ABSTRACT
Purpose: Report global adalimumab safety and efficacy outcomes in patients with non-infectious uveitis.
Methods: Adults with non-infectious intermediate, posterior, or panuveitis were randomized 1:1 to receive placebo or adalimumab in the VISUAL I (active uveitis) or VISUAL II (inactive uveitis) trials. Integrated global and Japan substudy results are reported. The primary endpoint was time to treatment failure (TF).
Results: In the integrated studies, TF risk was significantly reduced (hazard ratio [95% CI]) with adalimumab versus placebo (VISUAL I: HR = 0.56 [0.40–0.76], p < 0.001; VISUAL II: HR = 0.52 [0.37–0.74], p < 0.001). In Japan substudies, no consistent trends were observed between groups (VISUAL I: HR = 1.20 [0.41–3.54]; VISUAL II: HR = 0.45 [0.20–1.03]). Adverse event rates were similar between treatment groups in both studies (854 to 1063 events/100 participant-years).
Conclusions: Adalimumab lowered time to TF versus placebo in the integrated population; no consistent trends were observed in Japan substudies. Safety results were consistent between studies.
Uveitis is a heterogeneous collection of diseases with varied genetic and environmental influences. It has been estimated that uveitis accounts for about 10% of the visual handicap in the Western world, and up to 35% of all patients with uveitis suffer substantial visual impairment or legal blindness.Citation1 The annual prevalence of uveitis in the general population is estimated at 115–204 per 100,000 population, and the incidence is estimated at 17–52 per 100,000 population.Citation2 As uveitis often affects the young adult population in their most productive years of life, the personal and population burden of this sight-threatening disease is noteworthy.Citation3,Citation4 Conventional therapy with corticosteroids (CS) has been the mainstay to treat uveitis; although CS are often effective, ocular and/or systemic adverse effects limit their long-term use.Citation5–Citation7
Adalimumab (Humira®; AbbVie Inc., North Chicago, IL) is a recombinant human immunoglobulin (IgG1) monoclonal antibody that binds specifically to tumor necrosis factor (TNF) and neutralizes its biological function.Citation8–Citation11 Adalimumab’s safety and efficacy profile spans over 13 years for various approved inflammatory conditions.Citation12 Recently, adalimumab received approvals from the US Food and Drug Administration (FDA), European Medicines Agency (EMA), and Japan Pharmaceutical and Medical Devices Agency (PMDA) to treat adult participants with non-infectious intermediate, posterior, and panuveitis.
It is critical to prevent inflammation and vision loss in eyes with uveitis. The VISUAL I and VISUAL II studies were randomized, double-masked, placebo-controlled clinical trials designed to assess the efficacy and safety of adalimumab in inducing quiescence along with CS and maintaining the achieved quiescence in participants with active uveitis (VISUAL I) and as a steroid-sparing agent preventing relapse in participants dependent on CS to maintain inactivity (VISUAL II). The VISUAL program demonstrated adalimumab’s ability to lower the risk of uveitic recurrence and visual acuity loss in participants with active (VISUAL I) and an inactive disease state (VISUAL II) uveitis.Citation13,Citation14 The VISUAL program is the first successful program where the treatment effect of a non-CS medication could be demonstrated in two separate randomized controlled trials in non-infectious uveitis populations.
Due to regional heterogeneity and in agreement with the Japan PMDA, separate Japan substudies were conducted for the Japanese study participants. These substudies enrolled Japanese participants under a separate stratum to demonstrate directionality of substudy results when compared with those of the main studies. Results from the Japan substudies in context with the global integrated study results (main study and Japan substudy) from the VISUAL I and VISUAL II trials are reported.
METHODS
Study Design and Oversight
VISUAL I and VISUAL II were phase 3, randomized, double-masked, placebo-controlled studies conducted in 22 countries involving 74 and 72 study sites, respectively, between August 2010 and May 2015 (ClinicalTrials.gov identifiers, VISUAL I: NCT01138657 and VISUAL II: NCT01124838; registered May 2010). The study protocols were approved by the responsible ethics committees and internal review boards, and studies were performed in compliance with the Declaration of Helsinki, Good Clinical Practice (GCP) guidelines, and applicable local regulations.
