ABSTRACT
Purpose: To report the outcomes in eyes with noninfectious uveitis receiving dexamethasone implant at the time of pars plana vitrectomy (PPV).
Methods: Retrospective analysis of visual acuity (VA), intraocular pressure (IOP), vitreous haze score (VHS), and central subfield thickness (CST) at baseline and follow-up visits.
Results: Fourteen eyes received dexamethasone implant at the time of PPV. The CST was improved from 469 ± 182 µm at baseline to 320 ± 60 at 6 months (p = .0112) and 295 ± 46 at 12 months (p = .0728). Vitritis only recurred in 2 eyes at 6 months (18.2%) and 1 eye at 12 months (14.3%). The probability of VA improvement of ≥0.3 logMAR was 57% at 6 months and 66% at 12 months. Therapy for IOP rise was initiated in 6 eyes (42.9%).
Conclusions: Local delivery of dexamethasone implant with PPV is a feasible method to counteract postoperative inflammation and macular thickening.
Noninfectious uveitis is a sight-threatening disease, causing 10-15% of blindness in the developed world.Citation1 Uveitic macular edema, cataract, epiretinal membrane (ERM), and vitreous opacification are the main causes of visual loss. Pars plana vitrectomy (PPV) plays a role in the treatment of uveitis complications, including removal of secondary epiretinal membrane (ERM), and clearance of vitreous opacities (VO).Citation2–5 However, PPV in uveitic eyes can be associated with exacerbating inflammation and inducing postoperative CME due to increased tissue reactivity.Citation6,Citation7
In eyes with noninfectious uveitis, injection of intravitreal dexamethasone implant (Ozurdex®, Allergan, Inc., Irvine, CA, USA) has been shown to significantly reduce vitreous haze and macular edema.Citation8–11 Additionally, studies have shown that the pharmacokinetic properties of the dexamethasone implant are not different in vitrectomized eyes,Citation10 unlike intravitreal triamcinolone that has a significantly shortened half-life in the vitreous cavity following PPV surgery.Citation12
The intraoperative use of a sustained-release corticosteroid at the time of PPV could provide additional benefits to patients with noninfectious uveitis reinforcing the control of uveitis and preventing exacerbation of inflammation due to surgery. However, there is a paucity of literature describing the use of dexamethasone implant at the time of PPV. Zheng et al.Citation13 demonstrated the feasibility of combined vitrectomy and dexamethasone implant in a retrospective analysis of 15 eyes with different retinal problems including proliferative diabetic retinopathy, age-related macular degeneration, and retinal vein occlusion. Their study included five eyes with noninfectious uveitis but did not provide any analysis of uveitis descriptors such as vitreous haze score (VHS) or central retinal thickness (CRT) in these eyes.
We conducted this study to evaluate the benefits of using dexamethasone implant in eyes with noninfectious uveitis undergoing PPV surgery. The primary outcome measures in this study are VHS and CRT, while the secondary endpoint was visual acuity. In addition, we analyzed the complications, and durability of intravitreal dexamethasone implant when used in this context.
Methods
Data Extraction and Categorization
Following local Institutional Board Review approval, we retrieved data from electronic medical records of all eyes receiving intraoperative dexamethasone implant at the time of PPV from August 1, 2016, to June 30, 2018, at one tertiary referral uveitis center (University of Arkansas for Medical Sciences, Little Rock, AR). The study adhered to the tenets of the Declaration of Helsinki.
Of 99 eyes that underwent combined PPV and intraoperative dexamethasone implant over a 23-month study period, 81 were excluded due to a different diagnosis other than noninfectious uveitis (78 eyes had diabetic vitrectomy and 3 eyes had retinal vein occlusion related ERM). In addition, we excluded four eyes with noninfectious uveitis that underwent phacoemulsification at the time of combined PPV and intravitreal dexamethasone implant to eliminate any confounding effect of removing the cataract on visual acuity.
