ABSTRACT
The role of exogenous methyl jasmonate (MeJA) in alleviating drought stress was investigated on Huangguogan. Except for intercellular CO2 concentration, MeJA had little effect on net photosynthetic rate, stomatal conductance, and transpiration rate under drought stress. Compared with drought stress, MeJA significantly alleviated the decrease of chlorophyll content. However, chlorophyll a/b ratio was significantly increased. MeJA significantly increased proline and soluble sugar contents, significantly decreased the O2−· and H2O2 levels, and increased SOD and POD activities. In addition, the MDA content of drought stress was the highest of all treatments. MeJA significantly reduced MDA content in drought-stressed Huangguogan leaves. Although the Ascorbic acid (AsA) contents of 500 and 1000 mg L−1 MeJA treatments were lower than that of 250 mg L−1 MeJA, but all concentration of MeJA treatments delayed the decline of AsA content. Therefore, MeJA could induce drought stress tolerance by increasing the osmotic adjustment substances and antioxidant activities.
Graphical Abstract
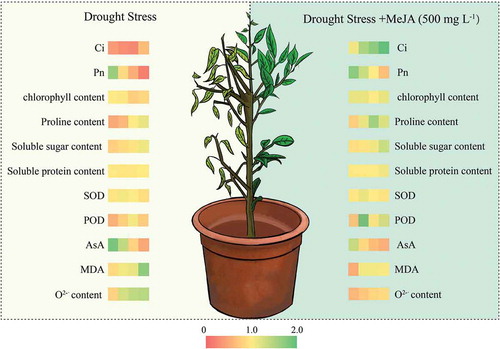
Difference in physiological indicators of drought resistance between drought stress (DS) and drought stress + 500 mg L-1 MeJA (DM) on the 5th, 8th, 11th and 14th day after treatment. Different colours represent different values of , and higher values are greener and lower are redder.
Due to the climate change, drought stress has long-term adverse effects on the production of worldwide crops [Citation1,Citation2]. Mild or moderate drought stress can significantly reduce plant growth and crop yields, while severe drought causes plants to wither, die, or even fail to harvest [Citation3]. Even under irrigation conditions, drought stress can also occur because the use or availability of irrigation water may be limited [Citation4,Citation5].
Citrus is one of the most important fruit crops in the family Rutaceae and the largest fruit industry over the world [Citation6,Citation7]. Drought may be one of the most influential factors on Citrus yield and quality in abiotic stress, results in leaf curl, transpiration area and fruit quality decrease, and leaves and fruit drop [Citation8]. Compared with other C3 plants, Citrus has lower water efficiency, and water lost from the fruit flows to the leaves under drought conditions [Citation9]. Meanwhile, the content of different ROS [superoxide (O2−·), hydrogen peroxide (H2O2), the hydroxyl radical (OH–), and singlet oxygen (1O2)] is enhanced. These ROS can cause photoinhibition or photooxidation, leading to uncontrolled oxidation of photosynthetic components [Citation10], and the reduction/oxidation state is determined by the availability of cells in the ROS scavenging system [Citation11]. In response to drought, citrus plants can activate a variety of protective mechanisms to increase resistance, such as osmotic adjustment [Citation12] and antioxidant enzyme system [Citation13].
Jasmonates (JAs), mainly including jasmonic acid (JA) and volatile methyl jasmonate (MeJA), is regarded as endogenous regulators in higher plants. The accumulation of JAs is also one of the mechanisms of plant resistance to drought stress [Citation13,Citation14]. The increased level of MeJA induces a jasmonic acid-dependent defense response, which is related to the enhancement of secondary metabolism [Citation15]. Therefore, exogenous MeJA is usually used in studies related to abiotic stress [Citation16,Citation17]. Some previous studies have found that MeJA can increase chlorophyll content, stomatal conductance (Gs), net photosynthetic rate (Pn), proline, soluble proteins, and soluble sugars to improve the tolerance of cowpea (Vigna sinensis) [Citation18] and Brassica napus [Citation19] under salinity stress. Other studies have found that MeJA enhanced the ability for water stress resistance in Cauliflower (Brassica oleracea L.) seedlings by promoting defense-related metabolism [Citation20], and MeJA also enhanced the ability of strawberry to resist deficit irrigation by increasing fructose content [Citation21]. MeJA improved drought tolerance of soybean plants as a potential growth regulator [Citation22]. However, the effects of JAs treatment vary with plant species [Citation23], not all MeJA treatments can significantly improve drought tolerance [Citation3,Citation24]. These effects vary depends on plant species and MeJA concentration [Citation13].
