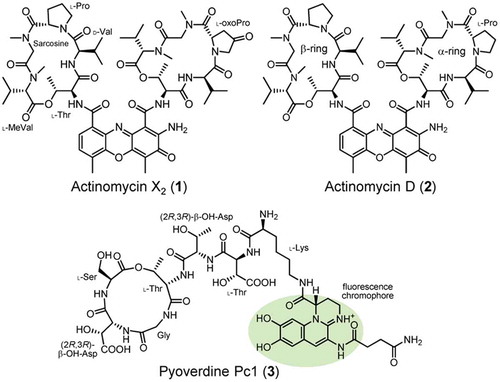
ABSTRACT
Pyoverdines, a group of peptide siderophores produced by Pseudomonas species, function not only in iron acquisition, but also in their virulence in hosts. Thus, chemical inhibition of pyoverdine production may be an effective strategy to control Pseudomonas virulence. In the plant pathogen Pseudomonas cichorii SPC9018 (SPC9018), pyoverdine production is required for virulence on eggplant. We screened microbial culture extracts in a pyoverdine-production inhibition assay of SPC9018 and found Streptomyces sp. RM-32 as a candidate-producer. We isolated two active compounds from RM-32 cultures, and elucidated their structures to be actinomycins X2 and D. Actinomycins X2 and D inhibited pyoverdine production by SPC9018 with IC50 values of 17.6 and 29.6 μM, respectively. Furthermore, pyoverdine production in other Pseudomonas bacteria, such as the mushroom pathogen P. tolaasii, was inhibited by the actinomycins. Therefore, these actinomycins may be useful as chemical tools to examine pyoverdine functions and as seed compounds for anti-Pseudomonas virulence agents.
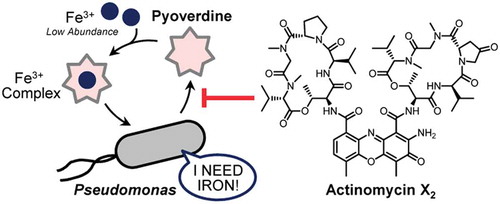
Actinomycins may be useful as chemical tools to examine pyoverdine functions and as seed compounds for anti-Pseudomonas virulence agents.
Iron is an essential trace element for nearly all living organisms, and plays key catalytic and structural roles in proteins. Although it is relatively abundant in the Earth’s crust, free iron (Fe3+) acquisition poses a challenge to bacteria due to its toxicity and poor solubility [Citation1]. As a result, bacteria have evolved synthetic pathways to produce and secrete high-affinity sequestering agents termed siderophores, which bind Fe3+ and are actively transported back into the cell [Citation2]. The yellow-green fluorescent siderophore pyoverdines (an example is shown in ) are the main iron uptake system of Pseudomonas bacteria [Citation3–Citation6]. Thus far, more than 100 pyoverdines have been reported. They are composed of a highly conserved dihydroxyquinoline fluorescent chromosphere attached to a variable peptide chain. In the opportunistic human pathogen Pseudomonas aeruginosa, iron acquisition by pyoverdines is essential for infection and virulence in different experimental models [Citation7]. Similarly, the pyoverdine-deficient mutants of Pseudomonas syringae pv. tabaci 6605 exhibited reduced virulence on tobacco plants due to the low production of virulence factors [Citation8]. Therefore, targeting pyoverdine production may be a good strategy to reduce Pseudomonas virulence, and has thus received much attention.
The analysis of Pseudomonas genomes suggested that there are analogous biosynthetic pathways for pyoverdines in Pseudomonas bacteria [Citation3–Citation6]. Under iron starvation, the δ-factor PvdS activates the transcription of pyoverdine biosynthetic genes [Citation9,Citation10]. PvdL, the first nonribosomal peptide synthetase (NRPS) involved in the assembly of the pyoverdine backbone, is highly conserved among all fluorescent pseudomonads, suggesting that the first steps of siderophore assembly – attachment of a fatty acid to an L-Glu, followed by the anchoring of L-Tyr and L-Dab (L-2,4-diaminobutyric acid) forming the dihydroxyquinoline chromophore – are common to all pyoverdines [Citation3–Citation6,Citation11]. On the other hand, the NRPS genes pvdI, pvdJ, and pvdD, and the export ABC transporter pvdE are highly divergent, explaining the diversity of the peptide sequence among pyoverdines [Citation3–Citation6]. Therefore, inhibitors of pyoverdine production should target the transcriptional regulation or chromophore construction pathway, including the PvdL enzyme, rather than the other Pvd NRPS enzymes and transporter.
The “multi-host” plant bacterium Pseudomonas cichorii SPC9018 causes necrotic lesions on eggplant leaves and rot symptoms on lettuce leaves [Citation12,Citation13]. The development of necrotic lesions on eggplant following P. cichorii SPC9018 infection is induced by programmed cell death (PCD) [Citation12]. The generation of intracellular reactive oxygen species and caspase III-like protease activity in eggplant cells are required for this PCD. However, heterochromatin aggregation and genomic DNA laddering followed by P. cichorii SPC9018 infection lead to PCD and rot development in lettuce [Citation13]. In addition, the rot symptoms observed on lettuce leaves may be synergistically increased by both apoptosis – and necrosis-like PCD. Therefore, P. cichorii SPC9018 may have several virulence strategies depending on plant species, and alternatively each host plant may differentially respond to the bacterial infection.