We analyzed data on gender, age, laterality, race, surgical indication, surgical complications, uveitis category and etiology, prior and current local and systemic therapy, history of steroid hypertensive response, lens status, intraocular pressure (IOP), prior IOP-lowering treatment, visual acuity (VA), central subfield thickness (CST) assessed by optical coherence tomography (OCT), and VHS. We also noted other safety parameters such as hypotony (≤5 mmHg), and postoperative fibrin syndrome (POFS).
As data were gathered from routine clinical setting, visual acuity was determined as the best visual response with current habitual correction or pinhole as measured by Snellen chart. We converted Snellen visual acuity to logMAR equivalents for the purpose of analyses. Per the Standardization of Uveitis Nomenclature (SUN), clinically significant improvement in visual acuity was defined as improvement of ≥0.3 logMAR units (approximately 3 Snellen lines), and improvement of vitreous haze score is defined as a 2-step improvement or complete inactivation of inflammation (0.5 to 0).Citation14 Standardized VHS was recorded in concordance with the SUN guidelines.Citation15
Central subfield thickness was determined by OCT on either Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, California, USA) or Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany) devices. For eyes with missing or unattainable data, no values were substituted. We defined macular edema (ME) as CST ± 2 standard deviations from the mean (≥300 µm on Zeiss Cirrus and ≥320 µm on Heidelberg Spectralis) irrespective of the presence of cystoid spaces.Citation16 Resolution of macular edema was achieved at less than 2 standard deviations below the normative mean (i.e. 260 µm on both machines), and clinically significant improvement in ME was defined as ≥20% reduction in macular thickness from baseline.Citation17
We approximated the follow-up time periods to the nearest month, for example, 1 month (20–45 days), 2 months (46–75 days), 3 months (76–105 days), and so forth.
Surgical Technique
In brief, we performed PPV using 27-gauge (2/14 eyes, 14.3%), 25-gauge (11 eyes, 78.6%), or 23-gauge (1 eye, 7.1%) transconjunctival approach and a non-contact wide-angle viewing system. Trocars were placed at 3.5 or 4 mm behind the limbus in pseudophakic and phakic eyes, respectively. In 5 eyes (35.7%), posterior vitreous detachment was not present and posterior hyaloid separation was performed using the vitreous cutter. Vitrectomy was completed in the usual manner including core and peripheral vitreous removal. Retinal peripheral examination with scleral depression was performed in all cases to identify preexisting or surgery-related peripheral retinal breaks. We performed ERM peel in 12 of 14 eyes (85.7%). Additional internal limiting membrane (ILM) peel was performed in 10 of these eyes (71.4%), mainly when ILM wrinkles were noted after ERM removal, assisted by indocyanine green staining. Air fluid exchange was performed in four eyes, while the remaining eyes were left filled with balanced salt solution. Regarding the implant injection technique, in all but two eyes, we injected the implant via the superotemporal sclerotomy after removing the vitrectomy cannula under direct visualization, aiming nasally away from the macula the implant. In one eye, due to the presence of an anterior chamber intraocular lens, we surgically secured the implant to the sclera to prevent anterior migration.Citation18 In the remaining eye, we injected the dexamethasone implant through the preexisting 23-gauge vitrectomy cannula.Citation19 Of the four air-filled eyes, air was exchanged for sulfur hexafluoride (SF6) gas in one eye after concurrent ILM peel for tamponade due to the cystic appearance of the fovea. At the conclusion of surgery, sutures were placed for sclerotomy leakage in 7 (50.0%) eyes.
Statistical Methods
We used paired t test to compare mean changes in VA, CRT, and IOP before and after the combined procedure. We used Kaplan-Meier survival curves to represent subsequent events after combined vitrectomy and dexamethasone implant.Citation20 A p value of less than 0.05 was considered statistically significant for all tests. We performed the statistical analyses using SPSS software (SPSS Inc, Chicago, Illinois, USA), and GraphPad Prism 8.0 (La Jolla, California, USA).
