ABSTRACT
Diabetic Retinopathy (DR) is an insidious neurovascular disorder secondary to chronic glycemic dysregulation in elderly diabetic patients. In the later stages of DR, the disease manifests as fluid infiltrating the macula, culminating in the leading cause of irreversible visual impairment in working age adults. With the current mainstay treatments preoccupied with slowing down the progression of DR, this presents an unsustainable solution from both an economic and quality of life perspective. Although the exact mechanisms by which hyperglycemia leads to retinal tissue insult are unknown, the evidence suggests that chronic low-grade inflammation in diabetic eye is in part driving the constellation of symptoms present in DR. Of the innate immune system within the eye, the NLR Family Pyrin Domain Containing 3 Inflammasome (NLRP3) has been identified in retinal cells as a causal factor in the pathogenesis of DR. Multiple pathways appear to be present in the diabetic eye that instigate prolonged activation of the NLRP3 which subsequently exerts its deleterious effects by upregulating the release of Interleukin-1Beta and Interleukin-18. In this review, we highlight the current understanding of the pathophysiology of DR, the dysregulation of the NLRP3 secondary to hyperglycemic stress in retinal cells, and novel therapeutic targets to alleviate overactivation of the inflammasome.
Background
Introduction
Secondary to a vast array of economic and cultural developments, certain health complications are becoming increasingly prevalent, namely diabetes. It is the complications from the prolonged hyperglycemia (HG) that leads to a deterioration in an individual’s quality of life. Currently, 2.6% of global blindness and the leading cause of blindness in working age adults can be directly attributed to Diabetic Retinopathy (DR).Citation1 DR is a neurovascular condition that can be subclassified based on the presence of certain anatomical hallmarks. The early stage of the disease is termed Non-Proliferative Diabetic Retinopathy (NPDR), characterized by the presence of intra-retinal hemorrhages, microaneurysms, and cotton wool spots from infarcted nerve tissue.Citation2,Citation3 NPDR has the potential to advance to a more severe stage of the disease called Proliferative Diabetic Retinopathy (PDR) which is distinguished by the presence of pathological retinal neovascularization.Citation4 The novel vessels often lack the conventional integrity and if left untreated, may lead to hemorrhages within the vitreous and precipitate tractional retinal detachment.Citation5,Citation6 At any point throughout the disease, an individual is also prone to developing Diabetic Macular Edema (DME) which is the leading cause of visual impairment from DR.Citation7 DME manifests itself as retinal thickening due to fluid accumulation in the macula – the most light sensitive area of the retina consisting of densely packed photoreceptors.Citation8 The built-up fluid within the neuronal tissue leads to the distortion and loss of high definition central vision.
Pathophysiology
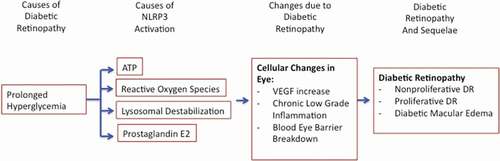
Although the pathophysiology of DR is not entirely known, the sequence of certain fundamental complications is known. Initially, due to sustained HG, the capillaries in the retina begin to deteriorate. The pericytes composing the capillary wall begin to drop out and leukocytes infiltrate, occlude, and attach to damaged vascular endothelial cells with the help of various adhesion molecules.Citation9,Citation10 This vascular degeneration induces hypoxia within the retina as a mismatch between the supply and demand of oxygen is now evident. To restore homeostasis in response to ischemic conditions, angiogenic growth factors are upregulated and secreted.Citation11,Citation12 Vascular Endothelial Growth Factor A (VEGF) in particular stimulates the formation of new vessels to supply the oxygen-deprived tissue. However, the ensuing vessels tend to have faulty tight junctions amongst endothelial cells and grow into crucially non-perfused regions of the retina.Citation3,Citation13 The complications of DR such as DME are instigated due to the hyperpermeable vessels and the subsequent breakdown of the blood-retina-barrier (BRB) resulting in fluid extravasation into the retina. With persistent hypoxia and edema, there is eventual apoptosis of retinal neurons and thus visual impairment. Of note, the visual complications from gross microvascular modifications tend to arise in the later stages of DR, despite subtle tissue insult from chronic HG being evident much earlier.Citation14 This highlights the potential for novel early biomarkers and emphasizes the necessity to understand the chronology of events if we wish to cure, rather than symptomatically address the condition.
