines. 2011 May;10(5):695–708.
- Torres VJ, Pishchany G, Humayun M, et al. Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. J Bacteriol. 2006 Dec;188(24):8421–8429.
- Verkaik NJ, van Wamel WJ, van Belkum A. Immunotherapeutic approaches against Staphylococcus aureus. Immunotherapy. 2011 Sep;3(9):1063–1073.
- Que YA, Haefliger JA, Piroth L, et al. Fibrinogen and fibronectin binding cooperate for valve infection and invasion in Staphylococcus aureus experimental endocarditis. J Exp Med. 2005 May 16;201(10):1627–1635.
- Yang Y, Qian M, Yi S, et al. Monoclonal antibody targeting staphylococcus aureus surface protein A (SasA) protect against staphylococcus aureus sepsis and peritonitis in mice. PloS One. 2016;11(2):e0149460.
- McDevitt D, Francois P, Vaudaux P, et al. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol. 1994 Jan;11(2):237–248.
- Brouillette E, Lacasse P, Shkreta L, et al. DNA immunization against the clumping factor A (ClfA) of Staphylococcus aureus. Vaccine. 2002 May 22;20(17–18):2348–2357.
- Dickinson RB, Nagel JA, McDevitt D, et al. Quantitative comparison of clumping factor- and coagulase-mediated Staphylococcus aureus adhesion to surface-bound fibrinogen under flow. Infect Immun. 1995 Aug;63(8):3143–3150.
- Elkhatib WF, Hair PS, Nyalwidhe JO, et al. New potential role of serum apolipoprotein E mediated by its binding to clumping factor A during Staphylococcus aureus invasive infections to humans. J Med Microbiol. 2015 Apr;64(Pt 4):335–343.
- Lee JB, Jang JE, Song MK, et al. Intranasal delivery of cholera toxin induces th17-dominated T-cell response to bystander antigens. PloS One. 2009;4(4):e5190.
- Meza-Sanchez D, Perez-Montesinos G, Sanchez-Garcia J, et al. Intradermal immunization in the ear with cholera toxin and its non-toxic beta subunit promotes efficient Th1 and Th17 differentiation dependent on migrating DCs. Eur J Immunol. 2011 Oct;41(10):2894–2904.
- Thiam F, Charpilienne A, Poncet D, et al. B subunits of cholera toxin and thermolabile enterotoxin of Escherichia coli have similar adjuvant effect as whole molecules on rotavirus 2/6-VLP specific antibody responses and induce a Th17-like response after intrarectal immunization. Microb Pathog. 2015 Dec;89:27–34.
- Kulis M, Gorentla B, Burks AW, et al. Type B CpG oligodeoxynucleotides induce Th1 responses to peanut antigens: modulation of sensitization and utility in a truncated immunotherapy regimen in mice. Mol Nutr Food Res. 2013 May;57(5):906–915.
- Klinman DM. Use of CpG oligodeoxynucleotides as immunoprotective agents. Expert Opin Biol Ther. 2004 Jun;4(6):937–946.
- De Gregorio E, Tritto E, Rappuoli R. Alum adjuvanticity: unraveling a century old mystery. Eur J Immunol. 2008 Aug;38(8):2068–2071.
- Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009 Apr;9(4):287–293.
- Miernikiewicz P, Owczarek B, Piotrowicz A, et al. Recombinant expression and purification of T4 phage Hoc, Soc, gp23, gp24 proteins in native conformations with stability studies. PloS One. 2012;7(7):e38902.
- Tosa N, Yoshimatsu K, Takahashi M, et al. Comparison of immune response in mice sensitized to an animal allergen, Can f 1, and to a food allergen, ovalbumin. Biomed Res. 2019;40(1):9–15.
- Esposito S, Pennoni G, Mencarini V, et al. Antimicrobial treatment of staphylococcus aureus in patients with cystic fibrosis. Front Pharmacol. 2019;10:849.
- Kuyucu E, Cabuk H, Guler Y, et al. Is intraarticular antibiotic administration effective in the treatment of methicillin-resistant staphylococcus aureus? Acta Chir Orthop Traumatol Cech. 2019;86(4):276–280.
- Mount A, Koernig S, Silva A, et al. Combination of adjuvants: the future of vaccine design. Expert Rev Vaccines. 2013 Jul;12(7):733–746.
- Srivastava AK, Yolcu ES, Dinc G, et al. SA-4-1BBL/MPL as a novel immune adjuvant platform to combat cancer. Oncoimmunology. 2016;5(1):e1064580.
- Tian Y, Li M, Yu C, et al. The novel complex combination of alum, CpG ODN and HH2 as adjuvant in cancer vaccine effectively suppresses tumor growth in vivo. Oncotarget. 2017 Jul 11;8(28):45951–45964.
- Pirahmadi S, Zakeri S, Mehrizi AA, et al. Cell-traversal protein for ookinetes and sporozoites (CelTOS) formulated with potent TLR adjuvants induces high-affinity antibodies that inhibit Plasmodium falciparum infection in Anopheles stephensi. Malar J. 2019 Apr 24;18(1):146.
- DeDent A, Kim HK, Missiakas D, et al. Exploring Staphylococcus aureus pathways to disease for vaccine development. Semin Immunopathol. 2012 Mar;34(2):317–333.
- Bagnoli F, Bertholet S, Grandi G. Inferring reasons for the failure of Staphylococcus aureus vaccines in clinical trials. Front Cell Infect Microbiol. 2012;2:16.