Results
Baseline Characteristics
A total of 14 eyes with noninfectious uveitis which underwent combined vitrectomy and dexamethasone implant were analyzed in this study. The demographics, surgery indications, uveitis classification, CME presence, and lens status of each patient are outlined in . The most common anatomic classification of uveitis was panuveitis (5/14, 35.7%) and posterior (5/14, 35.7%), followed by intermediate (3/14, 21.4%) and anterior uveitis (1/14, 7.1%). The most common etiology was undifferentiated (12/14, 85.7%). Macular edema was present in 13 of 14 eyes (92.9%). The most common surgical indications for PPV were uveitic ERM (11/14, 78.6%), and VO (8/14, 57.1%). At the time of surgery, only 3 eyes had active inflammation with VHS of 1 or worse, while the remaining eyes had a VHS of 0.5 or less.
Table 1. Baseline characteristics of eyes with noninfectious uveitis receiving intravitreal dexamethasone at the time of pars plana vitrectomy
Preoperative local treatment, including periocular and intraocular therapy, was used in 10 of 14 eyes (71.4%), with 7 eyes treated with orbital floor injection of triamcinolone acetonide (50.0%), 4 eyes treated with intravitreal dexamethasone implant (26.8%), 3 eyes treated with intravitreal triamcinolone acetate (21.4%), and 2 eyes treated with anti-VEGF (14.3%) (). Lens status at the time of surgery was either pseudophakic (12/14, 85.7%) or phakic (2/14, 14.3%). None of the eyes had a preoperative documentation of zonular defects, but one pseudophakic eye had an anterior chamber intraocular lens. Four eyes (28.6%) were on systemic therapy, 1 of which was treated solely with oral prednisone (7.1%), 1 on combined corticosteroids and immunomodulatory treatment (7.1%), and 2 on single, or dual, immunomodulatory treatment (14.3%) (). No patient required increased or additional systemic therapy, and 2 of 4 patients were able to discontinue systemic therapy due to the improvement of their uveitis.
Table 2. Treatments before and after intravitreal dexamethasone implant in uveitic eyes undergoing pars plana vitrectomy
Outcome Measures
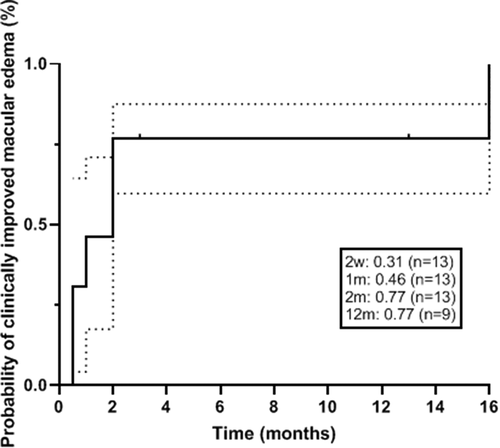
The mean ± SD CST improved from a baseline of 469 ± 182 µm to 380 ± 183 at 1 month (p = .0286), 338 ± 112 at 3 months (p = .0027), 320 ± 60 at 6 months (p = .0112), and 295 ± 46 at 12 months (p = .0728) (). Thirteen of 14 eyes had ME at baseline. All but 2 eyes (84.6%) achieved clinically improved ME (>20% reduction in CST). The probability of ME resolution was 46% at 1 month, 77% at 2 months, and remained at 77% at 6 and 12 months ().
Table 3. Clinical outcomes including visual acuity, vitreous haze score and central macular thickness after intravitreal dexamethasone implant in uveitic eyes undergoing pars plana vitrectomy
Figure 1. Probability of clinically improved macular edema after intravitreal dexamethasone implant treatment in uveitic eyes undergoing pars plana vitrectomy (defined as ≥20% reduction in central subfield thickness). Dotted lines represent the 95% confidence interval.
Vitritis (VHS ≥ 0.5) was present in 7 of 14 (50.0%) eyes at baseline (VHS = 0.5 in 4 eyes, 1+ in 2 eyes, and 2+ in 1 eye). At 2 weeks, VHS had resolved to 0 in 13 of 14 eyes. A single eye was found to have post-operative fibrin syndrome and was treated with intravitreal tissue plasminogen activator (tPA). Vitreous haze ≥0.5 was present in 1 eye at 3 months (7.7%), 2 eyes at 6 months (18.2%), and 1 eye at 12 months (14.3%) ().