Some previous researches about the effect of MeJA on citrus are related to salt and high temperature stress, and found that the biosynthesis and signaling pathways of JA were up-regulated, and JA, JA-isoleucine (JA-Ile) and 12-oxo-phytodienoic acid (OPDA) accumulation were induced in conditions of high salinity and heat stress. This response could further counteract heat effects by closing stomata, reducing transpiration and, thus, avoiding salt intoxication [Citation25,Citation26]. But there are few reports about the possible role of MeJA in drought resistance of Citrus plants. The ability of JA or MeJA to alleviate drought stress in Citrus is still not known. This study was conducted to further understand the response mechanisms of Citrus to drought stress and to clarify the alleviation of drought stress and the physiological mechanisms in Citrus cultivar (Huangguogan) treated with MeJA.
Materials and methods
Plant materials and Experimental design
Two-year-old Huangguogan plants grafted onto Trifoliate orange [Poncirus trifoliata (L.) Raf.] were selected for experiments. Each seedling was planted in a pot (25 × 25 cm). All Huangguogan plants received normal horticultural care for pest and disease control during the experiment. 45 Huangguogan trees with similar growth potential and no pests were transplanted into the greenhouse. This study used TWS-1 (Shanghai fa tai precision instrument co. LTD) to accurately control soil moisture. Irrigation was stopped three days before the onset of drought stress, allowing soil moisture reduces to 10%~15%, while it was maintained at 40–50% in the control group (CK). The soil moisture of all treatments was measured at 18:00 daily after the onset of drought stress, and then irrigated the lost water of evaporation to keep the soil moisture of each treatments at its own level.
After the beginning of drought stress, Huangguogan leaves were sprayed with MeJA (0, 250, 500 or 1000 mg L−1) at 9:00 am. (1) Normal irrigation + foliage spray with equal volume distilled water (CK), (2) Drought stress + distilled water (DS), (3) Drought stress + 250 mg L−1 MeJA, (4) Drought stress + 500 mg L−1 MeJA, (5) Drought stress + 1000 mg L−1 MeJA. Control plants were sprayed with distilled water. Methyl Jasmonate (95%) was purchased from Beijing Solarbio technology co. LTD. All chemicals were of analytical grade.
Leaf sampling
On 5, 8, 11 and 14 days (at 9:00 am) after the beginning of drought stress, more than 10 leaves with the same position and size were harvested from each treatment. After each sample collection, rinsed immediately with deionized water, cut off leaf edges and veins, and placed in sample bags. These collected leaves were frozen in liquid nitrogen immediately and stored at −80°C.
Gas exchange and Chlorophyll Content
Net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr), and intercellular CO2 concentration (Ci) of the leaves were monitored by portable photosynthesis measuring instrument (LI-6400; Licor, Lincoln, NE, USA). The photosynthetic active radiation and CO2 concentration was 1,200 µmol m−2 s−1 and 400 µmol m−2 s−1, respectively. Gs and Ci were determined at a saturated light intensity of 1,000 µmol m−2 s−1, and 70% relative humidity. The determination of chlorophyll a (Chl a) and chlorophyll b (Chl b) was performed based on the colorimetric method described by Huang et al [Citation27].
Measurement of Soluble Sugar and Soluble Protein
Soluble Protein were determined according to the method of Bradford et al [Citation28]. Soluble Sugar was determined according to the method of Wei et al [Citation29].