For VISUAL I, the Japan substudy was to end when either the 138th event of treatment failure had occurred in the VISUAL I main study or when the 19th event of treatment failure had occurred in the VISUAL I Japan substudy. For VISUAL II, the Japan substudy was to end when either the 96th event of treatment failure had occurred in the VISUAL II main study or when the 17th event of treatment failure had occurred in the VISUAL II Japan substudy.
Study Participants
VISUAL I
Participants (aged ≥ 18 years) with active non-infectious intermediate, posterior, or panuveitis were enrolled. Key inclusion criteria were active disease (active inflammatory chorioretinal or retinal vascular lesions, anterior chamber [AC] cell grade ≥ 2+, and/or vitreous haze [VH] grade ≥ 2+) and use of oral prednisone (≥ 10 mg/day to ≤ 60 mg/day) or a CS equivalent for ≥ 2 weeks before screening. Participants with isolated anterior uveitis or infectious uveitis, prior inadequate response to high-dose oral CS, or any ocular or systemic condition that would preclude safe participation in the study or interfere with study assessments were excluded.
VISUAL II
Participants (aged ≥ 18 years) with inactive non-infectious intermediate, posterior, or panuveitis were enrolled. Key inclusion criteria were inactive disease ≥ 28 days prior to the baseline visit and daily oral prednisone ≥ 10 to ≤ 35 mg to maintain inactive uveitis. Inactive uveitis was defined as no active inflammatory chorioretinal and/or retinal vascular lesions, AC cell grade ≤ 0.5+, and/or VH grade ≤ 0.5 +. To demonstrate CS dependency, the participant should have had a documented history of experiencing at least 1 disease flare during or within 28 days of tapering steroids, within 18 months of the screening visit. Participants with isolated anterior or infectious uveitis or any condition precluding safe participation in the study or interfering with study assessments were excluded.
Randomization and Study Treatments
For the main studies, at the baseline visit participants were randomized to placebo or adalimumab treatment groups in a 1:1 ratio stratified by baseline immunosuppressant use with an interactive voice/web response system that assigned allocation numbers and treatments. Japanese participants were randomized in a separate stratum and due to the small sample size in the Japan substudies no stratification by baseline immunosuppressant use was performed. Adalimumab and placebo were supplied in pre-filled syringes and were administered subcutaneously. The adalimumab group received an 80-mg baseline loading dose followed by 40-mg doses every other week starting at week 1 for the duration of the study. For VISUAL I, all participants received a standardized, 60 mg/day prednisone burst at study entry followed by a mandatory prednisone taper to 0 mg by week 15. For VISUAL II, participants were on 10 to 35 mg/day of oral prednisone at baseline and from week 2, all participants underwent a mandatory prednisone taper to 0 mg by week 19. In both studies, topical steroids were allowed at study entry, but participants were to undergo mandatory taper from week 1 to week 9.
Study Visits and Endpoints
Clinic visits were scheduled at screening; baseline; week 1 (VISUAL I), week 2 (VISUAL II), week 4, and approximately every 4 weeks thereafter. Participants were assessed until treatment failure was determined or completion of the 80-week study. Participants were assessed for treatment failure starting at week 6 for VISUAL I and week 2 for VISUAL II (). The primary efficacy endpoint and ranked secondary endpoints were tested and are listed in .
TABLE 1. Criteria for treatment failure for VISUAL I and VISUAL II clinical trials.
TABLE 2. Primary endpoint and ranked secondary endpoints for VISUAL I and VISUAL II trials.
Adalimumab immunogenicity was evaluated at multiple time points throughout the study. Adverse events (AEs) were monitored throughout the study and reported from the first dose of study drug until 70 days after the last dose of study drug or until participants were rolled into a separate extension study. Serious AEs were collected from the time of informed consent. AEs were tabulated using Medical Dictionary for Regulatory Activities (MedDRA) version 17.0 system organ class and preferred terms.