Despite glycemic control being the initial treatment for DR as the severity of the condition is initially a function of the degree of HG, glucose management tends to be ineffective in the later stages as chronic HG eventually produces irreversible damage.Citation2,Citation11 The mainstay treatment for PDR and DME is anti-VEGF antibody injections into the vitreous chamber. Granted the antibodies are more effective than traditional laser retinal ablation therapy, the antibodies have their fair share of downfalls including a hefty price tag, a short half-life entailing frequent injections and consequential poor patient compliance.Citation15 Not to mention, the antibodies are not particularly effective for all patients. For DME patients, in the DRCR Protocol Study 1, approximately 40% still had persistent edema after 6 months of treatment.Citation2 In fact, considering that VEGF is required for homeostatic maintenance of retinal vessels, prolonged suppression may inadvertently reduce choroidal thickness.Citation16
For refractory cases of DME and PDR not responding to anti-VEGF antibodies, the previous gold standard, pan-retinal-photocoagulation is still employed sparsely. While the exact mechanism of action is unknown, it is thought that the lasers directly close microaneurysms, stimulate the retinal pigment epithelium (RPE), and ablation of peripheral neural tissue improves oxygenation of remaining tissue.Citation17,Citation18 Unfortunately, the potential side effects include reduced night vision capacity due to destruction of the peripheral retina – the primary site of rod photoreceptors.
Additional treatments for refractory NPDR and DME include intraocular injections of anti-inflammatory corticosteroids; however, as a limited second-line therapy given the risk profile from prolonged usage.Citation19 Risks include an increased incidence of cataracts, elevated intraocular pressure, vitreous hemorrhages, and glaucoma.Citation20,Citation21 It is interesting to note that the success of anti-inflammatory therapy suggests that an inflammatory process is at least partially propelling DR. Given the increasing prevalence of diabetes, rising healthcare costs, and therapeutic efforts aimed at symptomatic management of DR, the current strategy does not represent a sustainable nor effective long-term action plan.
Inflammation in the Eye
Chronic low-grade inflammation has been implicated to be an ongoing process throughout the various stages of DR. Of particular importance is the inflammasome, a multiprotein molecular platform that modulates the innate immune system by regulating the processing and secretion of pro-inflammatory cytokines.Citation22,Citation23 The inflammasome is assembled in response to a variety of pathogen- and danger-associated molecular patterns (PAMP and DAMP).Citation24,Citation25 NOD-like receptors (NLRs), which are intracellular microbial and danger sensing proteins,Citation26 have a central nucleotide-binding and oligomerization (NACHT) domain, an effector domain at their amino terminus, and multiple leucine-rich repeats (LRRs) domain at their carboxyl terminus.Citation27 Amino terminus domains include either acidic transactivation domain, baculoviral inhibitor repeat-like domain, caspase activation and recruitment (CARD) domain, or the pyrin domain.Citation28 Upon detection of a PAMP or DAMP, the LRRs auto-phosphorylate and recruit ASC adaptor protein which in return recruits procaspase 1. The complex of NACHT, LRRs, apoptosis speck-like protein (ASC), and Procaspase 1 is collectively known as the inflammasome.Citation27 The CARD domain of ASC cleaves procaspase 1 into caspase 1 which is then able to catalyze the cleavage of pro-Interleukin-1Beta and pro-Interleukin-18 into active Interleukin-1Beta (IL-1β) and Interleukin-18 (IL-18), respectively.Citation27 Of the various inflammasomes, the NLR Family Pyrin Domain Containing 3 Inflammasome (referred to as NLRP3) is the most abundantly studied in relation to pathological conditions of the eye. When discussing the activation of the NLRP3, it is in fact a two-step process. The initial extracellular priming signal induced by the toll-like receptor (TLR)/nuclear factor (NF)-kB pathway upregulates cellular transcription of the NLRP3 Inflammasome component proteins.Citation29,Citation30 The secondary activation signal – transduction of a PAMP or DAMP – promotes the assembly of the component proteins.Citation29,Citation31 Common secondary signals include changes in potassium ion flux, reactive oxygen species (ROS), lysosomal destabilization, and certain post-translational protein modifications.Citation22