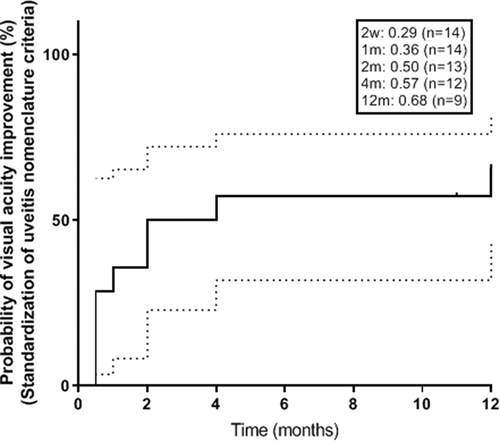
Median VA at baseline was 20/80 and improved to 20/40 at 12 months. Visual acuity improved from a mean ± SD of 0.84 ± 0.64 logMAR at baseline to 0.65 ± 0.55 at 1 month (p = .1950), 0.46 ± 0.38 at 3 months (p = .0496), 0.42 ± 0.36 at 6 months (p = .0390), and 0.56 ± 0.72 at 12 months (p = .3798). The probability of 3-line Snellen VA (≥0.3 logMAR) improvement was 36% at 1 month, 50% at 3 months, 57% at 6 months, and 66% at 12 months ().
Safety Measures
A single eye experienced an intraoperative complication of the intravitreal dexamethasone implant adhering to the retinal surface after PPV surgery combined with gas. In this eye, the air-fluid exchange was performed after placing the implant into the vitreous cavity. Because of the proximity of the implant to the fovea, we undertook repeat PPV and repositioned the implant away from the macular area using intraocular forceps.Citation21 One eye developed postoperative fibrin syndrome that was treated with tPA, and one eye that was left fluid-filled at the time of surgery developed transient hypotony related to a sclerotomy site leak which subsequently resolved at 1 week after placing a bandage contact lens. There was no migration of the dexamethasone implant in any of the pseudophakic eyes.
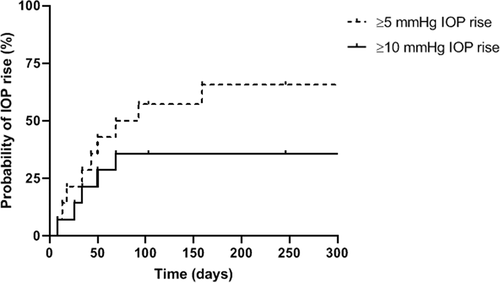
Elevation in IOP greater than 10 mmHg from baseline at any point during the study period occurred in 7 of 14 eyes (50.0%). Mean IOP was 16, 20, 18, 12, and 13 mmHg at baseline, 1 month, 3 months, 6 months, and 12 months, respectively. At final follow-up, only one eye had an IOP greater than 10 mmHg from baseline (7.1%) (). Nine patients had mild elevation of IOP with a 66% probability of ≥5 mmHg pressure rise from baseline at 6 months. The probability of IOP increase of 10 mmHg or greater was 36% at 2 months, and no increased probability was seen thereafter (). Intraocular pressure-lowering therapy was initiated in 42.9% of eyes (6/14) at a median time of 1 month (range 1–3 months). None of the study eyes required any glaucoma laser or incisional surgery. Neither of the 2 phakic eyes required cataract surgery over a median follow-up time of 8.5 months, postoperatively.