Procedures
Presence or absence of inflammatory chorioretinal and/or retinal vascular lesions and VH grade were determined by dilated indirect ophthalmoscopy, and AC cell counts were assessed using slit-lamp biomicroscopy and graded using Standardization of Uveitis Nomenclature (SUN)-adapted National Eye Institute (NEI) criteria.Citation15,Citation16
Statistical Analysis for VISUAL I and VISUAL II
Efficacy endpoints were analyzed in the intent-to-treat (ITT) data set (all participants randomized to treatment, excluding six participants from two non-compliant sites in VISUAL I and three participants from two non-compliant sites in VISUAL II).
Time to treatment failure was compared between treatment groups using a log-rank test. A proportional hazards model was fitted to estimate the hazard ratio (HR) with its 95% confidence interval (CI). Ranked secondary endpoint changes in AC cell grade, VH grade, and best corrected visual acuity (BCVA) were compared between groups by analysis of variance with treatment as factor adjusted for clustered observations within a participant. For analysis of secondary variables, missing data were imputed using last observation carried forward.
Testing of the primary and ranked secondary endpoints was conducted at a two-sided significance level of 5% in hierarchical order. In case of a non-significant test, the confirmatory multiple test procedure was stopped and p-values of secondary endpoints further down in the hierarchy were considered exploratory and descriptive in nature.
Participant information was summarized using descriptive statistics, continuous variables were compared by analysis of variance, and discrete variables were analyzed using chi-square tests or Fisher’s exact test. Treatment-emergent AEs were summarized descriptively by treatment group. AEs were presented as events per 100 participant-years (100 PY) to avoid confounding by between-group differences in duration of exposure to study treatment. Analyses were performed by the study sponsor using SAS software (SAS Institute Inc., Cary, NC). Data reported here reflect final study data.
RESULTS
Participants
VISUAL I
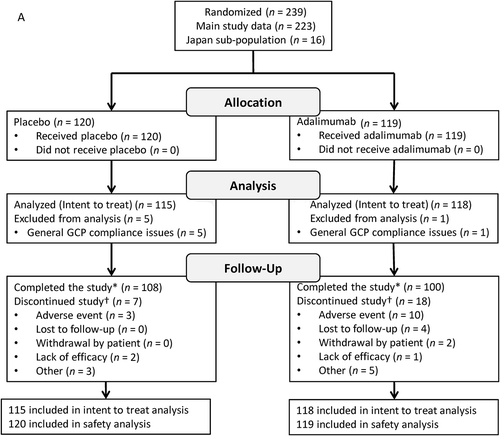
Of 239 participants randomized to treatment, 233 (217 main study and 16 Japan substudy) participants were included in the ITT analyses (six participants were excluded from non-compliant sites; placebo, n = 115 and adalimumab, n = 118). Most participants were female (58%) and white (75%); 47% were diagnosed with panuveitis. Mean participant age was 43.2 years, and mean duration of uveitis was 46 months. There were no significant differences between randomized groups in demographics and baseline characteristics (). Seven participants receiving placebo and 18 receiving adalimumab discontinued the study. AEs were the most common reason for discontinuation in both treatment groups (). The majority of the participants in the Japan substudy were diagnosed with panuveitis (13/16 [81.3%]), including all participants in the placebo group, compared with 97/217 (44.7%) in the main study. The three participants without panuveitis in the Japan substudy were in the adalimumab group and were diagnosed with intermediate (n = 1) or posterior uveitis (n = 2). Seven participants in the Japan substudy were diagnosed with idiopathic uveitis (placebo, n = 4; adalimumab, n = 3); one participant in the substudy receiving adalimumab was diagnosed with Vogt Koyanagi Harada disease; six participants (placebo, n = 4; adalimumab, n = 2) were diagnosed with sarcoidosis; and two participants receiving adalimumab were diagnosed with Behçet’s disease ().
TABLE 3. Participants’ demographics and baseline characteristics (intent-to-treat population).