Measurement of Proline and Ascorbate contents
Proline contents were determined according to the method of Bates et al [Citation30]. Leaves were homogenized in 3% sulfosalicylic acid and then centrifuged at 11,500 × g. The supernatant was mixed with acidic ninhydrin, glacial acetic acid, and phosphoric acid. After the sample was incubated at 100°C for 1 h and cooled, toluene was added. After several minutes, chromophore-containing toluene was analyzed spectrophotometrically at 520 nm. Ascorbate contents were calculated as describing by Huang et al [Citation31].
Measurement of H2O2, O2−· and malondialdehyde contents, and antioxidant enzyme activity
The levels of leaf O2−· were measured according to the procedure used by Orozco et al [Citation32]. And the H2O2 contents were analyzed using another published procedure [Citation33]. The lipid peroxidation level was evaluated by estimating the content of MDA with thiobarbital acid by Heath et al [Citation34]. with modifications [Citation35]. The SOD and POD activities were measured as described by Duan et al [Citation36].
Data analysis
The data were analyzed using Duncan’s multiple range test in the SPSS v.24 (IBM SPSS, Chicago, IL, USA) at the p < 0.05 level of significance.
Results
Photosynthesis and Chlorophyll Content
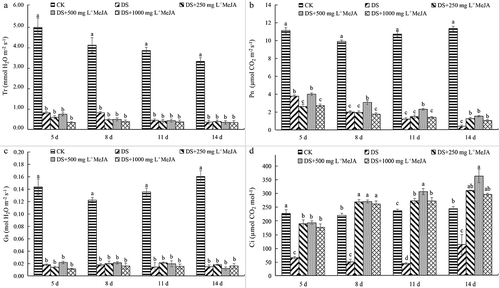
Under drought stress, the photosynthesis of Huangguogan leaves was seriously affected, and the gas exchange parameters are significantly reduced (). Compared with CK, Pn of DS was decreased by nearly 65% at 5th day under drought stress, and decreased more than 95% at 14 day, and MeJA treatment at different concentrations slowed down the rate of Pn decline. The Pn of 500 mg L−1 MeJA was significantly higher than that of other treatments ()). With the increase of drought stress days, Tr and Pn decreased gradually, while Ci increased slowly. And the Ci was significantly higher than that of CK at the 8th day after MeJA treatment (250, 500 and1000 mg L−1) ()). Compared with CK, the Tr and Gs of each experimental group were significantly decreased, but the difference was not significant in each treatment group, indicating that MeJA had little effect on Tr and Gs of Huangguogan leaves under drought stress ().
Figure 1. The change of Gs, Pn, Tr, and Ci of Huangguogan leaves during drought. Bars labeled with different lowercase letters are significantly different (p < 0.05) according to Duncan’s multiple range test.
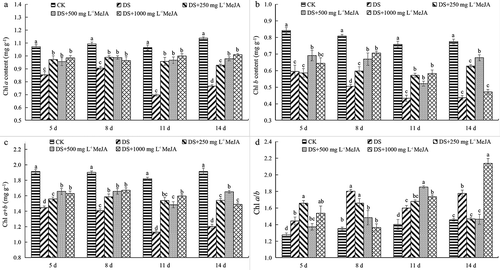
Under drought conditions, Chl a, Chl b, and Chl a + b content of Huangguogan leaves reduced rapidly. Compared with DS, MeJA significantly alleviated the decrease (). The Chl a content of Huangguogan leaves treated with 250, 500 and 1000 mg L−1 MeJA was maintained at about the same level, significantly higher than that of DS ()). The chl b content of Huangguogan leaves was increased by MeJA treatment, it was the most stable in 500 mg L−1 MeJA-treated, and was significantly higher than 250 and 1000 mg L−1 MeJA-treated at the 14th day after MeJA treatment (). However, the Chl a/b ratios were significantly increased ().
