ABSTRACT
Purpose: To analyze the efficacy of high dose (≥ 15mg/m2/week) methotrexate (MTX) versus low dose (<15mg/m2/week) MTX in relation to time to remission on medication.
Methods: Retrospective observational cohort study of pediatric patients with auto-immune uveitis with or without underlying systemic disease treated with MTX at the University Medical Center Groningen (the Netherlands) between 1990 and 2014. Primary outcome was time to remission on medication, which was defined as an observable inactive disease in the affected eye for longer than 3 months without the use of systemic corticosteroids.
Results: A total of 42 patients were included. Mean age at uveitis diagnosis was 6.5 years (range 1.7 – 14.4), and 22 (52.4%) patients were male. Bilateral disease was found in 33 patients. Most patients (n=25) had anterior uveitis. JIA was the underlying systemic disease in 21 patients. Overall, 28 (66.7%) patients reached remission on medication in (median) 22.5 months (IQR 10.4- 45). Time to remission on medication in the low dose group (median 35.2, IQR 20.5 – 72.1 months) was significantly longer than in the high dose group (median 16.6, IQR 7.8 – 22.5 months) (p= 0.01). No statistically significant differences in ocular complications, steroid-sparing effect, cumulative dosage and side effects of MTX were found between the high and low dose groups.
Conclusion: In this retrospective study on pediatric auto-immune uveitis, high dose MTX was associated with a shorter time to remission on medication as compared to low dose MTX, while side effects were comparable in both groups.
INTRODUCTION
Uveitis is an inflammatory disorder of the eye, involving the uveal tract. It is classified as anterior, intermediate, posterior or panuveitis, depending on the part of the eye affected by the inflammatory process. Uveitis can be associated with a systemic auto-immune disease, can be caused by an infection, and it can occur as an isolated ocular condition. In the developed countries, 87–89% of the pediatric uveitis cases are non-infectious and the majority (41.5%) are related to juvenile idiopathic arthritis (JIA).Citation1
Pediatric uveitis is a potentially blinding disorder and accounts for 3.2–15.2% of all cases of legal blindness in the affected eye(s) in the United States.Citation2,Citation3 Many children with uveitis do not report any symptoms. Citation4 This may lead to a delay in diagnosis and treatment, resulting in complications such as band keratopathy, posterior synechiae, cataract, glaucoma and amblyopia, which give a guarded prognosis.Citation5 Early detection and aggressive treatment of uveitis can prevent visual loss and ocular complications. Citation6
The first line of treatment are local corticosteroids. If these are insufficient, local injections with corticosteroids can be considered. Systemic prednisone is started in case of severe uveitis or in case of failure of the local therapy. In case of chronic uveitis, steroid sparing immunosuppressive therapy may be indicated. Because of its long track record and good safety profile, methotrexate (MTX) is the steroid sparing immunosuppressive agent of first choice in almost all cases of non-infectious pediatric uveitis. Citation7
MTX is an efficacious drug, since remission on medication is reached in about 70% of pediatric non-infectious uveitis cases. Citation8 However, MTX also has side-effects such as gastro-intestinal discomfort (nausea and vomiting), which are frequently reported, and the less common hepatic toxicity and bone marrow suppression. Citation9,Citation10 Also, anticipatory nausea and needle phobia in case of subcutaneous administration of MTX are common.
In pediatric uveitis patients, evidence regarding optimal dosage of MTX is scarce. Citation8,Citation11 Frequently used medication regimens start with low-dose MTX, with increasing doses at 2–6 monthly intervals in case of insufficient effectiveness. In the treatment of JIA there is evidence on the effectiveness of higher starting doses and faster dose-escalation schemes. Citation11–Citation13 Therefore, it would be relevant to evaluate whether such schemes would also be more efficient in the treatment of pediatric uveitis.
Optimizing the treatment of pediatric uveitis patients would be relevant because vision threatening complications are directly related to uveitis activity. Citation6,Citation14 Shortening the time to remission on medication will probably reduce or postpone long-term ocular complications and may improve visual prognosis. Also, it seems likely that a higher steroid sparing effect will be achieved with less side effects and complications of systemic corticosteroids. The present retrospective study aims primarily to evaluate the effectiveness of high-dose (≥15 mg/m2/week) and that of low-dose MTX (<15 mg/m2/week) in relation to time to remission on medication. Secondarily, the steroid sparing effect, cumulative dosage of MTX, side effects of MTX treatment, ocular complications and visual acuity are evaluated.