Table 4. Adverse effects after intravitreal dexamethasone implant in uveitic eyes undergoing pars plana vitrectomy
Durability of Dexamethasone Implant
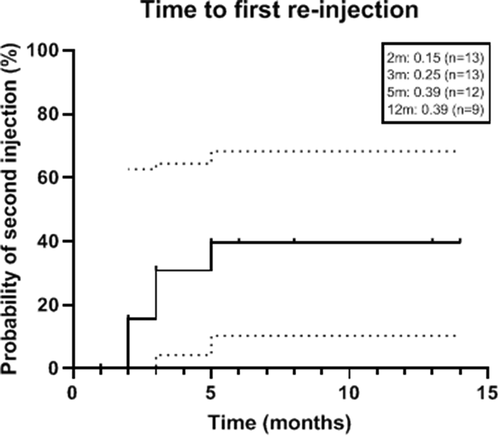
Five eyes (38.5%) required repeat intravitreal steroid depot injections. Probability of needing the second injection was 39.4% at 5 months ().
Discussion
This study looked at the feasibility of intraoperative dexamethasone implant use in eyes with noninfectious uveitis undergoing PPV. Our results demonstrate that the use of dexamethasone implant in this context was associated with optimum control of perioperative inflammation, as demonstrated by the absence of vitreous cavity inflammation (VHS of 0 at 2 weeks in 92.3% of eyes) and a significant decrease in macular thickness (mean decrease in CST by 89 µ at 1 month, p = .029), postoperatively. We also observed an acceptable safety profile when using dexamethasone implant intraoperatively with postoperative IOP rise being the most frequent complication.
We observed an approximately 30% probability of clinically reduced macular edema (defined as >20% CST reduction) as early as 2 weeks, and 77% probability of ME reduction at 2 months. Vitreous cavity inflammation was well controlled with all eyes achieving and maintaining a VHS of 0 as early as 4 weeks postoperatively. It is possible that vitrectomy surgery may have contributed to the near-immediate decrease in vitreous haze by removing vitreous opacities, but there was minimal reaccumulation of vitreous inflammation and early reduction in CRT in the short-term postoperative phase, consistent with a steroid-related effect.
Overall, mean VA in this cohort improved at 12 months from baseline. Visual acuity had the most improvement over the first 3 months then experienced a plateau effect (). According to the SUN criteria, there was a 50% and 68% probability of VA improvement by 2 months and 12 months, respectively (). Visual acuity is a secondary outcome of this study and should be interpreted with caution as it may be influenced by the effect of PPV treatment for vitreous opacities or ERM.
The results observed in this study regarding the rapid onset and the durability of the dexamethasone implant when used during PPV surgery concur with the previous feasibility study by Zheng et al.Citation13 and extends the evidence that concurrent PPV surgery and placement of the implant in air- or gas-filled eyes does not alter the function of the implant. Chiang-Lin et al.Citation22 used an animal model to show dexamethasone implants in vitrectomized eyes achieve an approximately 30% of peak concentration 2 days after injection and reach a maximum concentration at 22 days, suggesting a potentially rapid yet sustained effect after vitrectomy. Pelegrín et al.Citation10 found no significant change in durability between vitrectomized and non-vitrectomized eyes with dexamethasone implant. The median time of repeat steroid implant in our study was approximately 4 months, consistent with previous uveitis studies.Citation23
Retinal trauma due to the injector velocity has been reported in vitrectomized eyes.Citation24,Citation25 The case we encountered in this study where the implant was found adherent to the macula makes us suspect that performing air-fluid exchange with the implant already in the eye has resulted in its adherence onto the retina.Citation21 Other than this instance there was no intraoperative complication from the implant use.
Intraocular pressure was the main adverse effect in our series (), with a 36% probability of pressure rise at 2 months and no further increase by 12 months. Intraocular pressure-lowering therapy was necessary for 6 eyes (42.9%), which is consistent with prior literature involving intravitreal dexamethasone in eyes with uveitis.Citation9 No eye underwent laser or incisional glaucoma surgery.
There are limitations to this study including its small scale and uncontrolled retrospective design. We did not include a control group of uveitic eyes that are only treated with PPV. However, while it has been suggested PPV alone may result in uveitis remission in some cases,Citation26 a systematic review of recent literature on PPV in uveitis by Henry et al.Citation2 demonstrated that it was not possible to conclude if PPV is beneficial for controlling inflammatory activities and whether anti-inflammatory therapy can be reduced following surgery. Further, PPV was performed for different indications in our study including ERM and VO, which could influence the visual outcomes.