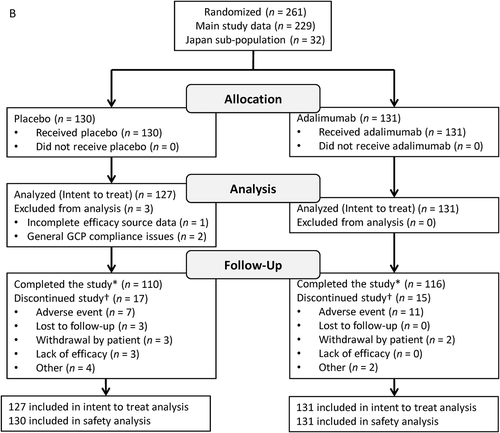
FIGURE 1. Participant disposition. VISUAL I (A) and VISUAL II (B). GCP = Good Clinical Practice.
*Includes participants who experienced treatment failure, reached 80 weeks of treatment without treatment failure, or had to terminate the study because the planned number of treatment failures was reached.†Some participants had multiple reasons for discontinuation; total counts for reasons for discontinuation exceed the total number of discontinuations.
VISUAL II
Of 261 participants randomized to treatment, 258 (226 main study and 32 Japan substudy) participants were included in the ITT analyses (three participants were excluded from non-compliant sites) (placebo, n = 127; adalimumab, n = 131). More participants were female (61%) and white (73%); 51% were diagnosed with panuveitis. Mean participant age was 43.1 years, and mean duration of uveitis was 59 months. There were no significant differences between randomized groups in demographics and baseline characteristics (). Seventeen participants receiving placebo and 15 receiving adalimumab discontinued the study. AEs were the most common reason for discontinuation in both treatment groups (). The majority of the participants in the Japan substudy were diagnosed with panuveitis (29/32 [90.6%]), compared with 103/226 (45.6%) in the main study. Three participants in the Japan substudy were diagnosed with posterior uveitis (placebo, n = 1; adalimumab, n = 2). Eight participants in the substudy were diagnosed with idiopathic uveitis (placebo, n = 4; adalimumab, n = 4); 12 participants were diagnosed with Vogt Koyanagi Harada disease (placebo, n = 4; adalimumab, n = 8); 10 participants were diagnosed with sarcoidosis (placebo, n = 6; adalimumab, n = 4); and one participant in the placebo group was diagnosed with Behçet’s disease ().
Efficacy
VISUAL I
Median time to treatment failure was significantly different between the placebo (3 months) and adalimumab (4.8 months) groups for the integrated study. The risk of treatment failure for participants in the adalimumab group was reduced by 44% compared with participants in the placebo group (HR = 0.56; 95% CI, 0.40–0.76; p < 0.001). In the Japan substudy, time to treatment failure was shorter (placebo, 2.8 months and adalimumab, 2.4 months) than the non-Japanese (main study) participants, and did not show a clear difference between the adalimumab group and placebo group (HR = 1.20; 95% CI, 0.41–3.54). In the Japan substudy there was one outlier participant in the placebo group who did not meet treatment failure and strongly impacted the outcome of the primary endpoint, given the small number of participants in the substudy. The HR (95% CI) in the Japan substudy for the primary endpoint was 0.73 (0.22–2.40) when this participant was excluded ().
TABLE 4. Primary endpoint for main study, integrated study, and Japan substudy for VISUAL I and VISUAL II trials.
Hierarchical testing of the ranked secondary outcomes showed that worsening of AC cell grade, VH grade, and BCVA were significantly reduced in participants receiving adalimumab versus placebo (p < 0.05). The Japan substudy had similar directionality as the main study after excluding the outlier participant, except for AC cell grade ().
TABLE 5. Summary of ranked secondary efficacy variables*.
VISUAL II
Similar to results in VISUAL I, there was a robust and statistically significant difference between adalimumab and placebo groups in the VISUAL II integrated study. Median time to treatment failure was 5.6 months for placebo and not estimable (> 18 months) for adalimumab, as more than half of the adalimumab-treated participants did not experience treatment failure for the integrated study. The risk of treatment failure for participants in the adalimumab group was reduced by 48% compared with participants in the placebo group (HR = 0.52; 95% CI, 0.37–0.74; p < 0.001). In the Japan substudy, time to treatment failure (placebo, 2.1 months and adalimumab, 2.9 months) had similar directionality as in the main study, with adalimumab reducing the risk of treatment failure (HR = 0.45; 95% CI, 0.20–1.03; ).