PATIENTS AND METHODS
We performed a retrospective observational cohort study on pediatric patients treated with MTX for uveitis between 1990 and 2014 at the University Medical Center of Groningen, The Netherlands. This study reflects the daily practice in a tertiary center. The Medical Ethical Committee of the University Medical Center of Groningen ruled that approval was not required for this study. Patients were identified from the digital uveitis database of the University Medical Center of Groningen. All patients who were younger than 18 years of age at the start of their uveitis and who were treated with MTX for longer than 6 months were included. MTX treatment was classified as high (≥15 mg/m2/week, maximum of 25 mg/week/sc) or low (<15 mg/m2/week) dose, based on the MTX dose given before remission on medication was reached or medication was switched. Based on this classification, patients were divided into a high (≥15 mg/m2/week) or low (<15 mg/m2/week) -dose group. Before 2007, most patients received low-dose MTX, and hereafter most patients were treated with high-dose MTX. This reflects evolving treatment strategies. Data collection was done from the pediatric and ophthalmological medical records. The diagnosis of uveitis was made by an ophthalmologist specialized in uveitis and dedicated to this patient group. During the follow up period two other ophthalmologists were occasionally involved in the ophthalmological care for these patients. Classification of uveitis was done according to the Standardization of Uveitis Nomenclature (SUN) criteria Citation15 and was based on the available information in the ophthalmological medical record. Children were evaluated for the presence of an underlying systemic disease by a pediatric rheumatologist. When JIA was diagnosed, it was classified according to the ILAR (International League of the Association for Rheumatology) criteria. Citation16
General Descriptives
For each patient the following descriptives were recorded: age, gender, ethnicity, date of first diagnosis of uveitis (further referred to as: uveitis onset), type of uveitis, laterality, date of onset of arthritis, diagnosis and subtype of underlying systemic disease, weight and length (at several time points during follow-up), anti-nuclear antibody (ANA) serologic status, HLA B27 status and ophthalmological findings at presentation. Prognostic signs for a worse outcome (young age, male gender, severity of uveitis at presentation, vitreous involvement and oligo arthritis) were recorded. Citation5
Uveitis Diagnosis and Classification
Uveitis was diagnosed when cells could be observed in the anterior chamber (AC) or in the vitreous. Activity of AC inflammation (cells) evaluated by standard slit-lamp examination was recorded according to the recommendations of the SUN working group.Citation15 Cells in vitreous humor were scored as being present or not. The diagnosis of posterior and panuveitis was made by fundoscopy and in some cases fluorescein angiography (FA) was performed.
Treatment
MTX dosage and route of administration at the start was recorded as mg/m2/week/orally or subcutaneously. Indications (uveitis, arthritis or both) and date for MTX dosage changes were documented. The MTX dosage was related to body surface area (BSA) at the moments of dosage change. Body surface area was calculated by the Mosteller formula [BSA (m2) = (Height(cm) × Weight(kg)/3600)½].Citation17 Measurements of length and weight were performed at the start of the treatment and during follow up. Length and weight values were plotted routinely in the growth curves corrected for age, sex and race. When a value was missing the growth line was plotted between the two existing values. Cumulative dosages of MTX were calculated by multiplying the time (weeks) to remission on medication by the dose in mg/m2 of MTX. Route of administration (oral or subcutaneous) and – in case the route of administration was switched – the indications for switch were noted. Side effects and indications to stop MTX were recorded. Initially, liver enzyme testing is done after four weeks, and thereafter every 3 months. In case of elevated liver enzymes, testing is more frequently performed. The steroid sparing effect of MTX was evaluated by calculating/counting the number of weeks in which patients were treated with oral corticosteroids in a dosage of more than 0.5 mg/kg/day.
In case of cataract or glaucoma surgery (Baerveldt glaucoma implant), patients were given intravenous corticosteroids during surgery followed by a tapering dosage of oral corticosteroids in the period thereafter. In case of glaucoma surgery MTX was stopped for 2 months prior to surgery and re-introduced after the Baerveldt implant was functional. During this period, patients were treated with oral corticosteroids.