In our previous study, P. cichorii SPC9018 was found to produce pyoverdines under iron starvation conditions, and application of the phytosiderophore mugineic acid reduced its population growth in culture [Citation14,Citation15]. Furthermore, mugineic acid increased the expression of fecA (involved in citrate-mediated iron uptake), pvdL, and pvdR (pyoverdine exporter), but reduced cell adhesion in P. cichorii SPC9018. These results suggested that mugineic acid addition leads to the iron-limited conditions in P. cichorii SPC9018 cells. Co-injection of P. cichorii SPC9018 with mugineic acid reduced its in vivo growth, leading to reduced virulence on eggplant. Therefore, iron acquisition may also play an important role in P. cichorii SPC9018 virulence on eggplant.
The aim of the present study was to find compounds capable of controlling pyoverdine production in Pseudomonas bacteria. The compounds that specifically regulate pyoverdine production have been sought in medical and agrochemical pharmacology because they may prevent virulence without directly killing Pseudomonas bacteria, thereby avoiding the emergence of drug-resistant strains [Citation16]. Therefore, we screened actinomycete strains isolated from soil for the inhibition of pyoverdine production in P. cichorii SPC9018 cultures. Actinomycins X2 (1) and D (2) () were found to inhibit the production of pyoverdines in P. cichorii SPC9018, and possibly several strains of Pseudomonas bacteria. These are the first reported natural products possessing inhibitory activity on pyoverdine production in Pseudomonas bacteria. We also elucidated the absolute configuration of pyoverdine Pc1 (3) (), a major pyoverdine in P. cichorii strains.
Materials and methods
General information
All purchased chemicals were used without further purification. Column chromatography was performed on a Wakogel C-200 gel (Wako Pure Chemical Industries, Osaka, Japan). Fluorescence measurements were performed on an FP-6200 spectrofluorometer (Jasco, Tokyo, Japan). NMR spectra were recorded on a JNM-AL400 (JEOL, Tokyo, Japan). Chemical shifts are reported as δ values (ppm); solvent signals (CDCl3: δH = 7.26, δC = 77.0) were used as internal references. HPLC experiments were performed using a LaChrom Elite HPLC system (Hitachi High-Technologies, Tokyo, Japan) and Prominence HPLC system (Shimadzu, Kyoto, Japan). LC/MS data were obtained using an LCMS-2020 spectrometer (Shimadzu). Solvents for HPLC and LC/MS were purchased from Wako Pure Chemical Industries.
Preparation of the extracts of actinomycete cultures
Actinomycete strains used for screening were isolated from soil collected in Osaka (Japan). Streptomyces sp. RM-32 was identified by 16S rRNA gene analysis (TechnoSuruga Laboratory, Shizuoka, Japan). The actinomycete strains isolated from soil samples were grown in medium (D-glucose 1 g, dextrin hydrate 2 g, soytone 1.5 g, yeast extract 0.1 g, and CaCO3 0.1 g, in 100 mL of water, pH 7.0) for 5 days at 30°C. Culture broth was extracted using an equal volume of acetone and the filtrates were used in the following inhibition assay.
Inhibition assays for pyoverdine production
P. cichorii SPC9018 cells grown in PY medium (peptone 0.5 g and yeast extract 0.2 g, in 100 mL of water) at 30°C for 4–6 h were diluted to an OD600 of 0.05 in iron-limited GASN medium (L-asparagine·H2O 0.2 g, D-glucose 0.7 g, Na2HPO4·12H2O 242 mg, KH2PO4 44 mg, MgSO4·7H2O 20 mg, in 100 mL of water, pH 7.0) [Citation14]. A sample (50 μL) of actinomycete culture extract was added to a P. cichorii cell suspension (2 mL). The cultures in 15-mL test tubes were incubated for 24 h at 20°C with shaking. After measuring the OD600, the fluorescence Ex280/Em490 values of the cell free supernatants were measured. The inhibitory activities of fractionated samples and actinomycins were also evaluated using this method. The IC50 values of actinomycins were calculated by fitting the data points to a logistic curve using GraphPad Prism 6J software (San Diego, CA, USA).
Isolation and identification of actinomycins
Seed culture of Streptomyces sp. RM-32 (20 mL) was inoculated into new medium for actinomycetes (400 mL) and incubated for 2 days at 30°C with shaking. The culture broth was extracted with EtOAc (400 mL ×3) and the combined extract was dried over Na2SO4. After evaporation of EtOAc, the concentrate (198 mg) was chromatographed on Wakogel C-200 gel by eluting with n-hexane/EtOAc/MeOH (20% stepwise). The fractions eluted with 100% EtOAc and 20% MeOH inhibited pyoverdine production by P. cichorii SPC9018. These fractions were combined (151 mg) and subjected to preparative HPLC (column, CAPCELL PAK C18 MGII (250 × 10 mm, 5 μm, Shiseido, Tokyo, Japan); eluent, 70% aq. MeOH; flow rate, 4 mL/min) to give actinomycins X2 (1) (2.7 mg) and D (2) (4.5 mg).