Hierarchical testing of the ranked secondary outcomes showed no statistically significant differences for the ranked secondary endpoints. Similar to VISUAL I, exploratory analyses on 1–3 secondary endpoints were carried out for hypothesis-generating purposes and showed that all the results favored adalimumab-treated eyes. The Japan substudy had similar directionality as the main study ().
Safety
VISUAL I
The incidence of AEs was comparable between treatment groups (placebo, 960 E/100 PY; adalimumab, 1063 E/100 PY) in the VISUAL I safety analysis set (). Serious AEs were reported at rates of 12.7 E/100 PY in the placebo group and 29.5 E/100 PY in the adalimumab group. The most frequently reported AE was injection site reaction (placebo, 14.8 E/100 PY; adalimumab, 43.5 E/100 PY). Serious infections occurred at a similar rate between groups. Two malignancies (one event each of carcinoid tumor of the gastrointestinal tract and glioblastoma multiforme) and one event each of active and latent tuberculosis were reported in the adalimumab group. One participant with intermediate uveitis in the adalimumab group reported demyelinating disorder. One event of lupus or lupus-like reaction was reported in the adalimumab group. Four participants (3.3%) in the placebo group and 11 (9.2%) in the adalimumab group discontinued study drug due to AEs. One death due to end-stage chronic renal disease was reported in the adalimumab group. Four participants (3.4%, n = 4/118) had anti-adalimumab antibodies (AAA+) during the study. The four AAA+ participants experienced treatment failure at 16, 20, 44, and 48 weeks, respectively; median time to treatment failure was 21 weeks in AAA– participants (n = 114).
TABLE 6. Summary of adverse events (safety population).
VISUAL II
The incidence of AEs was comparable between treatment groups (placebo, 884 E/100 PY; adalimumab, 854 E/100 PY) in the VISUAL II safety analysis set (). Serious AEs were reported at rates of 14.8 E/100 PY in the placebo group and 13.6 E/100 PY in the adalimumab group. The most frequently reported AEs were injection site reactions (placebo, 21.5 E/100 PY; adalimumab, 36.0 E/100 PY). Serious infections occurred at a similar rate between groups. One death due to two AEs of aortic dissection and cardiac tamponade leading to death was reported in the adalimumab group. Two malignancies (one event each of non-serious squamous cell carcinoma of skin and serious lung adenocarcinoma Stage IV) in the adalimumab group and one and three events each of latent tuberculosis were reported in the placebo and adalimumab groups, respectively. No lupus or lupus-like reaction or demyelinating disorders were reported. Seven participants (5.4%) in the placebo group and 11 (8.4%) in the adalimumab group discontinued study drug due to AEs. Eight participants (6.1%, n = 8/131) had anti-adalimumab antibodies (AAA+) during the study. Six out of eight AAA+ participants experienced treatment failure at 10, 13, 16, 16, 24, and 31 weeks, respectively; median time to treatment failure was not estimable for AAA– participants, as more than half of the AAA– participants did not experience treatment failure (n = 123).
In both Japan substudies, adalimumab was generally safe and well tolerated, and the AE profile was consistent with the safety profile established across other approved indications. No new safety signals were identified. Furthermore, AE rates for the global VISUAL I and VISUAL II trials were comparable with the respective Japan substudy ().
DISCUSSION
In both VISUAL I and VISUAL II integrated data sets, treatment with adalimumab effectively reduced the risk of treatment failure compared with placebo in participants with both active and controlled uveitis. Both clinical trials had a similar but unique study design, large study population, range of uveitis diagnoses, and a comprehensive stringent, multi-component primary endpoint. In both studies, participants receiving adalimumab were significantly less likely to experience treatment failure and had fewer reasons for treatment failure compared with participants receiving placebo. The median time to treatment failure for adalimumab was 60% and 87% longer for the integrated population and main study populations when compared with their respective placebo populations for the VISUAL I trial.
