ABSTRACT
Purpose: To describe a case series of ocular complications associated with upper respiratory tract infections.
Methods: Four patients aged 21–61 years (three females, one male) had confirmed ocular complications connected with a general upper respiratory tract infection with myalgia and fever. Ophthalmological examination, including a visual acuity test, a slit-lamp exam, intraocular pressure measurements, fluorescein and indocyanine green angiography, optical coherence tomography (OCT), and diagnostic tests for influenza were performed in the patients (RT-PCR, HAI).
Results: Acute posterior multifocal placoid pigment epitheliopathy (APMPPE) was diagnosed in three patients and serous macular detachment (SME) in one. Influenza virus infection was confirmed by molecular biological methods (RT-PCR) or the hemagglutination inhibition test (HAI) in two patients. All patients were treated with systemic prednisone.
Conclusion: A coincidence between APMPPE and SME epitheliopathy and influenza virus infection was observed in different months of a given epidemic season.
Influenza viruses can produce a wide spectrum of diseases in humans, from mild symptoms resembling the common cold to acute respiratory failure or severe neurological, cardiovascular, ENT, or renal complications. Not infrequently influenza exacerbates pre-existing disease or causes new-onset disease and in some cases the outcome may be fatal.Citation1–Citation5
In case of suspecting acute phase of influenza virus infection nasopharyngeal swabs can be taken to confirm it with molecular biology methods (RT-PCR) by identifying genetical material of influenza virus type A or B. Other way of confirming influenza virus infection is testing serum samples with in example HAI test. HAI test is done for three kinds of influenza virus type A (H1H1), A (H3N2), and B that are contained in anti-influenza vaccine for current epidemic season. However, that method can be trustful only if samples are taken during acute and convalescent phase of illness and demands fourfold or greater rise in antibodies level between those samples for confirmation of influenza virus etiology according to WHO guidlines.Citation6
A literature review has shown that infection with different influenza viruses can be followed by ocular manifestations, but such cases are only rarely reported.Citation7–Citation13 The infection usually results from direct contact with the infectious material or may be contracted by breathing in air containing virus particles exhaled by the infected individuals.
In this article, we present four cases of ocular complications associated with influenza infection.
MATERIALS AND METHODS
Four patients aged 21–61 years (three females, one male) were seen at the Department of Ophthalmology, Medical University of Warsaw for ocular problems associated with a systemic infection. The ocular manifestations developed following an upper respiratory tract infection with sinusitis, fever, and myalgia, treated with systemic non-steroidal anti-inflammatory drugs and three antibiotics in one case (Patient 1). Ophthalmological examination was performed in all patients, including a visual acuity test, a slit-lamp exam, intraocular pressure measurements, fluorescein and indocyanine green angiography, and optical coherence tomography (OCT).
According to the WHO guidance influenza virus infection is diagnosed by molecular assays and serologic testing.Citation6 The presence of influenza viral RNA was detected by RT-PCR assay in nasopharyngeal swab in two patients. Serum tests (HAI) were done in all patients.
Both tests were done by National Influenza Centre, National Institute of Public Health-National Institute of Hygiene.
RESULTS
Following the ophthalmological examination, the diagnosis of acute posterior multifocal placoid pigment epitheliopathy (APMPPE) was established in three patients and of serous macular detachment (SME) in one patient.
Testing for influenza viruses confirmed an earlier infection with the influenza virus in two patients (no 1 and no 2) by RT-PCR assay and seral test (HAI).
In patient no 3 and no 4 HAI tests titer results were: 1:40 from 1st sample and 0 from control sample. Those patients do not meet the WHO criteria for the diagnosis of influenza infection.
In those patients (no 3 and no 4), nasopharyngeal swabs for RTC-PCR assay and serum samples could not be obtained in the acute phase of the disease as they reported to the Department of Ophthalmology in convalescent phase, without preceding contact with any physician.
During the follow-up period, none of the patients developed other complications such as cardiovascular, pulmonary, or neurological disorders. None of the patients had been vaccinated against influenza. Because of significant visual acuity deterioration, systemic prednisone was prescribed at the starting dose of 60 mg/day. Patient 2 was initially treated topically with steroidal and non-steroidal anti-inflammatory eye drops. Gradual resolution of the active inflammatory lesions and their scarification or absorption of subretinal fluid and edema were observed and the visual acuity returned to the level of 9/10–10/10.