Marfey’s analysis of pyoverdine Pc1
Compound 3 (2 mg) was treated at 110°C for 12 h with 6 M HCl (2 mL) [Citation17]. After cooling to room temperature, the sample was dried under a vacuum. The residue was dissolved in 1 M NaHCO3 (500 μL) and reacted with L-FDAA (1-fluoro-2,4-dinitrophenyl-5-L-alaninamide) (120 μL of 10 mM acetone solution) at 40°C for 2 h. After cooling, the sample was quenched with 2 M HCl and dried under a vacuum. The solid residue was dissolved in 50% aq. MeCN and analyzed by LC/MS (column, InertSustain C18 (150 × 2.1 mm, 3 μm, GL Sciences, Tokyo, Japan); eluent, 10 − 50% MeCN in 0.1% aq. formic acid; flow rate, 200 μL/min).
Virulence assay
Eggplant plants (Solanum melongena cv. Senryo-Nigo) were grown in pots containing a high-grade potting mix (Tsuchitaro, Sumitomo Forestry Landscaping, Aichi, Japan) at 28°C in an incubator. Light (16 h/day) was supplied at 10,000 lx throughout the experimental period. Five-week-old plants were inoculated by leaf infiltration using a 1-mL disposable syringe with P. cichorii SPC9018 cell suspension (20 μL). The inoculum concentrations of P. cichorii cells were adjusted to an OD600 of 0.1. Following inoculation, plants were incubated at 28°C and inspected for symptoms 3 days after inoculation.
Iron-chelation assay
Test compound (0.1 mg) in 50% MeCN (0.5 mL) was added to 1 μM FeCl3 solution (10 μL) and mixed gently. The mixture was analyzed by LC/MS (column, InertSustain C18 (150 × 2.1 mm, 3 μm); eluent, 20−95% MeCN in 0.1% aq. formic acid for actinomycins and 5% MeCN in 0.1% aq. formic acid for pyoverdine Pc1; flow rate, 200 μL/min).
Results
Determination of the absolute configuration of the pyoverdine produced by P. cichorii SPC9018
We previously reported that P. cichorii SPC9018 produces pyoverdine Pc1 (3) () as a major siderophore in iron-limited GASN media [Citation14,Citation15]. Compound 3 was expected to be identical to the pyoverdine produced by P. cichorii LMG8401; however, the absolute configurations of Thr (×2) and β-OH-Asp (β-hydroxyaspartic acid, ×2) residues in 3 have yet to be examined [Citation18]. Thus, we first elucidated the absolute configuration of 3 by Marfey’s analysis [Citation19,Citation20]. Pyoverdine Pc1 (3) isolated from P. cichorii SPC9018 cultures was acid hydrolyzed and the free amino acids in the hydrolysate were derivatized with L-FDAA. The LC/MS analysis of the L-FDAA derivatives confirmed the presence of Gly, L-Ser, and L-Lys (). The absolute configurations of Thr residues were determined to be L-Thr by comparing the LC/MS retention times with the L-FDAA derivatives of the four stereoisomers ()). In the case of β-OH-Asp, the erythro diastereomers were not commercially available, and therefore prepared by epoxide aminolysis from (2R,3R)-diethyl 2,3-epoxysuccinate (), upper). The L-FDAA derivative of β-OH-Asp from 3 had an identical retention time to that of (2R,3R)-β-OH-Asp (), lower). Taken together, we clarified the complete structure of pyoverdine Pc1 (3), as shown in .
Figure 2. Elucidation of the absolute configurations of amino acid residues in pyoverdine Pc1 (3). (a) Marfey’s analysis of Gly, Ser, and Lys residues in 3. The peak identity was confirmed by comparisons of LC/MS retention times with those of the L-FDAA derivatives of standard amino acids. (b) Determination of the absolute configuration of Thr residue in 3. The L-FDAA derivative of Thr from 3 had an identical LC/MS retention time to that of L-Thr. In the case of Thr/allo-Thr, L-isomers gave faster retention times than those of D-isomers [Citation19]. (c) Preparation of erythro-β-OH-Asp and determination of the absolute configuration of β-OH-Asp residue in 3. The L-FDAA derivative of β-OH-Asp from 3 had an identical LC/MS retention time to that of (2R,3R)- β-OH-Asp. In the case of threo-/erythro-β-OH-Asp, D-isomers gave faster retention times than those of L-isomers [Citation19].