In VISUAL I, among Japanese participants (n = 16), time to treatment failure was shorter than the main study participants, and there was no clear difference between the adalimumab group and the placebo group. The potential explanation for such rapid time to treatment failure is that the majority of the participants in the Japan substudy were diagnosed with panuveitisCitation17,Citation18 (13/16 [81.3%]), including all participants in the placebo group, compared with 97/217 (44.7%) in the main study. This difference between the uveitis types in two populations is statistically significant and clinically meaningful. In the main study, among participants with panuveitis, intermediate, or posterior uveitis receiving placebo, the rates of treatment failure were 100%, 84%, and 69%, respectively, by month 9. Despite the clear risk reduction of treatment failure in the panuveitis subpopulation receiving adalimumab in the main studies (VISUAL I: HR = 0.45, p < 0.001; VISUAL II: HR = 0.44, p = 0.003), participants with panuveitis trended toward earlier treatment failure compared with other anatomical locations, which may explain why the Japan substudy had shorter time to treatment failure than the main study population. Additionally, when compared with the panuveitis population from the main study (median time to treatment failure: placebo, 2.9 months and adalimumab, 3.8 months), the Kaplan-Meier curves for time to treatment failure were similar to the Japanese participants (median time to treatment failure: placebo, 2.8 months and adalimumab, 4.6 months).
Additionally, in the Japan substudy, one participant in the placebo group did not reach treatment failure during the maximum double-masked treatment period of 80 weeks. This participant was confirmed as an outlier with Smirnov-Grubbs’ test. This one outlier out of the eight participants in the placebo group strongly impacted the outcome of the study. The HR in the Japan substudy for the primary endpoint was 0.73 when excluding this participant. This is a similar directionality to the main study (0.50) and integrated study (0.56) results. Given the small sample size of 16 participants, this single outlier participant drove the result of this substudy. In the VISUAL II Japan substudy (n = 32), a trend was observed between the adalimumab and placebo groups in favor of adalimumab (HR = 0.45). The median time to treatment failure for the integrated study was not estimable for the adalimumab group, as fewer than half of patients receiving adalimumab in the main study experienced treatment failure, but was significantly longer for adalimumab (> 18 months) compared with placebo (5.6 months). In the Japan subpopulation, median time to treatment failure was longer in patients receiving adalimumab (2.9 months) compared with those receiving placebo (2.1 months). No clear differences were expected within the Japan subpopulation, given the small sample sizes of 16 (VISUAL I) and 32 (VISUAL II) participants. The distribution of specific etiologies in Japan differed from that of the overall populations in the VISUAL I and VISUAL II studies, with sarcoidosis occurring as the most frequently identified diagnosis in VISUAL I after idiopathic, and Vogt Koyanagi Harada and sarcoidosis as the most frequent in VISUAL II.Citation17,Citation18 Despite the small sample size in the Japan substudies, the higher incidence of sarcoidosis observed in these studies agrees with the reported incidence in previous Japanese epidemiological studies.Citation17 The trend toward shorter time to treatment failure in the Japan substudy emphasizes the importance of characterizing geographical differences in uveitis diagnostic frequencies, which may be correlated with environmental and other exogenous factors.Citation18 As mentioned, these findings should be interpreted with caution due to the small size for each treatment group, particularly in VISUAL I (n = 8 per group).