Epidemiological, ophthalmological, virological, and serological data of the patients are presented in .
TABLE 1. Epidemiological, ophthalmological, virological and serological data of the patients.
The most severe systemic symptoms (an upper respiratory tract infection with arthralgia and myalgia, and fever of 40◦C) and ocular manifestations were observed in patient 2. Three members of her family had recently experienced an upper respiratory tract infection, but with lower fever and no changes in visual acuity.
Changes in the eye fundi of Patient 2 are presented in and (fluorescein and indocyanine green angiography) and in and (OCT).
FIGURE 1. (A) Color photograph of the right eye. (B) Fluorescein angiographic image. (C) indocyanine green angiographic image before treatment: slight dye leakage at the optic disk, small foci of inflammation at the periphery. (D) Color photograph. (E) Fluorescein angiographic image. (F) Indocyanine green angiographic image after 6 months of treatment: decreased severity of inflammation.
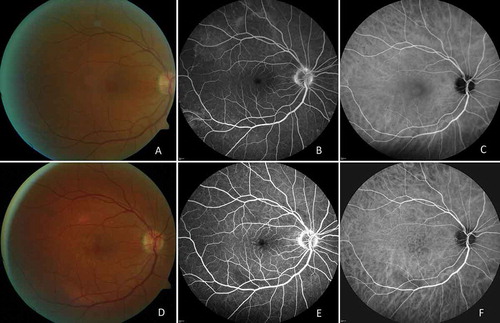
FIGURE 2. (A)Color photograph of the left eye. (B) Fluorescein angiographic image. (C) indocyanine green angiographic image before treatment: slight dye leakage at the optic disk, small foci of inflammation at the periphery. (D) Color photograph. (E) Fluorescein angiographic image. (F) Indocyanine green angiographic image after 6 months of treatment: decreased severity of inflammation.
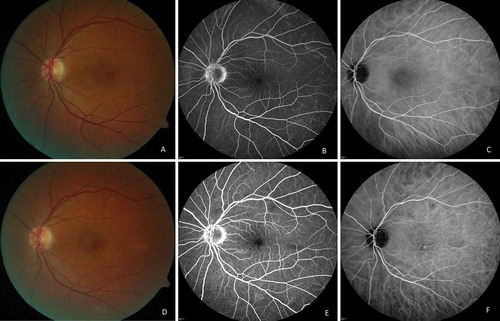
FIGURE 3. Optical coherence tomographic (OCT) images of the right eye. (A) At diagnosis: serous detachment of the neurosensory retina and intraretinal fluid. (B) Exudate decreased following the institution of topical treatment. (C) Recurrence of serous detachment of the neurosensory retina and intraretinal fluid prior to systemic treatment. (D) Exudate regressed after 6 months of systemic treatment.
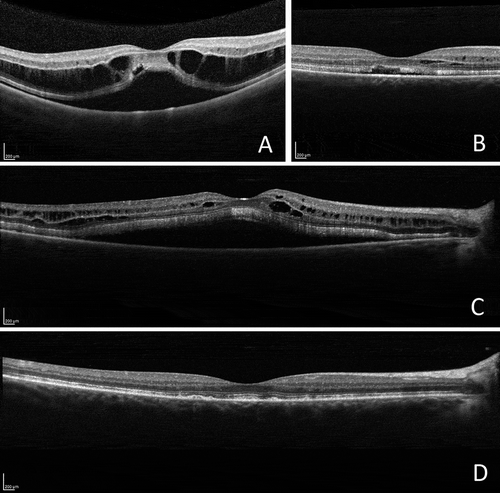
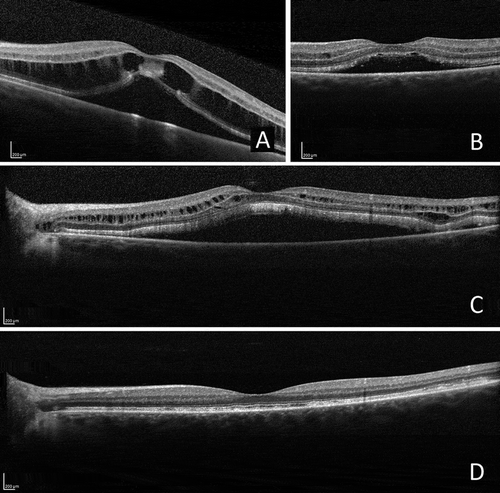
FIGURE 4. Optical coherence tomographic (OCT) images of the left eye: changes more pronounced than in the right eye. (A) At diagnosis: serous detachment of the neurosensory retina and intraretinal fluid. (B) Exudate decreased following the institution of topical treatment. (C) Recurrence of serous detachment of the neurosensory retina and intraretinal fluid prior to systemic treatment. (D) Exudate regressed after 6 months of systemic treatment.