Remission on Medication
Remission on medication was defined as an observable inactive disease in the affected eye for longer than 3 months without the use of systemic corticosteroids or local steroid injections (subtenon or subconjunctival). During this period local steroid medication such as eye drops or ointment were allowed in a maintenance dosage of less than 4 drops per eye daily. With this treatment regimen, sufficient compliance-adherence was expected and it was regarded as being compatible with daily activities.Citation18 Patients were advised to use the eye drops during mealtimes and – when necessary – before sleeping. A relapse was defined as a recurrence of the uveitis after a quiet episode described in the patient file. The total follow up time, time to remission on medication, time between dose adjustments and time to cataract and glaucoma surgery were documented.
Visual Acuity
The decimal equivalent of the Snellen visual acuity (VA) of the affected eyes was recorded at presentation, 6, 12 and 24 months and at last follow-up. Snellen VA was converted to logarithm of the minimum angle of resolution units (LogMAR) VA for calculations.
Ocular Complications
The following ocular complications were scored per eye; band keratopathy, posterior synechiae, cataract and amblyopia. Ocular hypertension was defined as an intra-ocular pressure above 21 mmHg without treatment. Citation15 Glaucoma was defined as glaucomatous changes to the optic nerve or visual field.Citation15 Surgery for medically uncontrollable intra-ocular pressure was separately scored.
Statistics
Statistical analysis was performed by SPSS® software version 22 (SPSS, Inc., Chicago, IL) .A P < 0.05 was considered statistically significant. Descriptive statistics were used to present mean and standard deviation (SD) in normally distributed data or median and range if data were abnormally distributed. For the differences between the nominal data in the high and low-dose groups we used the Chi-square test. In case of non-normally distributed linked samples, the Wilcoxon test for paired samples and the Mann-Whitney U test for independent samples were used. Analysis of VA at presentation compared to that at 6, 12 and 24 months and at the end of follow up was done by the independent samples T-test. A Kaplan-Meier survival analysis with a log rank test was used to analyze survival curves and to compare the two treatment groups. Finally, a multiple regression model was used to assess the weight and influence of treatment groups, age, gender, underlying disease and anatomic location of the uveitis on the time to remission on medication.
RESULTS
Patient, ocular and disease characteristics at uveitis onset are summarized in . A total of 44 (22 male) patients were primarily identified, two of whom (both female, Caucasian JIA- patients with longstanding bilateral anterior uveitis) were excluded because of incomplete data. In four patients with JIA-uveitis active arthritis (next to the uveitis) was the indication to start MXT. The 17 patients in the high-dose group were significantly younger (mean 5.0 ± 2.7 years) than the 25 patients in the low-dose group (mean 7.6 ± 3.5 years, P = 0.01). The follow up time was statistically significantly shorter in the high-dose group (median 4.0 years, range 0.9–19.2) as compared to the low-dose group (median 6.9 years, range 1.4–15.4, P = 0.02).
Table 1. Patient characteristics.
No differences were found between the high and low-dose groups regarding severity of the uveitis at presentation and the need for ocular surgery for cataract or medically uncontrollable intra ocular pressure during follow-up ().
The median starting dose and median maximum dose were both significantly lower in the low-dose group. The median starting dose in the low-dose group was 10.4 (min 5.7 – max 14.8) mg/m2/week and in the high-dose group 17.9 (min 11.8 – max 24.6) mg/m2/week (P < 0.001). The median maximum dose in the low-dose group was 13.4 (min 10.9 – max 14.9) mg/m2/week and in the high-dose group it was 20.7 (min 16.7 – max 25.3) mg/m2/week (P < 0.001). The time to maximum dose of MTX was -although not statistically significant -shorter in the high-dose group. The median time to maximum dose in the low-dose group was 20.9 (min 2.1 – max 120.1) months versus median 9.1 (min 4.6 – max 21.7, P = 0.10) in the high-dose group.