Osmotic adjustment substances
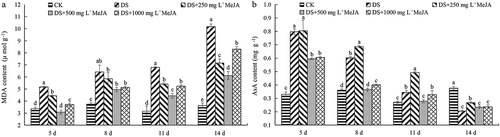
Compared with DS, MeJA significantly increased the osmotic regulatory substances of Huangguogan leaves under drought stress, especially the contents of soluble sugar and proline (). Among them, proline had the strongest response to drought stress, and the content of proline in Huangguogan leaves treated with 500 mg L−1 MeJA was increased by more than 4 times compared with CK at 11th days under drought stress. And the proline contents of Huangguogan leaves treated with 250 and 500 mg L−1 MeJA were significantly higher than that of DS and CK ()). Soluble sugar content was increased by drought stress, and that of low concentration methyl jasmonate treatments (250 and 500 mg L−1 MeJA) was significantly higher than DS. The soluble sugar content of Huangguogan leaves treated with 250 mg L−1 MeJA was significantly higher than that of 500 mg L−1 MeJA before the 11th day under drought stress, while it was reversed on the 14th day (). The 5th to 11th days under drought stress, there was no significant difference in the soluble protein content between DS and MeJA-treated Huangguogan leaves. Until the 14th day, the soluble protein content of the 250 mg L−1 MeJA treatment was significantly higher than that of DS ().
Figure 3. Effects of exogenous MeJA on the content of proline, soluble sugar and soluble protein of Huangguogan leaves during drought.
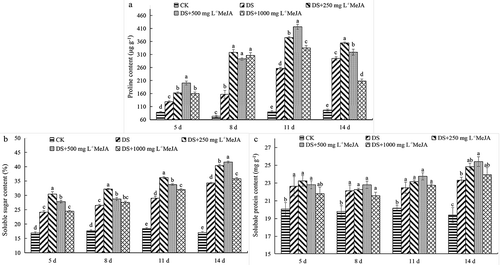
In summary, drought stress caused the accumulation of osmotic regulatory substances, especially proline, in the leaves of Huangguogan, while 250 and 500 mg L−1 MeJA treatments were more conducive to the rapid accumulation of osmotic regulatory substances, improving the osmotic potential of leaf cells, and thus enhancing the drought-resistance.
Antioxidant activities and reactive oxygen species
With the increase of drought stress time, the antioxidant activity of Huangguogan leaves showed a trend of increase and then decrease, and the maximum enzyme activity of SOD and POD were the 8th day, while the POD activity of DS was delayed 3 days to reach its maximum compared with other treatments. In addition, Huangguogan leaves treated with 500 mg L−1 MeJA showed the highest antioxidant activity ().
Figure 4. Effects of exogenous MeJA on the activities of superoxide dismutase (SOD), peroxidase (POD), superoxide anion (O2−·) and H2O2 content of Huangguogan leaves during drought.
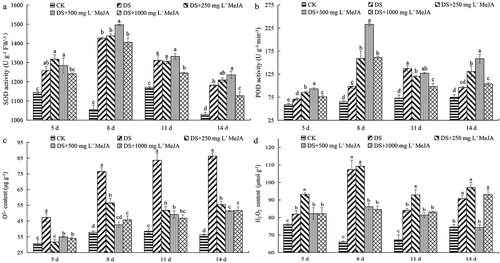
In the study, the content of superoxide anion (O2−·) was increased significantly under drought stress, and the content of O2−· was suppressed in Huangguogan leaves treated with MeJA (). With the extension of the drought time, the content of H2O2 was firstly increased, and then decreased. At the 14th day under drought stress, the H2O2 content of 500 mg L−1 MeJA treatment was significantly lower than other treatments, but there was no significant difference among DS, 250 and 1000 mg L−1 MeJA treatments in the final H2O2 content.