DISCUSSION
Ocular complications in humans can be caused by any type of the influenza virus, but most commonly they are associated with a systemic infection which may be accompanied by conjunctivitis. Such coincidence was observed in the course of infection with influenza A virus, A/H5N1/, A/H1N1 subtype, and in some cases of seasonal influenza.Citation11 Influenza type A infection may produce a range of conditions in the posterior segment, including retinopathy, retinitis, angiitis, or uveal effusion syndrome.Citation12–Citation15 Experimental studies of Chan, Belser, or Michaelis demonstrated the presence of the influenza virus not just in the conjunctiva but also in other tissues of the eye, that is the cornea, trabecular meshwork, or the retinal pigment epithelium.Citation16–Citation18
Acute multifocal placoid pigment epitheliopathy (APMPPE) is an inflammatory disease of the choroid and retinal pigment epithelium, which tends to improve spontaneously. In some patients, the development of APMPPE is preceded by manifestations of systemic viral infection, such as cough, fever, malaise, myalgia and arthralgia, and lymphadenopathy.
We first diagnosed a coincidence between AMPPE and influenza virus infection in 2014.Citation19 Since then, any ophthalmic patient who presents with systemic symptoms of respiratory infection is tested for influenza.
Serous macular detachment (SMD) is observed in circulatory disorders and excessive vascular permeability of retinal and/or choroidal circulation and retinal pigment epithelial (RPE) damage. In is seen in eyes with macular edema in patients with diabetic retinopathy, thrombosis, retinal vasculitis, hypotonia, and central serous chorioretinopathy.Citation20 In the patient reported in this article, serous macular detachment was most likely due to the blood-retinal barrier damage by the circulating immune complexes or the direct virus replication.Citation16
Ocular complications observed in the reported cases, with the significant visual acuity deterioration at presentation, are resolved with systemic steroid therapy and the visual acuity considerably improved.
Michaelis et al. by infecting human retinal pigment epithelial cells (RPE) with strains of seasonal and avian influenza studied the cytopathogenetic effects and the ability of influenza A viruses to induce apoptosis. They demonstrated virus replication in RPE cells with avian influenza strains replicating to high titers resulting in RPE cells apoptosis.Citation18
The literature review we have conducted suggests that the two cases reported in this article are the first well-documented cases with APMPPE and SME as consequence of influenza virus infection. All diagnosed patients had not been vaccinated against influenza.
Level of antihaemagglutin antibodies in serum is markable only after influenza virus infection or vaccination. Patients no 3 and 4 reported to the Department of Ophthalmology because of decreased vision after systemic infection with typical for influenza virus symptoms, during convalescent phase. Due to that fact obtaining samples from acute phase was impossible, HAI tests upon arrival resulted in low antibodies level, and finally negative.
Due to that facts influenza virus infection status in those patients (no 3 and no 4) couldn’t be confirmed by WHO criteria what limited certainty, and considering that their status was marked as probable.Citation6
Taking in to account that in APMPPE pathogenesis influenza viral etiology is considered, it can be assumed that in patient no 3 and no 4 infection was connected to later ophthalmological changes; despite that influenza virus etiology cannot be confirmed according to WHO guidelines due to late patients contact with physician.
Health care professionals should be aware of both health and economic aspects of influenza virus infection.Citation21 It should be emphasized that the cases of influenza infection and the associated ocular complications were reported in different months of a given epidemic season with no relation to the season of the year.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Additional information
Funding
References
- Brydak LB. Influenza, Pandemic Flu, Myth or Real Threat?. Warsaw, Poland: Rhythm; 2008; 1–492 (in Polish).
- Brydak LB. Profilaktyka grypy w praktyce lekarza rodzinnego; X Jubileuszowy Kongres Top Medical trends. Przewodnik Lekarza. 2016; 1:13–15. (in Polish). [Influenza prophylaxis in general practice].