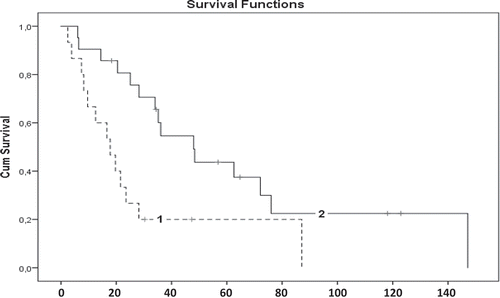
A total of 13 patients (76.6%) treated with high-dose MTX reached remission on medication after a median of 16.6 months (inter quartile range (IQR); 7.8–22.5). In the low-dose group 15 (60%) patients reached remission on medication in a median of 35.2 months (IQR; 20.5–72.1). The difference in time to remission on medication was statistically significant (P = 0.01) (, ).
Table 2. Remission on medication, cumulative MTX dose and steroid use.
Figure 1. Kaplan-Meier curves showing the time to and chance of remission on medication. Cum = cumulative.
1 = High dose (>15 mg/m2/week) methotrexate. 2 = Low dose (<15 mg/m2/week) methotrexate. The difference between the groups is statistically significant (P = 0.007, Log Rank test). HD = High dose, LD = Low dose
Patients (n = 12) treated with high-dose subcutaneous MTX had a statistically significantly shorter time to remission on medication (median 17.2 months) than patients (n = 7) who reached remission on medication on low-dose subcutaneous MTX (median 62.6 months; p = 0.001; ). Of the 9 patients who reached remission on medication on oral MTX, 1 was treated with high-dose and 8 with low-dose MTX. By using a multivariate linear regression model time to remission on medication was analyzed. Within this model; age, gender, anatomic location of the uveitis, presence of juvenile idiopathic arthritis and the two treatment groups were taken into account. With this model (RCitation2 = 0.4, P = 0.05, B = 68.6 (CI 24.8 – 112.4)) we found that treatment with a higher dose MTX was associated with a statistically significantly shorter time to remission on medication (P 0.008) ().
The cumulative dose of MTX, in the 28 patients reaching remission on medication, was lower in the high-dose group as compared to the low-dose group, but this difference was not statistically significant (). MTX related side effects were reported by 25 out of 41 patients (). No statistically significant differences regarding side effects were found between the high- and low-dose groups.
Table 3. MTX-related side effects.
Table 4. Ocular complications*.
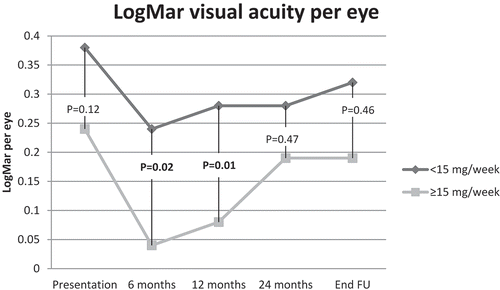
On average, patients used more than 0.5 mg/kg/day of oral corticosteroids for 17.2 weeks (); no statistically significant differences in steroid use were seen between the two groups (). The high-dose group had a better visual acuity at presentation (), but this difference was not statistically significant. At 6 and 12 months, visual acuity in the high-dose group was significantly higher than in the low-dose group. At later time points, this difference was no longer statistically significant ().
DISCUSSION
This study of 42 pediatric patients with non-infectious uveitis shows that patients who were treated with a high dose of MTX (≥15 mg/m2/week, maximum of 25 mg/week/sc) reached remission on medication sooner compared to patients who were treated with a low dose of MTX (<15 mg/m2/week). The data also indicate that an MTX dose of ≥15 mg/m2/week administered by subcutaneous injection is the most effective in establishing rapid remission on medication. With regard to visual acuity measurements at 6 and 12 months the data suggests a favorable outcome in the high-dose group. High- and low-dose groups were comparable with regard to severity of uveitis, incidence of ocular complications and surgery, steroid sparing capacity of MTX, cumulative dose of MTX and side effects.