![Figure 2. Elucidation of the absolute configurations of amino acid residues in pyoverdine Pc1 (3). (a) Marfey’s analysis of Gly, Ser, and Lys residues in 3. The peak identity was confirmed by comparisons of LC/MS retention times with those of the L-FDAA derivatives of standard amino acids. (b) Determination of the absolute configuration of Thr residue in 3. The L-FDAA derivative of Thr from 3 had an identical LC/MS retention time to that of L-Thr. In the case of Thr/allo-Thr, L-isomers gave faster retention times than those of D-isomers [Citation19]. (c) Preparation of erythro-β-OH-Asp and determination of the absolute configuration of β-OH-Asp residue in 3. The L-FDAA derivative of β-OH-Asp from 3 had an identical LC/MS retention time to that of (2R,3R)- β-OH-Asp. In the case of threo-/erythro-β-OH-Asp, D-isomers gave faster retention times than those of L-isomers [Citation19].](/cms/asset/42978039-1fae-4dfd-ae62-793920f8c1d1/tbbb_a_1785839_f0002_oc.jpg)
Screening of actinomycete isolates for pyoverdine-production inhibitors
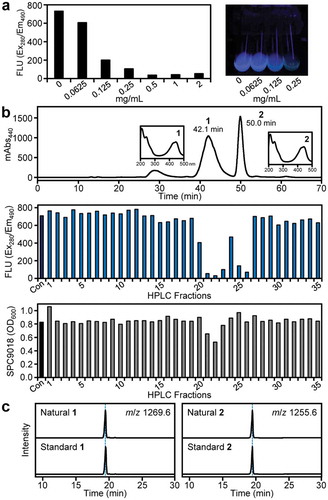
To find microbial compounds that inhibit pyoverdine production by P. cichorii SPC9018, we used a fluorescence-based assay (excitation 280 nm/emission 490 nm) to detect the dihyroxyquinolinone chromophore in pyoverdine Pc1 (3) and its derivatives. In our continuing studies on bioactive microbial metabolites [Citation21], we have isolated many actinomycete strains from soil samples collected in Osaka. We screened the acetone extracts of their culture broths (200 strains), and found that strain RM-32 produces compounds capable of inhibiting the accumulation of fluorescent pyoverdines in P. cichorii SPC9018 cultures in a dose-dependent manner (). Strain RM-32 was identified as Streptomyces sp. by 16S rRNA gene analysis. We also obtained two additional actinomycete strains that inhibited pyoverdine production of P. cichorii SPC9018, but their microscopic observation suggested that these strains are identical to strain RM-32 or its derivatives. Therefore, we selected strain RM-32 for further evaluation.
Figure 3. Identification of actinomycins X2 (1) and D (2) as pyoverdine-production inhibitors from Streptomyces sp. RM-32. (a) Inhibitory activity of RM-32 culture extract on pyoverdine production by P. cichorii SPC9018 (left). Test tubes containing P. cichorii SPC9018 cultures under UV352 irradiation are shown as a representative assay image (right). (b) HPLC purification of actinomycins X2 (1) and D (2) (upper). UV spectra of 1 and 2 are also shown as insets. Inhibition of pyoverdine production in P. cichorii SPC9018 by the HPLC fractions (middle). The fluorescence values (Ex280/Em490) from pyoverdines were measured. Effects of the HPLC fractions on P. cichorii SPC9018 growth (lower). Con: control. (c) LC/MS comparison of natural and standard actinomycins X2 (1) (left) and D (2) (right).
Isolation and identification of actinomycins X2 and D
To identify the active compounds produced by Streptomyces sp. RM-32, we prepared a large amount of the EtOAc extract of RM-32 cultures. Bioassay-guided fractionations by silica gel column chromatography and the subsequent reversed-phase HPLC revealed the presence of two active compounds (peaks 1 and 2) in the RM-32 culture extract ()). These compounds strongly inhibited fluorescence accumulation in P. cichorii SPC9018 cultures. Weak antibacterial activity was observed when the HPLC fractions containing peak 1 were assayed. We thus elucidated the structures of compounds 1 and 2.
The UV spectrum of compound 1 gave a λmax at 445 nm (), upper), suggesting that it possesses a characteristic chromophore. As the ESI-MS of 1 gave the [M + H]+ ion at m/z 1270 and the [M − H]− ion at m/z 1268, the molecular weight was 1269. The 1H-NMR spectrum (Table S1) demonstrated resonances for four amide protons (δH 7.13−8.20 ppm) and many α-methine/methylene protons (δH 3.55−6.54 ppm, containing downfield shifted proton signals), suggesting that the compound was a peptidyl secondary metabolite. The 1H- and 13C-NMR signals of 1 were assigned based on 1H−1H COSY, HMQC, and HMBC spectra, revealing that compound 1 possesses two macrocycles, Thr-Val-oxoPro (4-oxoproline)-sarcosine-MeVal (N-methylvaline) (α-ring) and Thr-Val-Pro-sarcosine-MeVal (β-ring), and a heterocyclic chromophore (Figure S1). Therefore, the compound was suggested to be actinomycin X2 [Citation22]. The structural identity including the absolute configuration was confirmed by LC/MS analysis of 1 and its commercially available standard (), left). Therefore, the characteristic UV absorption of 1 was revealed to be derived from a central phenoxazinone chromophore. Similarly, the 1H-NMR spectrum of 2 (Table S2) gave proton signals similar to those of 1 except for the substitution of an oxoPro residue with Pro one (α-ring), suggesting that compound 2 was actinomycin D [Citation22]. The identity of 2 was confirmed by LC/MS analysis using the commercially available standard (), right).