MDA and AsA content
The MDA content of DS was significantly higher than that of other treatments. At the 14th day, the MDA content of DS was more than 3 times that of CK. MeJA significantly reduced MDA content in drought-stressed Huangguogan leaves. The MDA content of Huangguogan leaves treated with 500 mg L−1 MeJA was the lowest one from the 5th to 14th day after treatment (). Ascorbic acid (AsA), an important non-enzymatic antioxidant, is a substrate for APX catalyzed H2O2 reactions. In this study, AsA content of Huangguogan leaves increased rapidly and then gradually decreased under drought stress condition, and was lower than that of CK at the 14th day ()). Although the AsA content of 500 and 1000 mg L−1 MeJA treatments was lower than that of 250 mg L−1 MeJA, but all concentration of MeJA treatments delayed the decline of AsA content ().
Discussion
In the study, we found that drought stress applied to Citrus plants caused significant changes on photosynthesis, antioxidant enzyme activity and the concentrations of cellular osmotic regulators. In response to drought stress, Pn, Gs, Tr, and Ci were declined and the content of proline, soluble sugar, soluble protein and antioxidant enzyme activity were increased, and the balance between intracellular active oxygen production and the defense system was broken down under drought stress.
Pn was sensitive to drought stress. In general, under drought conditions, there are stomatal and non-stomatal factors in the decrease of Pn. Stomatal factors are manifested by the decrease of Gs, Tr and Ci, while non-stomatal factors are usually caused by damage of photosynthetic structure [Citation37]. In this study, the Gs and Tr of Huangguogan leaves were decreased under drought stress, but complete stomatal closure did not occur, indicated that stomatal aperture size was reduced (). Gs was firstly decreased and then increased with the increase of drought stress time, indicated that stomatal factors mainly affected photosynthesis in the early stage of drought stress, perhaps because Chlorophyll degradation, the photosynthetic system was damaged, the efficiency of CO2 fixation was reduced in the later stage. Other studies have found that decreased photosynthesis in sugar beet (Beta vulgaris L.) plants due to drought is not related to stomatal closure [Citation3]. However, it is one of the restrictive stomatal factors in wheat [Citation38]. Obviously, MeJA could significantly increase Ci, which showed that MeJA reduced the damage to photosynthetic system by drought stress. The decrease in Pn was alleviated by the application of MeJA, which may be related to the change of chl b content, indicating that MeJA can increase the use of scattered light to enhance photosynthesis (). All MeJA concentration treatments had no effect on Tr and Gs in this work, which was consistent with the findings of Fugate et al [Citation3]. But other studies have found that MeJA can alleviate drought stress by promoting the production of H2O2 [Citation38], and regulating stomatal closure [Citation13].
Accumulation of Osmotic adjustment substances in response to drought stress has been observed in a variety of plants [Citation3,Citation22]. All osmotic adjustment substances are considered to have a role in adjusting cellular osmotic potential, thereby allowing plants to increase cell water potential and maintain cell pressure [Citation39], and stabilizing cellular membranes, protecting enzymes and scavenging free radicals to alleviate abiotic stress [Citation40]. In the present study, the proline the content of proline was more than twice that of the CK from the 5th to 14th day of drought stress, indicating that proline played a major role in osmotic regulation. MeJA treatment significantly increased osmotic regulatory substances and alleviated the adverse effects of drought on Huangguogan leaves, MeJA of 500 and 1000 mg L−1 had better drought resistance (). The increase of proline, soluble sugar and soluble protein contents in drought stress suggested that Huangguogan plants utilized these compounds to alleviate drought stress.
The content of O2−· and H2O2, which are highly reactive and poisonous molecules generated in cells, are accumulated in drought conditions [Citation41]. The increase of MDA content aggravated the degree of lipid peroxidation, indicated that the cell membrane had damaged [Citation42]. Under stress conditions, the dynamic balance of free radical content in plants will be broken and accumulated a large amount of reactive oxygen species (ROS), causing great damage to plant cells. Antioxidant enzyme system (SOD, POD, CAT, etc.) plays an important role in active oxygen metabolism to protect plants from damage of free radicals [Citation43]. AsA, which reacts with a range of ROS, including H2O2 and O2−·, is a vital water-soluble antioxidant in plant cells [Citation7,Citation44].