- Machała M, Wiatr E, Gawryluk D, Brydak LB. Significance of virological diagnostics for the effective treatment of hospitalized patients infected with influenza virus. Pneumonol Alergol Pol. 2006;74(2):166–170. Poland.
- Ciszewski J, Bilińska ZT, Brydak LB, et al. Influenza vaccination in secondary prevention from coronary ischaemic events in coronary artery disease: FLUCAD study. Eur Heart J. 2008 Jun;29(11):1350–1358.
- Rybicka K, Brydak LB. Encephalopathy and encephalitis–influenza-associated neurological sequels. Pol Merkur Lekarski. 2005;19(112):501–505.
- WHO Global Influenza Surveillance Network. Manual for the laboratory diagnosis and virological surveillance of influenza. 2011;59–62.
- Nakagawa H, Noma H, Kotake O, Motohashi R, Yasuda K, Shimura M. Optic neuritis and acute anterior uveitis associated with influenza A infection: a case report. Int Med Case Rep J. 2017 Jan 4;10:1–5.
- Breker DA, Stacey AW, Srinivasan A, Bursztyn LL, Trobe JD, Johnson MW. Vision loss caused by retinal and lateral geniculate nucleus infarction in H1N1 influenza. J Neuroophthalmol. 2015 Sep;35(3):265–269.
- Pula JH, Issawi A, Desanto JR, Kattah JC. Cortical vision loss as a prominent feature of H1N1 encephalopathy. J Neuroophthalmol. 2012 Mar;32(1):48–50.
- Lai CC, Chang YS, Li ML, Chang CM, Huang FC, Tseng SH. Acute anterior uveitis and optic neuritis as ocular complications of influenza A infection in an 11-year-old boy. J Pediatr Ophthalmol Strabismus. 2011 Jul 6;48 Online:e30–3. doi:10.3928/01913913-20110628-03.
- Belser JA, Rota PA, Tumpey TM. Ocular tropism of respiratory viruses. Microbiol Mol Biol Rev. 2013 Mar;77(1):144–156.
- Roesel M, Heinz C, H1n HA. 1 and uveal effusion syndrome. Ophthalmology. 2010;117(7):1467.
- Jo T, Mizota A, Hatano N, Tanaka M. Frosted branch angiitis-like fundus following presumed influenza virus type A infection. Jpn Ophthalmol. 2006;50(6):563–564.
- Rifkin L, H1n SS. 1-associated acute retinitis. Ocul Immunol Inflamm. 2012 Jun;20(3):230–232.
- Villegas VM, Monagas M, Rivera L, Santos C, Serrano L. Visual loss associated with influenza A: a case report and review of literature. P R Health Sci J. 2012 Mar;31(1):35–37. Review.
- Chan MC, Chan RW, Yu WC, et al. Tropism and innate host responses of the 2009 pandemic H1N1 influenza virus in ex vivo and in vitro cultures of human conjunctiva and respiratory tract. Am J Pathol. 2010;176(4):1828/40. Epub 2010 28 Jan. doi:10.2353/ajpath.2010.091087.
- Belser JA, Zeng H, Katz JM, Tumpey TM. Ocular tropism of influenza A viruses: identification of H7 subtype-specific host responses in human respiratory and ocular cells. J Virol. 2011 Oct;85(19):10117–10125.
- Michaelis M, Geiler J, Klassert D, Doerr HW, Cinatl J Jr. Infection of human retinal pigment epithelial cells with influenza A viruses. Invest Ophthalmol Vis Sci. 2009 Nov;50(11):5419–5425.
- Brydak-Godowska J, Gołębiewska J, Turczyńska M, et al. Observation and clinical pattern in patients with the white dot syndromes. The role of colour photography in monitoring ocular changes in long-term observation. Med Sci Monit. 2017 Mar 2;23:1106–1115.
- Burak T, Fatih CG, Tamer D, Ulku C. The causes of serous macular detachment excluding central serous chorioretinopathy. Clinical Optometry. 2010;2:51–54.
- Brydak LB. Skutki zdrowotne i ekonomiczne zakażeń grypą w aspekcie zdrowia publicznego. Polski Przegląd Nauk O Zdrowiu. 2016;4(49):401–407. (in Polish) [Public health and economic impact of influenza virus infections].