Evidence exists for chronic inflammation and the upregulated activity of the NLRP3 contributing to the pathogenesis of diabetes.Citation2,Citation32–Citation36 For example, in type 1 diabetes, the pancreatic expression of NLRP3 dependent IL-1β contributes to the autoimmune destruction of beta cells.Citation32,Citation37 In type 2 diabetes, Lee et al. provide compelling evidence for the activation of the NLRP3 in the pathogenesis of DR. Macrophages derived from type 2 diabetics exhibited significantly increased expression of the NLRP3 at both the mRNA and protein level and unsurprisingly, an increased tendency to process downstream inflammatory cytokines IL-1β and IL-18 when exposed to DAMPs.Citation33 While IL-1β plays a similar role of pancreatic destruction in type 2 diabetics,Citation34 further evidence for inflammation is provided by the diabetes drug metformin alleviating both diabetic clinical findings and concurrently attenuating NLRP3 and IL-1β activity.Citation33
When considering DR in particular, Loukovaara et al. provide conclusive evidence of NLRP3 overactivity stemming from a study based on vitreous donations from NPDR and PDR patients. The authors demonstrate the presence of elevated inflammasome component proteins and their downstream inflammatory cytokines IL-1β and IL-18 in the vitreous of DR patients.Citation38 Interestingly, the measured levels of NLRP3 activity clearly correlated with disease severity. Patients with PDR exhibited significantly higher levels of NLRP3 proteins and IL-18 activity than patients with NPDR.Citation38 Within PDR, the presence of TRD exhibited similar results, as TRD patients tended to have higher levels of NLRP3 activity when compared to those with a fully intact retina.Citation38 In a study conducted by Chen et al., vitreous and fibrovascular membrane samples collected from NPDR and PDR patients feature congruent results at both the protein and mRNA level for the NLRP3 activity when compared to normal controls.Citation39 Similarly, the prognostic implications of NLRP3 activity are supported by Chen et alCitation39 who also demonstrate significantly higher expression of IL-1β and IL-18 in patients with PDR than those with NPDR. Moreover, in a study with PDR patients, Song et al. noted a correlation between IL-18 levels and PDR severity.Citation39 However, the study by Chen et al. presents conflicting results with Loukovaara et al. who suggest no difference in IL-1β between PDR and NPDR patients, only with IL-18. Considering the infancy of the research in NLRP3 activity and the presence of confounding variables such as diabetes type and temporal relations, more work is clearly required to elucidate accurate results. With the information at hand, it is possible that IL-18 provides a robust prognostic indicator. Loukovaara et al. suggest that IL-18 increases early on in the disease process and its measured levels correlate with disease severity as indicated by all three of Loukovaara et al., Chen et al., and Song et al.Citation38–Citation40
Although the exact role of a dysregulated NLRP3 in DR is not entirely known, current attention to this area has produced numerous promising studies. For instance, human retinal microvascular endothelial cells (HRMEC) exposed to HG demonstrate a statistically significant spike in both the protein and mRNA of the NLRP3.Citation36 Similar results are evident for the NLRP3’s direct downstream inflammatory cytokines IL-1β and IL-18 and secondary mediators such as VEGF and Tumor Necrosis Factor Alpha.Citation36 In order to model type 1 diabetes, mice are often injected with streptozotocin (STZ) to destroy pancreatic beta cells. In such models with simultaneous blockade of the NLRP3 via shRNA, the models tend to exhibit reduced vascular permeability, pro-apoptotic caspase 3, caspase 1, IL-1β, IL-18, and VEGF.Citation36 Within the retina, the NLRP3 appears to be active in the inner and outer nuclear layers, within the resident immune cells, microglia.Citation11,Citation41,Citation42 Muller cells, the primary retinal glial type, are particularly important considering retinal caspase 1 is primarily located within Muller inner cell processes.Citation43 With HG, retinal Muller cells exhibit a spike in caspase 1, processing of IL-1β and IL-18, and prolonged gliosis.Citation43,Citation44 While few studies exist exploring the effects of the NLRP3 activity on the RPE in DR patients, extrapolating related studies provides key insights. In a HG environment, RPE cells tend to display elevated NLRP3 and IL-1β activity.Citation45 The destruction of the RPE which represents the outer BRB may lead to various complications in the pathogenesis of DR. For instance, DR patient may experience vascular complications, edema, activation of compensatory angiogenic pathwaysCitation46 and eventual pyroptosis and apoptosis of the RPE cells as seen in studies evaluating NLRP3 activity in age-related macular degeneration.Citation47
Upstream Activators of the NLRP3
While the exact mechanism by which the NLRP3 becomes dysregulated in DR is not entirely known, it is proposed that prolonged HG increases the prevalence of prominent activating DAMPs, which are discussed below.