In the study, the antioxidant enzyme activity of Huangguogan leaves was increased to adapt to the drought stress, and the activity of SOD and POD was further increased by MeJA treatment, it was consistent with previous research results in other abiotic stresses [Citation45]. AsA was also rapidly rising as a substance for scavenging H2O2, and the rate of active oxygen removal was not as fast as the generation rate with the passage of drought stress time (). AsA was continuously consumed, and the generated AsA was insufficient to maintain the removal of ROS, led to the accumulation of O2−· and H2O2. Moreover, accumulation of MDA further damaged intracellular nucleic acids, proteins and lipids, and the excessive reactive oxygen attacked enzyme proteins, resulting in the decrease of antioxidant enzyme activity [Citation46,Citation47]. In this experiment, MeJA improved photosynthesis, osmotic regulation and antioxidant activity of Huangguogan leaves under drought conditions, but the change trend of MeJA treatment at three concentrations was consistent with DS. This suggests that exogenous MeJA can further enhance resistance and slow down the rate of damage within a certain range.
We also found that the gas exchange parameters have little relation with the concentration of MeJA, especially in Pn, Gs, and Tr. This may be because the gas exchange parameters are relatively low due to stomatal closure after drought stress, while exogenous MeJA treatment could only enhance drought stress resistance by improving related indicators of resistance.
Conclusion
Drought stress decreased Chl a, Chl b, Chl a + b content and photosynthesis, and increased the osmotic adjustment substances, antioxidant activity and ROS. The content of proline and AsA, and the activity of SOD and POD were significantly increased by exogenous MeJA application, which resulted in decreasing MDA, H2O2, and O2−· contents and cell membrane permeability under drought stress. Therefore, exogenous MeJA application could induce drought stress tolerance by increasing the osmotic adjustment substances, antioxidant activities and photosynthesis.
Author contribution
Bo Xiong, Yuan Wang, and Yue Zhang conceived and planned the experiments. Mengmeng Ma, Yifei Gao, and Zhiyang Zhou contributed to samples collection. Yuan Wang, Yue Zhang, Mengmeng Ma, and Bozhi Wang carried out the experiments. Honghong Deng, Tie Wang, and Jin Wang analyzed the data. Bo Xiong, Yuan Wang, and Xiulan Lv took the lead in writing the manuscript. Bo Xiong, Xun Wang and Zhihui Wang designed the study and gave final approval of the version to be submitted. All authors provided critical feedback and helped shape the research, analysis and manuscript. Bo Xiong and Yuan Wang contributed equally to this work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Nath R, Nath D, Li Q, et al. Impact of drought on agriculture in the Indo-Gangetic plain, India. Adv Atmos Sci. 2017 Mar;34(3):335–346. .
- Ullah A, Nisar M, Ali H, et al. Drought tolerance improvement in plants: an endophytic bacterial approach. Appl Microbiol Biot. 2019 Sep;103(18):7385–7397. .
- Fugate KK, Lafta AM, Eide JD, et al. Methyl jasmonate alleviates drought stress in young sugar beet (Beta vulgaris L.) plants. J Agron Crop Sci. 2018 Dec;204(6):566–576. .
- Ober ES, Rajabi A. Abiotic stress in sugar beet. Sugar Tech. 2010;12(3–4):294–298.
- Tarkalson DD, King BA. Effects of tillage and irrigation management on sugarbeet production. Agron J. 2017 Sep-Oct;109(5):2396–2406. .
- Naseer MAUR, Mehdi M, Ashfaq M, et al. Effect of marketing channel choice on the profitability of citrus farmers: evidence form Punjab-Pakistan. Pak J Agr Sci. 2019 Dec;56(4):1003–1011. .