In our study, we found an overall success rate of 67% of MTX in the treatment of uveitis, which is similar to the effectiveness of 70% described in the literature. Citation8 More patients reached disease remission on subcutaneously administered MTX when compared to oral administration. Time to disease remission on medication did not significantly differ between patients on oral and subcutaneous administration. Patients treated with high-dose MTX had a significantly shorter time to remission on medication than the patients treated with low-dose MTX. A shorter time to remission on medication may be favorable for the prognosis of an inflammatory disease as was shown for rheumatoid arthritis (RA) in several studies. Citation19–Citation22 By analogy, it would be plausible to assume that achieving early remission on medication will help to prevent or delay secondary complications of uveitis and to preserve visual function. Citation14,Citation23
Our retrospective study did not find statistically significant differences in the prevalence of ocular complications at any time point between high and low-dose groups, including complications already present at presentation. Therefore and because high-dose MTX was mainly given from 2007 onwards, we assume that MTX dose was mainly based on evolving treatment strategies and not primarily on the severity or complications of the uveitis. We did find a statistically significantly better visual acuity in the high-dose group at 6 and 12 months, which is suggestive of a better outcome favorable for the daily functioning and development of a child. However, there may be some inclusion bias, since the high-dose group had a better, though not statistically significantly better, visual acuity at presentation.
A faster remission reached by a predefined quick dose escalation scheme is probably more rewarding and motivating for a patient than a slow escalation scheme based on dose adjustments because of persisting disease activity. Also, the frequently reported MTX intolerance after longer use of MTX, might be prevented if remission on medication is sooner reached.Citation24,Citation25 Finally, MTX failure will be apparent after a shorter time interval when a faster dose escalation scheme is used, thus enabling an earlier switch in therapy. Our high-dose group is comparable to the intermediate MTX dose group of Ruperto et al. Citation12 who evaluated the effectiveness and side effects of MTX in JIA. They found a better effect of an intermediate (15 mg/m2/week) MTX dose as compared to a low MTX dose (10 mg/m2/week). In addition, they observed that increasing the MTX dose to 30 mg/m2/week (with a maximum of 40 mg/week by intra muscular or subcutaneous administration) was not associated with any therapeutic benefit and resulted in more adverse events.Citation12 In line with that study, our maximum MTX dose is 25 mg/week by subcutaneous administration. Our findings indicating a positive effect of higher MTX dosages in pediatric non-infectious uveitis are in line with the results of a systematic reviewCitation8 that showed that the proportion of children responding to MTX is the highest in the studies with an MTX dosage of ≥15 mg/m2/week.Citation26–Citation29
No significant differences were found in steroid sparing effect, cumulative MTX dosage until remission on medication and side effects of MTX between our two study groups. The first is possibly explained by our reluctance to use systemic steroids in children, since they were mainly given in case of severe uveitis at presentation and peri-operatively. The second reflects that remission on medication is reached sooner in the high-dose group as compared to the low-dose group. And the latter may be explained by the lower cumulative MTX dosage in the high-dose group.
The results of the current study are limited by the fact that the study is retrospective, the numbers of patients are small and there is a large variability in follow up time. The better outcome in the high-dose group is possibly influenced by positive developments in treatment options and improved screening programs for JIA uveitis. The reporting of side-effects is influenced in an uncertain way because of the retrospective study design and variability in follow up time. All patients were included from a tertiary center and two patients had to be excluded because of missing data, therefore this study does not represent the total spectrum of pediatric non-infectious uveitis. Also, personal experience or preferences of ophthalmologists and pediatric rheumatologist may have influenced the choice of treatment. The strengths of this study are the systematic way in which data were collected, its adherence to the SUN classification system and guidelines for publications and the dose adjustment for body surface area.
Based on our findings, we would recommend an MTX starting dose of ≥15 mg/m2/week with a maximum of 25 mg/week by subcutaneous administration in the treatment of pediatric non-infectious uveitis. After reaching remission on medication a lower (10–15 mg) – possibly oral – maintenance dosage can be considered to maintain remission. Earlier publications about the efficacy of low-dose MTX in rheumatoid arthritis are supporting this.Citation30–Citation33 Because of the lower and varying bioavailability of oral MTX when compared to subcutaneous administrationCitation34–Citation36 the effect of switching from subcutaneous to oral administration is difficult to predict. Ayuso et al.Citation29 described a higher relapse rate after withdrawal of MTX in pediatric non-infectious uveitis. Their results indicate that the period of inactivity before withdrawal should be preferably longer than 2 years.Citation29 They do not describe a dose reduction after remission on medication is reached. By sharing our treatment experiences and advising on steps to optimize treatment regimens, we hope to make a contribution to the improvement of care for children with non-infectious uveitis.