Adenosine Triphosphate
Adenosine triphosphate (ATP) is hypothesized to be working at both the NLRP3 priming and activating DAMP signals.Citation22,Citation48 Extracellular ATP stimulates P2X7 ATP-gated ion channels to allow an efflux of potassium ionsCitation49,Citation50 and thereby stimulating the NLRP3. In particular, the literature focuses on the role of Connexin hemichannels, of which Connexin43 is the most prominent isotype in humans and upregulates early on in DR due to ischemia.Citation51 Connexin hemichannels are six protein subunits which oligomerize to form a hexamer termed a Connexon or HemichannelCitation51 that may restrict the movement of anything larger than 1KdaCitation48; implying that ATP can freely pass through. The hemichannels are constitutively expressed in HRMECs, glial, RPE, and astrocytesCitation51 within the retina; all of which are essential in the maintenance of the BRB.Citation48 Under hypoxic or ischemic conditions, the hemichannels open to form membranous pores,Citation52,Citation53 which facilitates ATP outflow and thus an autocrine activation of the NLRP3 inflammasome.Citation48 The results from DR studies blocking the Connexin43 hemichannel by Peptide 5Citation48 or XiflamCitation54 indicate a reduction in NLRP3 Inflammasome activity (fewer retinal aneurysms, reduced gliosis), pro-inflammatory cytokines, and VEGF to basal levels.Citation48,Citation54 Interestingly, the Connexin43 hemichannels may be involved in a viscous positive feedback cycle. The presence of pro-inflammatory cytokines such as IL-1β processed by the NLRP3 trigger the opening,Citation48 increase the transcription, and availability of the hemichannels on the cell membrane.Citation48 As expected, this leads to an increase in available extracellular ATP for autocrine NLRP3 activation.
Reactive Oxygen Species
Reactive Oxygen Species (ROS) are a known DAMP which instigate the NLRP3.Citation45,Citation55,Citation56 In the diabetic retina, as the cells attempt to dispose of excess glucose, the upregulated mitochondrial activity is the prominent source of byproduct ROS.Citation57,Citation58 It is proposed that ROS indirectly prompt the NLRP3 through dysregulation of the anti-oxidant Thioredoxin (TRX) system.Citation59 Under normoxidative conditions, the anti-oxidant TRX is negatively regulated by Thioredoxin Interacting Protein (TXNIP) physically creating a disulfide bond at the redox domain of TRX.Citation60,Citation61 As such, the anti-oxidative interactions of TRX with other molecules are inhibited.Citation59,Citation60 However, with excessive oxidative stress, ROS eventually oxidize the disulfide bond, liberating TXNIP.Citation60,Citation61 Unlike bound TXNIP, free TXNIP is able to activate the NLRP3Citation29,Citation45,Citation56,Citation59,Citation62 by binding its LRRs.Citation23
For diabetics, this activation of the NLRP3 by TXNIP may be further compounded as indicated by HG upregulating TXNIP expression in Muller and endothelial cells via genomic modifications.Citation36,Citation44 For instance, in a HG environment, cells exhibit stimulatory histone acetylation at the histone activation marks H3K9AC at the TXNIP promoter region by P300 Histone Acetyltransferase.Citation63–Citation65 Likewise, HG in the form of glucose-6-phosphate enhances the translocation of carbohydrate response element-binding protein (ChREBP) into the nucleus which promotes the expression of the carbohydrate response element in the TXNIP promoter regionCitation59,Citation64 consequently enhancing TXNIP transcription which is then able to subsequently activate the NLRP3 in diabetics.