- Xiong B, Ye S, Qiu X, et al. Exogenous spermidine alleviates fruit granulation in a Citrus cultivar (Huangguogan) through the antioxidant pathway. Acta Physiologiae Plantarum. 2017;4(39):98.
- Silva CC, Molina RO, Back L, et al. The effect of drought conditions on sweet orange (Citrus sinensis) plants infected with citrus tristeza virus (CTV). Trop Plant Pathol. 2019 Aug;44(4):335–342. .
- Doria MS, de Sousa AO, Barbosa CD, et al. Citrus tristeza virus (CTV) causing proteomic and enzymatic changes in sweet orange variety “Westin”. Plos One. 2015 Jul 24;10(7):e0130950.
- Jozwiak W, Politycka B. Effect of selenium on alleviating oxidative stress caused by a water deficit in cucumber roots. Plants-Basel. 2019Jul;8:7.
- Bodega G, Alique M, Puebla L, et al. Microvesicles: ROS scavengers and ROS producers. J Extracell Vesicles. 2019;8(1):1626654.
- Pinto-Marijuan M, Munne-Bosch S. Ecophysiology of invasive plants: osmotic adjustment and antioxidants. Trends Plant Sci. 2013 Dec;18(12):660–666. .
- Yu XX, Zhang W, Zhang Y, et al. The roles of methyl jasmonate to stress in plants. Funct Plant Biol. 2019;46(3):197–212.
- Savchenko TV, Rolletschek H, Dehesh K. Jasmonates-mediated rewiring of central metabolism regulates adaptive responses. Plant Cell Physiol. 2019 Dec 1;60(12):2613–2620. .
- Kanagendran A, Chatterjee P, Liu B, et al. Foliage inoculation by Burkholderia vietnamiensis CBMB40 antagonizes methyl jasmonate-mediated stress in Eucalyptus grandis. J Plant Physiol. 2019Nov;242:153032.
- Jiang YF, Ye JY, Li S, et al. Methyl jasmonate-induced emission of biogenic volatiles is biphasic in cucumber: a high-resolution analysis of dose dependence. J Exp Bot. 2017 Jul 20;68(16):4679–4694. .
- Shi J, Ma CY, Qi DD, et al. Transcriptional responses and flavor volatiles biosynthesis in methyl jasmonate-treated tea leaves. Bmc Plant Biol. 2015 Sep;30;15(1):1–10. .
- Sadeghipour O. Amelioration of salinity tolerance in cowpea plants by seed treatment with methyl jasmonate. Legume Res. 2017 Dec;40(6):1100–1106. .
- Ahmadi FI, Karimi K, Struik PC. Effect of exogenous application ofmethyl jasmonate on physiological and biochemical characteristics of Brassica napus L. cv. Talaye under salinity stress. S Afr J Bot. 2018Mar;115:5–11.
- Wu HL, Wu XL, Li ZH, et al. Physiological evaluation of drought stress tolerance and recovery in cauliflower (Brassica oleracea L.) seedlings treated with methyl jasmonate and coronatine. J Plant Growth Regul. 2012 Mar;31(1):113–123. .
- Gine-Bordonaba J, Terry LA. Effect of deficit irrigation and methyl jasmonate application on the composition of strawberry (Fragaria x ananassa) fruit and leaves. Sci Hortic-Amsterdam. 2016 Feb 16;199: 63–70. .
- Mohamed HI, Latif HH. Improvement of drought tolerance of soybean plants by using methyl jasmonate. Physiol Mol Biol Pla. 2017 Jul;23(3):545–556. .
- Rohwer CL, Erwin JE. Horticultural applications of jasmonates: a review. J Hortic Sci Biotech. 2008 May;83(3):283–304. .
- Riemann M, Dhakarey R, Hazman M, et al. Exploring jasmonates in the hormonal network of drought and salinity responses. Front Plant Sci. 2015Dec;1:6.
- Balfagon D, Zandalinas SI, Gomez-Cadenas A. High temperatures change the perspective: integrating hormonal responses in citrus plants under co-occurring abiotic stress conditions. Physiol Plant. 2019 Feb;165(2):183–197. .