Inhibition of pyoverdine production by actinomycins
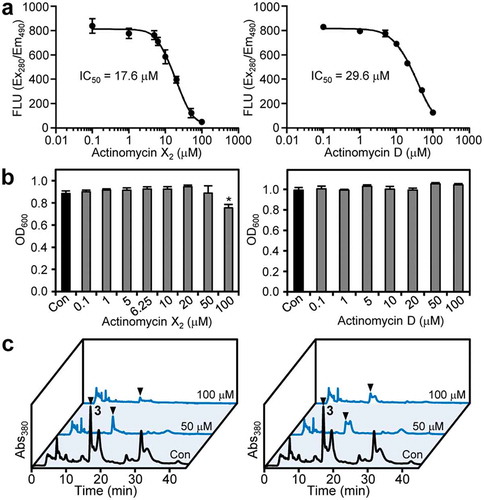
The inhibitory activity of actinomycins X2 (1) and D (2) on pyoverdine production was evaluated quantitatively using their commercially available standards. As shown in ), these compounds exhibited inhibitory effects on pyoverdine production by P. cichorii SPC9018 in a dose-dependent manner. The IC50 values of 1 and 2 were 17.6 and 29.6 μM, respectively. The significant growth inhibition of P. cichorii SPC9018 by actinomycin X2 (1) was observed only at 100 μM, and actinomycin D (2) had no such influence on bacterial growth at the concentrations used ()). Therefore, the antibacterial activity observed at the HPLC purification step may have been due to impurities. Therefore, the actinomycins inhibited the production of pyoverdines without markedly affecting bacterial growth.
Figure 4. Inhibitory effects of actinomycins X2 (1) and D (2) on pyoverdine production by P. cichorii SPC9018. (a) Dose-response curves of actinomycins X2 (1) (left) and D (2) (right) regarding to the inhibition of pyoverdine production by P. cichorii SPC9018. Error bars are the mean ±SD (n = 3). (b) Effects of actinomycins X2 (1) (left) and D (2) (right) on P. cichorii SPC9018 growth. Error bars are the mean ±SEM (n = 3). *p < 0.05 versus control (Dunnett’s test). (c) Comparison of HPLC profiles of P. cichorii SPC9018 cultures incubated with actinomycins X2 (1) (left) and D (2) (right). The arrowheads indicate the peaks of pyoverdine Pc1 (3).
To investigate further, we compared the HPLC profiles (absorbance at 380 nm) between control and actinomycin-supplemented cultures of P. cichorii SPC9018. Pyoverdine Pc1 (3) was detected at 16.9 min by HPLC analysis of the control culture extract ()). The peak area of 3 decreased according to the concentrations of actinomycins X2 (1) and D (2) applied. In addition, other HPLC peaks, which may be corresponding to unidentified pyoverdines, exhibited similar dynamics in response to actinomycin application. Therefore, the actinomycins may inhibit the common step(s) of pyoverdine production, including transcriptional regulation, in P. cichorii SPC9018.
Evaluation of iron-chelating activity of actinomycins
To the best of our knowledge, it was unclear whether actinomycins can chelate Fe3+. Thus, these compounds may compete against the iron chelation of Pseudomonas pyoverdines. We therefore evaluated the iron-chelating activity of actinomycins X2 (1) and D (2). After incubation with Fe3+, pyoverdine Pc1 (3) formed a complex with Fe3+, and exhibited a mass shift and decrease in hydrophobicity ()). On the other hand, both actinomycins had no such mass shift or change in hydrophobicity after incubation with Fe3+ ()). Therefore, actinomycins X2 (1) and D (2) do not act as iron chelators.
Figure 5. Evaluation of iron-chelation and virulence-inhibition activities of actinomycins. (a) LC/MS analysis of pyoverdine control and after incubation with Fe3+. The detection of pyoverdine Pc1 and its Fe3+ complex were based on [M + H]2+ and [M − 2H+Fe]2+, respectively. (b) LC/MS analysis of actinomycin control and after incubation with Fe3+. The data of actinomycins X2 (1) (upper) and D (2) (lower) are shown. (c) Effects of actinomycins X2 (1) (left) and D (2) (right) on the attenuation of P. cichorii SPC9018 virulence. P. cichorii cell suspensions with/without the actinomycins were inoculated into eggplant leaves.