The efficacy results for these controlled trials are supported by previous uncontrolled studies. In a retrospective study in participants with refractory chronic uveitis, adalimumab effectively controlled inflammation in 35% of participants who were refractory to previous treatment with infliximab or etanercept.Citation19 In a prospective open-label pilot study of 19 participants with active intraocular inflammation treated with adalimumab, 33 eyes (86%) had cystoid macular edema by optical coherence tomography at baseline. After 1 year of treatment visual acuity improved by −0.3 logMAR in 12/38 (31%) eyes, inflammation was significantly reduced in 63% of participants, and cystoid macular edema completely resolved in 55%.Citation20 In a multicenter study of 131 participants with a mean age of 27 years, adalimumab therapy significantly improved anterior chamber and vitreous inflammation, and it was possible to taper CS.Citation21 In another non-comparative open-label prospective study of 31 participants with refractory uveitis, 68% of participants were clinical responders at 10 weeks, and sustained response at 50 weeks was seen in 39% of the participants.Citation22 The French uveitis network recently published a multicenter study of 160 participants with refractory uveitis treated with anti-TNFα (infliximab and adalimumab) agents. The participants had an overall response rate of 93% at 12 months.Citation23 Most recently, the main study data from VISUAL I and VISUAL II studies also demonstrated the efficacy and safety of adalimumab (anti-TNFα) to treat uveitis.Citation13,Citation14
Low adalimumab immunogenicity was observed in both VISUAL I and VISUAL II studies and was in the range of rates observed in other disease states.Citation13,Citation14 The safety profile of adalimumab was similar to the established safety profile for approved adalimumab indications,Citation24 and the rates of AEs were similar compared with placebo. In participants with active uveitis (VISUAL I), the overall rates of adalimumab-associated AEs were higher than the placebo group, as were serious AEs and AEs leading to discontinuation. In participants with inactive uveitis (VISUAL II), injection-site reactions were the most frequently reported AEs with adalimumab, consistent with other reports.Citation21,Citation25 The strengths and the limitations of the VISUAL I and VISUAL II studies have been described and published.Citation13,Citation14 Additionally, in the Japan substudies, the sample sizes of 16 participants (VISUAL I) and 32 participants (VISUAL II) were very low and no clear differences were expected. Furthermore, to receive 60 mg of prednisone per the VISUAL I protocol, participants needed to be hospitalized, which was one of the reasons for low enrollment in the VISUAL I study in Japan.
In conclusion, adalimumab lowered the risk of uveitic flare or visual acuity loss in participants with active and controlled uveitis who are at risk of the long-term side effects of CS. Based on results taken together from VISUAL I and VISUAL II trials, there is no reason to believe that adalimumab treatment in Japanese participants with non-infectious uveitis would have different effects from those in non-Japanese participants. Safety data from the two studies in Japanese and non-Japanese populations are consistent with the safety profile established across other approved indications. On the basis of the results from the VISUAL studies, adalimumab received approvals from the FDA, EMA, and Japan PMDA, and is the first non-CS biologic to be licensed for the treatment of non-infectious uveitis.Citation14,Citation23
Declaration of Interest
Hiroshi Goto has served on advisory boards for AbbVie. Masahiro Zako has no disclosures of interest. Kenichi Namba has served on advisory boards for AbbVie. Noriyasu Hashida has no disclosures of interest. Toshikatsu Kaburaki has served on advisory boards for AbbVie. Masanori Miyazaki has no disclosures of interest. Koh-Hei Sonoda has served on advisory boards for AbbVie. Toshiaki Abe has no disclosures of interest. Nobuhisa Mizuki has served on advisory boards for AbbVie. Koju Kamoi has no disclosures of interest. Antoine P. Brezin has served on advisory boards and as a consultant for AbbVie. Andrew Dick has served on advisory boards for AbbVie. Glenn Jaffe has served as a consultant for AbbVie and Sivida. Quan Dong Nguyen has served on the Scientific Advisory Board for AbbVie, Santen, XOMA, Bausch & Lomb, and chairs the Steering Committee for the VISUAL studies. Noritaka Inomata is a former employee of AbbVie and may hold AbbVie stock or options. Samir Tari is a former AbbVie Employee and may hold AbbVie stock or options. Martina Kron, Alex Song, Anne Camez, and Nisha Kwatra are AbbVie employees and may hold AbbVie stock or options. Shigeaki Ohno has served on advisory boards for AbbVie and Santen.
Additional information
Funding
References
- Wakefield D, Chang JH. Epidemiology of uveitis. Int Ophthalmol Clin. 2005;45:1–13.
- Jabs DA. Epidemiology of uveitis. Ophthalmic Epidemiol. 2008;15:283–284. doi:10.1080/09286580802478724.
- Kempen JH, Gewaily DY, Newcomb CW, et al. Remission of intermediate uveitis: incidence and predictive factors. Am J Ophthalmol. 2016;164:110–117 e112. doi:10.1016/j.ajo.2015.12.034.