- Vives-peris V, Marmaneu D, GÓmez-cadenas A, et al. Characterization of Citrus WRKY transcription factors and their responses to phytohormones and abiotic stresses. Biol Plant. 2018;62(1):33–44.
- Huang Y, Sheng J, Yang F, et al. Effect of enzyme inactivation by microwave and oven heating on preservation quality of green tea. J Food Eng. 2007;78(2):687–692. .
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 07;72(1–2):248–254.
- Wei KL, Ma C, Sun K, et al. Relationship between optical properties and soluble sugar contents of apple flesh during storage. Postharvest Biol Tec. 2020 Jan 159. DOI:10.1016/j.postharvbio.2019.111021
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973 August 01;39(1):205–207.
- Huang C, He W, Guo J, et al. Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J Exp Bot. 2005;56(422):3041–3049.
- Orozco-Cardenas LM. Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell. 2001;13(1):179–191.
- Chen F, Wang F, Wu F, et al. Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol Biochem. 2010;48(8):663–672.
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. 1968;125(1):189–198.
- Hasanuzzaman M, Hossain MA, Fujita M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol Rep. 2011;5(4):353–365.
- Duan J, Li J, Guo S, et al. Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J Plant Physiol. 2008;165(15):0–1635.
- Farquhar GD, Sharkey TD. Stomatal Conductance and Photosynthesis. Annurevplant Physiol. 1982;33(1):317–345.
- Ma C, Wang ZQ, Zhang LT, et al. Photosynthetic responses of wheat (Triticum aestivum L.) to combined effects of drought and exogenous methyl jasmonate. Photosynthetica. 2014 Sep;52(3):377–385.
- Wang XP, Liu HL, Yu FL, et al. Differential activity of the antioxidant defence system and alterations in the accumulation of osmolyte and reactive oxygen species under drought stress and recovery in rice (Oryza sativa L.) tillering. Sci Rep-Uk. 2019 Jun 12;9. doi:10.1038/s41598-019-44958-x
- Wu SW, Hu CX, Tan QL, et al. Drought stress tolerance mediated by zinc-induced antioxidative defense and osmotic adjustment in cotton (Gossypium Hirsutum). Acta Physiol Plant. 2015 Aug;37(8):8.
- Choudhury FK, Rivero RM, Blumwald E, et al. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017 Jun;90(5):856–867.
- Wei TL, Wang Y, Xie ZZ, et al. Enhanced ROS scavenging and sugar accumulation contribute to drought tolerance of naturally occurring autotetraploids in Poncirus trifoliata. Plant Biotechnol J. 2019 Jul;17(7):1394–1407.
- Al-Saman MA, Abdella A, Mazrou KE, et al. Antimicrobial and antioxidant activities of different extracts of the peel of kumquat (Citrus japonica Thunb). J Food Meas Charact. 2019 Dec;13(4):3221–3229.
- Liao L, Dong T, Qiu X, et al. Antioxidant enzyme activity and growth responses of Huangguogan citrus cultivar to nitrogen supplementation. Biosci Biotechnol Biochem. 2019Jun;27:1–13.
- Miranshahi B, Sayyari M. Methyl jasmonate mitigates drought stress injuries and affects essential oil of summer savory. J Agr Sci Tech-Iran. 2016 Nov-Dec;18(6):1635–1645.
- Payton P, Webb R, Kornyeyev D, et al. Protecting cotton photosynthesis during moderate chilling at high light intensity by increasing chloroplastic antioxidant enzyme activity. J Exp Bot. 2001 Dec;52(365):2345–2354.
- Garmash EV, Velegzhaninov IO, Grabelnych OI, et al. Expression profiles of genes for mitochondrial respiratory energy-dissipating systems and antioxidant enzymes in wheat leaves during de-etiolation. J Plant Physiol. 2017Aug;39:110–121.