In summary, the results of this study demonstrate that intravitreal dexamethasone implant is a feasible treatment option to counteract postoperative exacerbation of inflammation and macular edema in eyes with noninfectious uveitis undergoing PPV. Since the implant has a rapid onset of action, and its durability is not affected by concurrent vitreous surgery, it provides an ideal method for covering PPV surgery in uveitic eyes as compared to intravitreal triamcinolone or systemic steroids. Similar to its use in non-vitrectomized eyes, dexamethasone implant use during PPV has an overall acceptable safety profile, with the main side effects related to postoperative IOP rise.
Declaration of Interest
No conflict of interest or financial disclosures exist for any of the authors.
References
- de Smet MD, Taylor SR, Bodaghi B, et al. Understanding uveitis: the impact of research on visual outcomes. Prog Retin Eye Res. 2011;30(6):452–470. doi:https://doi.org/10.1016/j.preteyeres.2011.06.005.
- Henry CR, Becker MD, Yang Y, Davis JL. Pars plana vitrectomy for the treatment of uveitis. Am J Ophthalmol. 2018;190:142–149. doi:https://doi.org/10.1016/j.ajo.2018.03.031.
- Kiryu J, Kita M, Tanabe T, Yamashiro K, Miyamoto N, Ieki Y. Pars plana vitrectomy for cystoid macular edema secondary to sarcoid uveitis. Ophthalmology. 2001;108(6):1140–1144. doi:https://doi.org/10.1016/S0161-6420(01)00558-9.
- Wiechens B, Nolle B, Reichelt JA. Pars-plana vitrectomy in cystoid macular edema associated with intermediate uveitis. Graefes Arch Clin Exp Ophthalmol. 2001;239(7):474–481. doi:https://doi.org/10.1007/s004170100254.
- Quinones K, Choi JY, Yilmaz T, Kafkala C, Letko E, Foster CS. Pars plana vitrectomy versus immunomodulatory therapy for intermediate uveitis: a prospective, randomized pilot study. Ocul Immunol Inflamm. 2010;18(5):411–417. doi:https://doi.org/10.3109/09273948.2010.501132.
- Scott RA, Haynes RJ, Orr GM, Cooling RJ, Pavesio CE, Charteris DG. Vitreous surgery in the management of chronic endogenous posterior uveitis. Eye (Lond). 2003;17(2):221–227. doi:https://doi.org/10.1038/sj.eye.6700299.
- Callaway NF, Gonzalez MA, Yonekawa Y, et al. Outcomes of pars plana vitrectomy for macular hole in patients with uveitis. Retina. 2018;38(Suppl 1):S41–S48. doi:https://doi.org/10.1097/IAE.0000000000001942.
- Adan A, Pelegrin L, Rey A, et al. Dexamethasone intravitreal implant for treatment of uveitic persistent cystoid macular edema in vitrectomized patients. Retina. 2013;33(7):1435–1440. doi:https://doi.org/10.1097/IAE.0b013e31827e247b.
- Nobre-Cardoso J, Champion E, Darugar A, Fel A, Lehoang P, Bodaghi B. Treatment of non-infectious uveitic macular edema with the intravitreal dexamethasone implant. Ocul Immunol Inflamm. 2017;25(4):447–454. doi:https://doi.org/10.3109/09273948.2015.1132738.
- Pelegrin L, de la Maza MS, Molins B, Rios J, Adan A. Long-term evaluation of dexamethasone intravitreal implant in vitrectomized and non-vitrectomized eyes with macular edema secondary to non-infectious uveitis. Eye (Lond). 2015;29(7):943–950. doi:https://doi.org/10.1038/eye.2015.73.