- Pasadhika S, Rosenbaum JT. Update on the use of systemic biologic agents in the treatment of noninfectious uveitis. Biologics. 2014;8:67–81. doi:10.2147/btt.s41477.
- Becker MD, Smith JR, Max R, Fiehn C. Management of sight-threatening uveitis: new therapeutic options. Drugs. 2005;65:497–519.
- Cervantes-Castaneda RA, Jawed N, Foster CS. Immunosuppressive therapy for noninfectious uveitis. Retinal Physician. 2007;4:30–38.
- Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130:492–513.
- Beckham JC, Caldwell DS, Peterson BL, et al. Disease severity in rheumatoid arthritis: relationships of plasma tumor necrosis factor-alpha, soluble interleukin 2-receptor, soluble CD4/CD8 ratio, neopterin, and fibrin D-dimer to traditional severity and functional measures. J Clin Immunol. 1992;12:353–361.
- Brennan FM, Feldmann M. Cytokines in autoimmunity. Curr Opin Immunol. 1992;4:754–759.
- Rabinovich CE. Use of tumor necrosis factor inhibitors in uveitis. Curr Opin Rheumatol. 2007;19:482–486. doi:10.1097/BOR.0b013e32825f5481.
- Turan B, Gallati H, Erdi H, Gurler A, Michel BA, Villiger PM. Systemic levels of the T cell regulatory cytokines IL-10 and IL-12 in Bechcet’s disease; soluble TNFR-75 as a biological marker of disease activity. J Rheumatol. 1997;24:128–132.
- Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda AP. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517–524. doi:10.1136/annrheumdis-2011-201244.
- Jaffe GJ, Dick AD, Brezin AP, et al. Adalimumab in patients with active noninfectious uveitis. N Engl J Med. 2016;375:932–943. doi:10.1056/NEJMoa1509852.
- Nguyen QD, Merrill PT, Jaffe GJ, et al. Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet. 2016;388:1183–1192. doi:10.1016/S0140-6736(16)31339-3.
- Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–516.
- Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92:467–471.
- Goto H, Mochizuki M, Yamaki K, Kotake S, Usui M, Ohno S. Epidemiological survey of intraocular inflammation in Japan. Jpn J Ophthalmol. 2007;51:41–44. doi:10.1007/s10384-006-0383-4.
- Ohguro N, Sonoda KH, Takeuchi M, Matsumura M, Mochizuki M. The 2009 prospective multi-center epidemiologic survey of uveitis in Japan. Jpn J Ophthalmol. 2012;56:432–435. doi:10.1007/s10384-012-0158-z.
- Tynjala P, Kotaniemi K, Lindahl P, et al. Adalimumab in juvenile idiopathic arthritis-associated chronic anterior uveitis. Rheumatology (Oxford). 2008;47:339–344. doi:10.1093/rheumatology/kem356.
- Diaz-Llopis M, Garcia-Delpech S, Salom D, et al. Adalimumab therapy for refractory uveitis: a pilot study. J Ocul Pharmacol Ther. 2008;24:351–361. doi:10.1089/jop.2007.0104.
- Diaz-Llopis M, Salom D, Garcia-de-Vicuna C, et al. Treatment of refractory uveitis with adalimumab: a prospective multicenter study of 131 patients. Ophthalmology. 2012;119:1575–1581.
- Suhler EB, Lowder CY, Goldstein DA, et al. Adalimumab therapy for refractory uveitis: results of a multicentre, open-label, prospective trial. Br J Ophthalmol. 2013;97:481–486.doi:10.1136/bjophthalmol-2012-302292.
- Vallet H, Seve P, Biard L, et al. Infliximab versus adalimumab in the treatment of refractory inflammatory uveitis: A multicenter study from the French Uveitis Network. Arthritis Rheumatol. 2016;68:1522–1530. doi:10.1002/art.39667.
- HUMIRA (adalimumab). Full Prescribing Information. North Chicago, IL: AbbVie Inc.; 2014.
- Diaz-Llopis M, Gallego-Pinazo R, Garcia-Delpech S, Salom-Alonso D. General principles for the treatment of non-infectious uveitis. Inflamm Allergy Drug Targets. 2009;8:260–265.