![Figure 5. Evaluation of iron-chelation and virulence-inhibition activities of actinomycins. (a) LC/MS analysis of pyoverdine control and after incubation with Fe3+. The detection of pyoverdine Pc1 and its Fe3+ complex were based on [M + H]2+ and [M − 2H+Fe]2+, respectively. (b) LC/MS analysis of actinomycin control and after incubation with Fe3+. The data of actinomycins X2 (1) (upper) and D (2) (lower) are shown. (c) Effects of actinomycins X2 (1) (left) and D (2) (right) on the attenuation of P. cichorii SPC9018 virulence. P. cichorii cell suspensions with/without the actinomycins were inoculated into eggplant leaves.](/cms/asset/f44ab9f5-7080-4257-945b-e154e5c6572f/tbbb_a_1785839_f0005_oc.jpg)
Effects of actinomycins on P. cichorii virulence
The above in vitro assays revealed that actinomycin X2 (1) and D (2) may cause the iron starvation in P. cichorii SPC9018 cells by inhibiting pyoverdine production, but not by chelating Fe3+. The virulence of P. cichorii SPC9018 on eggplant can be evaluated by a leaf-inoculation assay [Citation14,Citation15]. Using this method, we investigated the in vivo activity of the actinomycins on P. cichorii virulence. However, the actinomycins were highly phytotoxic to eggplant and necrosis developed more rapidly on the leaves than that due to PCD caused by P. cichorii virulence ()). Therefore, the in vivo effectiveness of actinomycin X2 (1) and D (2) on P. cichorii virulence was unable to be evaluated.
Effects of actinomycins on pyoverdine production in other Pseudomonas bacteria
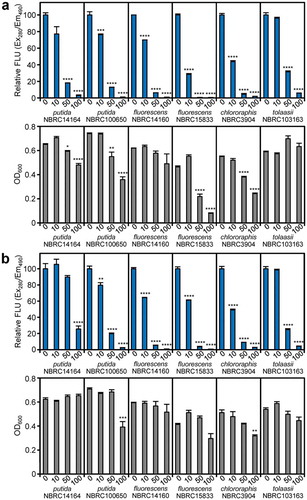
Whether the actinomycins are also active against pyoverdine production in other Pseudomonas bacteria was also of interest. Therefore, we examined the effects of the actinomycins on soil Pseudomonas species, P. putida NBRC14164, P. putida NBRC100650, P. fluorescens NBRC14160, P. fluorescens NBRC15833, P. chlororaphis NBRC3904, and P. tolaasii NBRC103163 (a mushroom pathogen) [Citation23]. Actinomycin X2 (1) exerted inhibitory effects on pyoverdine production in these strains ()). Similarly, actinomycin D (2) inhibited the production of pyoverdines in strains NBRC100650, NBRC14160, NBRC3904, and NBRC103163, but the effects were slightly lower than those of actinomycin X2 (1) in strains NBRC14164 and NBRC15833 ()). In particular, pyoverdine production in P. fluorescens NBRC14160 and P. tolaasii NBRC103163 was inhibited without a notable reduction of bacterial growth. It is difficult to assess whether the observed growth inhibition by the actinomycins (especially in P. fluorescens NBRC158333 and P. chlororaphis NBRC3904) was due to their antibiotic effects or the marked limitation of iron acquisition. Taken together, these results suggested that the actinomycins also inhibit pyoverdine production in other Pseudomonas bacteria.
Figure 6. Inhibitory activities of actinomycins on pyoverdine production and growth of Pseudomonas bacteria. (a) Effects of actinomycin X2 (1) on pyoverdine production and bacterial growth. (b) Effects of actinomycin D (2) on pyoverdine production and bacterial growth. Error bars are the mean ±SEM (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 versus 0 μM control (Dunnett’s test).
Discussion
The production of pyoverdines is an attractive drug target because iron acquisition by pyoverdines is highly important for Pseudomonas virulence but not essential for normal bacterial growth [Citation7]. Prof. Gulick and colleagues reported several compounds that can inhibit the enzymes involved in pyoverdine biosynthesis [Citation24,Citation25]. Among them, ML318 was found as an inhibitor of PvdQ (an N-acyl hydrolase involved in pyoverdine maturation) by high-throughput screening, and it inhibited the production of a pyoverdine in P. aeruginosa cells with an IC50 value of 1.9 μM [Citation25]. Moreover, the PvdQ inhibitor limited the growth of P. aeruginosa under iron-limited conditions. Although the in vivo effectiveness of ML318 was not confirmed, this study suggested that targeting siderophore production in general can be a suitable strategy to induce iron starvation in Pseudomonas cells.
Actinomycins are a well-known class of chromopeptide produced by Streptomyces species. These compounds were the first to be isolated and identified from a soil actinomycete by Waksman and Woodruff in 1940 [Citation26,Citation27]. Actinomycins inhibit RNA synthesis as a result of their ability to bind DNA at the transcription initiation complex, with their specificity favoring the 5ʹ-GC-3ʹ sequence [Citation28,Citation29]. This is the main mode of action of actinomycins for antitumor effects. In addition, actinomycin D (2) was reported to exhibit antibacterial activity against many Gram-positive bacteria [Citation30]. Recently, Ogasawara et al. reported that actinomycin D (2) can inhibit the Xanthomonas oryzae MurD2 enzyme involved in peptidoglycan biosynthesis and resulted in antibacterial activity [Citation31]. Therefore, actinomycins may have additional biological activities, which have been overlooked due to their strong antitumor and antibacterial activities.