In conclusion, children with non-infectious uveitis can benefit from early treatment with high-dose MTX (≥15 mg/m2/week, maximum 25 mg/week/sc) preferably by subcutaneous administration. Such a strategy may lead to a shorter time to remission on medication, a higher rate of remission on MTX and similar rates of side effects as in low-dose MTX treatment strategies. Future studies, most preferably randomized controlled trials, are needed to confirm these findings.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
REFERENCES
- Mehta PJ, Alexander JL, Sen HN. Pediatric uveitis: new and future treatments. Curr Opin Ophthalmol. 2013;24(5):453–462. doi:10.1097/ICU.0b013e3283641ede.
- Sauberan DP. Pediatric uveitis. Int Ophthalmol Clin. 2010;50(4):73–85. doi:10.1097/IIO.0b013e3181f0f2b5.
- de Boer J, Wulffraat N, Rothova A. Visual loss in uveitis of childhood. Br J Ophthalmol. 2003;87:879–884.
- Wentworth BA, Freitas-Neto CA, Foster CS. Management of pediatric uveitis. F1000Prime Rep. 2014;6:41. eCollection 2014. doi:10.12703/P6-41.
- Rosenberg KD, Feuer WJ, Davis JL. Ocular complications of pediatric uveitis. Ophthalmology. 2004;111(12):2299–2306. doi:10.1016/j.ophtha.2004.06.014.
- Gregory AC 2nd, Kempen JH, Daniel E, et al. Risk factors for loss of visual acuity among patients with uveitis associated with juvenile idiopathic arthritis: the systemic immunosuppressive therapy for eye diseases study. Ophthalmology. 2013;120(1):186–192. doi:10.1016/j.ophtha.2012.07.052.
- Wong VG. Methotrexate treatment of uveal disease. Am J Med Sci. 1966;251:239–241.
- Simonini G, Paudyal P, Jones GT, Cimaz R, Macfarlane GJ. Current evidence of methotrexate efficacy in childhood chronic uveitis: a systematic review and meta-analysis approach. Rheumatology (Oxford). 2013;52(5):825–831. doi:10.1093/rheumatology/kes186.
- Visser K. van der Heijde D. Optimal dosage and route of administration of methotrexate in rheumatoid arthritis: a systematic review of the literature. Ann Rheum Dis. 2009;68(7):1094–1099. doi:10.1136/ard.2008.092668.
- Chan ES, Cronstein BN. Methotrexate–how does it really work? Nat Rev Rheumatol. 2010;6(3):175–178. doi:10.1038/nrrheum.2010.5.
- Hashkes PJ, Becker ML, Cabral DA, et al. Methotrexate: new uses for an old drug. J Pediatr. 2014;164(2):231–236. doi:10.1016/j.jpeds.2013.10.029.
- Ruperto N, Murray KJ, Gerloni V, et al. A randomized trial of parenteral methotrexate comparing an intermediate dose with a higher dose in children with juvenile idiopathic arthritis who failed to respond to standard doses of methotrexate. Arthritis Rheum. 2004;50(7):2191–2201. doi:10.1002/art.20288.
- Visser K, Katchamart W, Loza E, et al. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E initiative. Ann Rheum Dis. 2009;68(7):1086–1093. doi:10.1136/ard.2008.094474.
- Smith JA, Mackensen F, Sen HN, et al. Epidemiology and course of disease in childhood uveitis. Ophthalmology. 2009;116(8):1544–51, 1551.e1. doi:10.1016/j.ophtha.2009.05.002.
- Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. results of the first international workshop. Am J Ophthalmol. 2005;140:509–516.
- Petty RE, Southwood TR, Manners P, et al. International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, edmonton, 2001. J Rheumatol. 2004;31:390–392.
- El Edelbi R, Lindemalm S, Eksborg S. Estimation of body surface area in various childhood ages–validation of the mosteller formula. Acta Paediatr. 2012;101(5):540–544. doi:10.1111/j.1651-2227.2011.02580.x.
- Sharma SM1, Ad D, Ramanan AV. Non-infectious pediatric uveitis: an update on immunomodulatory management. Paediatr Drugs. 2009;11:229–241.
- van Nies JA, Tsonaka R, Gaujoux-Viala C, Fautrel B, van der Helm-van Mil AH. Evaluating relationships between symptom duration and persistence of rheumatoid arthritis: does a window of opportunity exist? Results on the Leiden early arthritis clinic and ESPOIR cohorts. Ann Rheum Dis. 2015;74(5):806–812. doi:10.1136/annrheumdis-2014-206047.