- Tomkins-Netzer O, Taylor SR, Bar A, et al. Treatment with repeat dexamethasone implants results in long-term disease control in eyes with noninfectious uveitis. Ophthalmology. 2014;121(8):1649–1654. doi:https://doi.org/10.1016/j.ophtha.2014.02.003.
- Boyer DS, Faber D, Gupta S, et al. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina. 2011;31(5):915–923. doi:https://doi.org/10.1097/IAE.0b013e318206d18c.
- Zheng A, Chin EK, Almeida DR, Tsang SH, Mahajan VB. Combined vitrectomy and intravitreal dexamethasone (Ozurdex) sustained-release implant. Retina. 2016;36(11):2087–2092. doi:https://doi.org/10.1097/IAE.0000000000001063.
- Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature working G. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140:509–516.
- Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92(4):467–471. doi:https://doi.org/10.1016/S0161-6420(85)34001-0.
- Thorne JE, Sugar EA, Holbrook JT, et al. Periocular triamcinolone vs. Intravitreal triamcinolone vs. Intravitreal dexamethasone implant for the treatment of uveitic macular edema: the PeriOcular vs. INTravitreal corticosteroids for uveitic macular edema (POINT) trial. Ophthalmology. 2019;126(2):283–295. doi:https://doi.org/10.1016/j.ophtha.2018.08.021.
- Sugar EA, Jabs DA, Altaweel MM, et al. Identifying a clinically meaningful threshold for change in uveitic macular edema evaluated by optical coherence tomography. Am J Ophthalmol. 2011;152(6):1044–1052 e1045. doi:https://doi.org/10.1016/j.ajo.2011.05.028.
- Mateo C, Alkabes M, Bures-Jelstrup A, et al. Scleral fixation of dexamethasone intravitreal implant (OZURDEX) in a case of angle-supported lens implantation. Int Ophthalmol. 2014;34(3):661–665. doi:https://doi.org/10.1007/s10792-013-9841-4.
- Uwaydat SH, Wang H, Sallam AB. Intraoperative injection of intravitreal dexamethasone implant using a vitrectomy tracer-assisted technique. Retina. 2019;39(1):S123–S124. doi:https://doi.org/10.1097/IAE.0000000000001853.
- Kaplan EL, Meier P. Nonparametric-estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. doi:https://doi.org/10.1080/01621459.1958.10501452.
- Uwaydat SH, Sallam AB, Wang H, Goyal S. Retinal Indentation by a dexamethasone implant in a gas-filled eye: report of an unusual complication. JAMA Ophthalmol. 2017;135(10):1125–1127. doi:https://doi.org/10.1001/jamaophthalmol.2017.3276.
- Chang-Lin JE, Burke JA, Peng Q, et al. Pharmacokinetics of a sustained-release dexamethasone intravitreal implant in vitrectomized and nonvitrectomized eyes. Invest Ophthalmol Vis Sci. 2011;52(7):4605–4609. doi:https://doi.org/10.1167/iovs.10-6387.
- Zarranz-Ventura J, Carreno E, Johnston RL, et al. Multicenter study of intravitreal dexamethasone implant in noninfectious uveitis: indications, outcomes, and reinjection frequency. Am J Ophthalmol. 2014;158(6):1136–1145 e1135. doi:https://doi.org/10.1016/j.ajo.2014.09.003.
- Christensen L, Sanders R, Olson J. “Magic bullet”: eccentric macular hole as a complication from dexamethasone implant insertion. Case Rep Ophthalmol Med. 2016;2016:1706234.
- Panjaphongse R, Liu W, Pongsachareonnont P, Stewart JM. Kinematic study of ozurdex injection in balanced salt solution: modeling the behavior of an injectable drug delivery device in vitrectomized eyes. J Ocul Pharmacol Ther. 2015;31(3):174–178. doi:https://doi.org/10.1089/jop.2014.0134.
- Bansal L, Gupta A, Gupta V, et al. Safety and outcome of microincision vitreous surgery in uveitis. Ocul Immunol Inflamm. 2017;25(6):775–784. doi:https://doi.org/10.3109/09273948.2016.1165259.