How actinomycins X2 (1) and D (2) exert their inhibitory effects on pyoverdine production in P. cichorii SPC9018 is of interest. Under iron starvation, P. cichorii SPC9018 cells activate the expression of genes involved in pyoverdine production [Citation14]. Therefore, if the actinomycins act as specific (or selective) transcriptional inhibitors of biosynthetic gene expression, the production of pyoverdines in P. cichorii SPC9018 may be inactivated. Related to this, fluocytosine (a prodrug of fluorouracil, which is a chemotherapeutic drug used for solid cancers) was found to inhibit pyoverdine production by down-regulating the expression of the pvdS gene in P. aeruginosa [Citation32–Citation34]. Thus, the transcriptional regulator that controls the expression of pyoverdine biosynthetic genes may be a target of the actinomycins. The size of the actinomycins may be similar to that of pyoverdines, and both compounds are cyclic depsipeptides. Therefore, the possibility that the actinomycins directly inhibit the enzymes involved in pyoverdine production or their transporters was suggested. Further genetic and biochemical studies to examine these possibilities are needed in a future study.
The isolation and structural elucidation of pyoverdine Pc1 (3) was reported previously [Citation18]. However, the absolute configurations of several amino acid residues in 3 remained to be clarified. The present Marfey’s analysis with appropriate standards revealed the absolute configurations of the respective amino acid residues in pyoverdine Pc1 (3). The tentative identification of many pyoverdine compounds has been reported, in which pyoverdine structures were suggested by analyzing MS/MS fragmentation and/or biosynthetic gene clusters [Citation35,Citation36]. However, the absolute configurations of amino acids, especially unusual amino acids, in the pyoverdines remain to be elucidated. The β-OH-Asp residue plays an important role in chelating Fe3+; therefore, it is frequently found in pyoverdines and other peptide siderophores [Citation3–Citation6,Citation35]. Although Reitz et al. very recently reported the in silico prediction of β-OH-Asp stereochemistry by analyzing pyoverdine biosynthetic gene clusters [Citation37], the identification workflow used in this study will be a simple analytical methodology to establish the absolute configuration of β-OH-Asp residue in bacterial peptide siderophores.
In conclusion, we identified actinomycins X2 (1) and D (2) as pyoverdine-production inhibitors in P. cichorii SPC9018. These compounds may be model compounds to evaluate the true effectiveness of chemical inhibition of pyoverdine function in Pseudomonas virulence control. The fluorescence bioassay developed in this study may be useful to find new inhibitors of pyoverdine production. The complete structure of pyoverdine Pc1 (3) was also elucidated. Taken together, this study provides the basis of chemical control of pyoverdine production in pathogenic Pseudomonas bacteria, including P. cichorii strains.
Author contributions
K.K. designed the experiments. K.K. and R.M. performed the experiments and analyzed the data. K.K., R.M., S.T., and Y.H. contributed reagents and materials. K.K. wrote the paper with the help of the coauthors.
SOM200605.pdf
Download PDF (212.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Touati D. Iron and oxidative stress in bacteria. Arch Biochem Biohys. 2000;373:1–6.
- Andrews SC, Robinson AK, Rodríguez-Quiñones F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003;27:215–237.
- Ravel J, Cornelis P. Genomics of pyoverdine-mediated iron uptake in pseudomonads. Trends Microbiol. 2003;11:195–200.
- Visca P, Imperi F, Lamont IL. Pyoverdine siderophores: from biogenesis to biosignificance. Trends Microbiol. 2007;15:22–30.
- Ringel MT, Brüser T. The biosynthesis of pyoverdines. Microb Cell. 2018;5:424–437.
- Schalk IJ, Rigouin C, Godet J. An overview of siderophore biosynthesis among fluorescent Pseudomonads and new insights into their complex cellular organization. Environ Microbiol. 2020;22:1447–1466.
- Visca P. Iron regulation and siderophore signalling in virulence by Pseudomonas aeruginosa. In: Ramos JL, editor. Virulence and gene regulation. Boston, (MA): Springer; 2004. p. p. 69−123.
- Taguchi F, Suzuki T, Inagaki Y, et al. The siderophore pyoverdine of Pseudomonas syringae pv. tabaci 6605 is an intrinsic virulence factor in host tobacco infection. J Bacteriol. 2010;192:117–126.
- Cunliffe HE, Merriman TR, Lamont IL. Cloning and characterization of pvdS, a gene required for pyoverdine synthesis in Pseudomonas aeruginosa: pvdS is probably an alternative sigma factor. J Bacteriol. 1995;177:2744–2750.
- Stintzi A, Johnson Z, Stonehouse M, et al. The pvc gene cluster of Pseudomonas aeruginosa: role in synthesis of the pyoverdine chromophore and regulation by PtxR and PvdS. J Bacteriol. 1999;181:4118–4124.
- Mossialos D, Ochsner U, Baysse C, et al. Identification of new, conserved, non-ribosomal peptide synthetases from fluorescent pseudomonads involved in the biosynthesis of the siderophore pyoverdine. Mol Microbiol. 2002;45:1673–1685.
- Kiba A, Takata O, Ohnishi K, et al. Comparative analysis of induction pattern of programmed cell death and defense-related responses during hypersensitive cell death and development of bacterial necrotic leaf spots in eggplant. Planta. 2006;224:981–994.