- van Nies JA, Krabben A, Schoones JW, Huizinga TW, Kloppenburg M, van der Helm-van Mil AH. What is the evidence for the presence of a therapeutic window of opportunity in rheumatoid arthritis? A systematic literature review. Ann Rheum Dis. 2014;73(5):861–870. doi:10.1136/annrheumdis-2012-203130.
- Wallace CA, Giannini EH, Spalding SJ, et al. Trial of early aggressive therapy in polyarticular juvenile idiopathic arthritis. Arthritis Rheum. 2012;64(6):2012–2021. doi:10.1002/art.34343.
- Magnani A, Pistorio A, Magni-Manzoni S, et al. Achievement of a state of inactive disease at least once in the first 5 years predicts better outcome of patients with polyarticular juvenile idiopathic arthritis. J Rheumatol. 2009;36:628–634. doi:10.3899/jrheum.080560.
- Wolf MD, Lichter PR, Ragsdale CG. Prognostic factors in the uveitis of juvenile rheumatoid arthritis. Ophthalmology. 1987;94:1242–1248.
- van Dijkhuizen EH, Wulffraat NM. Prediction of methotrexate efficacy and adverse events in patients with juvenile idiopathic arthritis: A systematic literature review. Pediatr Rheumatol Online J. 2014;12:51–0096-12–51. eCollection 2014. doi:10.1186/1546-0096-12-51.
- van Dijkhuizen EH, Bulatovic Calasan M, Pluijm SM, et al. Prediction of methotrexate intolerance in juvenile idiopathic arthritis: a prospective, observational cohort study. Pediatr Rheumatol Online J. 2015;13:5–015-0002–3. eCollection 2015. doi:10.1186/s12969-015-0002-3.
- Malik AR, Pavesio C. The use of low dose methotrexate in children with chronic anterior and intermediate uveitis. Br J Ophthalmol. 2005;89(7):806–808. doi:10.1136/bjo.2004.054239.
- Foeldvari I, Wierk A. Methotrexate is an effective treatment for chronic uveitis associated with juvenile idiopathic arthritis. J Rheumatol. 2005;32:362–365.
- Heiligenhaus A, Mingels A, Heinz C, Ganser G. Methotrexate for uveitis associated with juvenile idiopathic arthritis: value and requirement for additional anti-inflammatory medication. Eur J Ophthalmol. 2007;17:743–748.
- Kalinina Ayuso V, van de Winkel EL, Rothova A, de Boer JH. Relapse rate of uveitis post-methotrexate treatment in juvenile idiopathic arthritis. Am J Ophthalmol. 2011;151(2):217–222. doi:10.1016/j.ajo.2010.08.021.
- Hoffmeister RT. Methotrexate in rheumatoid arthritis (abstract). Arthritis Rheumatol. 1972;15:114.
- Hoffmeister RT. Methotrexate therapy in rheumatoid arthritis: 15 years’ experience. Am J Med. 1983;(75):69–73. doi:10.1016/0002-9343(83)90477-1.
- Willkens RF, Watson MA, Paxson CS. Low dose pulse methotrexate therapy in rheumatoid arthritis. J Rheumatol. 1980;7:501–505.
- Willkens RF, Watson MA. Methotrexate: a perspective of its use in the treatment of rheumatic diseases. J Lab Clin Med. 1982;100:314–321.
- Tuková J, Chládek J, Nemcová D, Chládková J, Dolezalová P. Methotrexate bioavailability after oral and subcutaneous dministration in children with juvenile idiopathic arthritis. Clin Exp Rheumatol. Nov-Dec 2009;27(6):1047–1053.
- Braun J. Optimal administration and dosage of methotrexate. Clin Exp Rheumatol. 2010 Sep-Oct;28(5Suppl 61):S46–51. Epub 2010 Oct 28.
- Bello AE, Perkins EL, Jay R, Efthimiou P. Recommendations for optimizing methotrexate treatment for patients with rheumatoid arthritis. Open Access Rheumatol. 2017 Mar 31;9:67–79. doi:10.2147/OARRR.S131668.