- Kiba A, Sangawa Y, Ohnishi K, et al. Induction of apoptotic cell death leads to the development of bacterial rot caused by Pseudomonas cichorii. Mol Plant-Microbe Interact. 2006;19(2):112–122.
- Wali UM, Maenaka R, Mori Y, et al. Implication of limited iron acquisition by Pseudomonas cichorii strain SPC9018 in its reduced virulence on eggplant. J Gen Plant Pathol. 2015;81:136–141.
- Wali UM, Mori Y, Maenaka R, et al. The N-acetyltransferase gene-implicated iron acquisition contributes to host specificity of Pseudomonas cichorii strain SPC9018 and its virulence. Physiol Mol Plant Pathol. 2015;92:14–21.
- Wilson BR, Bogdan AR, Miyazawa M, et al. Siderophores in iron metabolism: from mechanism to therapy potential. Trends Mol Med. 2016;22:1077–1090.
- Murai Y, Mori S, Konno H, et al. Ralstonins A and B, lipopeptides with chlamydospore-inducing and phytotoxic activities from the plant pathogen Ralstonia solanacearum. Org Lett. 2017;19:4175–4178.
- Bultreys A, Gheysen I, Wathelet B, et al. The pyoverdins of Pseudomonas syringae and Pseudomonas cichorii. Z Naturforsch C J Biosci. 2004;59:613–618.
- Fujii K, Ikai Y, Oka H, et al. A nonempirical method using LC/MS for determination of the absolute configuration of constituent amino acids in a peptide: combination of Marfey’s method with mass spectrometry and its practical application. Anal Chem. 1997;69:5146–5151.
- Vijayasarathy S, Prasad P, Fremlin LJ, et al. C3 and 2D C3 Marfey’s methods for amino acid analysis in natural products. J Nat Prod. 2016;79:421–427.
- Hayashi H. Frontier studies on highly selective bio-regulators useful for environmentally benign agricultural production. Biosci Biotechnol Biochem. 2015;79:877–887.
- Matsui T, Tanaka J, Namihira T, et al. Antibiotics production by an actinomycete isolated from the termite gut. J Basic Microbiol. 2012;52:731–735.
- Savoie JM, Mata G, Largeteau M. Chapter 6 − New prospects in pathogen control of button mushroom cultures. In: Petre M, editor. Mushroom biotechnology developments and applications. Lodon: Academic Press; 2016. p. p. 93−110.
- Drake EJ, Gulick AM. Structural characterization and high-throughput screening of inhibitors of PvdQ, an NTN hydrolase involved in pyoverdine synthesis. ACS Chem Biol. 2011;6:1277–1286.
- Wurst JM, Drake EJ, Theriault JR, et al. Identification of inhibitors of PvdQ, an enzyme involved in the synthesis of the siderophore pyoverdine. ACS Chem Biol. 2014;9:1536–1544.
- Waksman SA, Woodruff HB. Bacteriostatic and bactericidal substances produced by a soil Actinomyces. Proc Soc Exp Biol Med. 1940;45:609–614.
- Reich E. Biochemistry of actinomycins. Cancer Res. 1963;23:1428–1441.
- Kirk JM. The mode of action of actinomycin D. Biochim Biophys Acta. 1960;42:167–169.
- Takusagawa F, Dabrow M, Neidle S, et al. The structure of a pseudo intercalated complex between actinomycin and the DNA binding sequence d(GpC). Nature. 1982;296(5856):466–469.
- Waksman SA, Geiger WB, Reynolds DM. Strain specificity and production of antibiotic substances: VII. Production of actinomycin by different actinomycetes. Proc Natl Acad Sci USA. 1946;32:117–120.
- Ogasawara Y, Shimizu Y, Sato Y, et al. Identification of actinomycin D as a specific inhibitor of the alternative pathway of peptidoglycan biosynthesis. J Antibiot (Tokyo). 2020;73:125–127.
- Imperi F, Massai F, Facchini M, et al. Repurposing the antimycotic drug flucytosine for suppression of Pseudomonas aeruginosa pathogenicity. Proc Natl Acad Sci USA. 2013;110:7458–7763.
- Kirienko DR, Revtovich AV, Kirienko NV. A high-content, phenotypic screen identifies fluorouridine as an inhibitor of pyoverdine biosynthesis and Pseudomonas aeruginosa virulence. mSphere. 2016;1:e00217−16.
- Kang D, Revtovich AV, Chen Q, et al. Pyoverdine-dependent virulence of Pseudomonas aeruginosa isolates from cystic fibrosis patients. Front Microbiol. 2019;10:2048.
- Fuchs R, Schäfer M, Geoffroy V, et al. Siderotyping − a powerful tool for the characterization of pyoverdines. Curr Top Med Chem. 2001;1:31–57.
- Fuchs R, Budzikiewicz H. Structural studies of pyoverdins by mass spectrometry. Curr Org Chem. 2001;5: 265–288.
- Reitz ZL, Hardy CD, Suk J, et al. Genomic analysis of siderophore β-hydroxylases reveals divergent stereocontrol and expands the condensation domain family. Proc Natl Acad Sci USA. 2019;116(40):19805–19814.