The physiology of ROS stimulated TXNIP activation of the NLRP3 hinges on time-dependent attempts by homeostatic processes to prevent HG-induced mitochondrial dysregulation.Citation36,Citation44 To dispose of the HG, there is an initial uptick in glucose processing by the cell resulting in increased production of ATP and ROS, which are swiftly neutralized by cellular antioxidants.Citation44 As antioxidants are overwhelmed by the persistent HG, ATP production drops as the cell switches to anaerobic glycolysis in an attempt to thwart ROS production, inadvertently generating a hypoxic environment.Citation44 Regardless of the cell’s homeostatic attempts, ROS continues to leak from mitochondria and the upregulated TXNIP due to persistent HG bind and inhibit the anti-oxidative capacity of TRX.Citation44 To preserve viability, the cell engages in autophagy and mitophagy to discard damaged organelles.Citation44,Citation66 However, fragmentation of damaged mitochondria contributes continued DAMPs such as additional ROS leakage and mitochondrial DNA.Citation62 While efficient autophagy and mitophagy are essential for homeostasis, in the diabetic retina, these cellular processes may be pathologically activated in part by the upregulated TXNIP. This is accomplished by TXNIP sustaining an environment with persistent oxidative stressCitation44 and culminating in inappropriate NLRP3 activity, pyroptosis, and thus progression of DR.
Lysosomal Destabilization
Lysosomal damage is a known activator of the NLRP3,Citation13,Citation67; possibly through the release of Cathepsin B into the cytoplasm following lysosomal rupture.Citation23 Lysosomal stress can be precipitated by numerous means such as declining autophagy efficiency,Citation68 irritants such as uric acid (UA),Citation69 and aging.Citation70 UA is the byproduct of purine metabolism and at serum levels above 356 (μmol/L), it forms into the NLRP3 detectable DAMP, monosodium urate (MSU) crystals.Citation69 According to Thounaojam et al., DR patients tend to have serum UA levels exceeding the nucleation threshold and postmortem DR vitreous samples indicate a significant positive correlation between UA levels and IL-1β activity.Citation69 To ascertain the implications of UA on the retina, Thounaojam et al. exposed STZ mice to allopurinol, an inhibitor of UA production, and benzbromarone, which enhances UA excretion.Citation69 In conjugation with a HG environment, the mice exposed to both hypouricemic drugs exhibited lower levels of IL-1β, VEGF, and NLRP3 activity. It is hypothesized that MSU crystals lead to NLRP3 activity by either directly causing lysosomal rupture or UA alone is a pro-inflammatory DAMP,Citation23,Citation69,Citation71,Citation72 possibly through the promotion of TXNIP dissociation from TRX in an ROS dependent manner. The preliminary results provide justification into exploring UA as a target, monitor, and early-stage prognostic biomarker in DR. Beyond UA, since age is the largest risk factor for DR, the deteriorating efficiency in general physiology may lead to lysosomal damage and successive NLRP3 activity. For example, the ability of the RPE to recycle photoreceptor tips declines with age and results in an accumulation of the lipid peroxidation byproduct lipofuscin, a lysosomal destabilizer resulting in enhanced NLRP3 activity.Citation70,Citation73
Prostaglandin E2
Prostaglandin E2 (PGE2) is a proinflammatory molecule that has been noted to be statistically elevated by up to 53% in the vitreous of DR patients.Citation72,Citation74,Citation75 Likewise, its parent enzyme cyclooxygenase 2 (COX2) and its target receptor EP2R follow suit.Citation75,Citation76 PGE2 is of importance because its inhibition has demonstrated an ability to suppress the progression of DR.Citation72 This is partly due to its possible role as an upstream NLRP3 activator.Citation75 Additionally, in the diabetic retina, PGE2 exhibits a positive correlation with VEGF and promotes leukocyte adhesion, neovascularization, impairment of retinal vessels, retinal edema, and apoptosis.Citation72,Citation74,Citation75,Citation77,Citation78 Upon application of PGE2 receptor antagonists, the effects of PGE2 are diminished possibly due to an inhibited association of the NLRP3 with ASC oligomers.Citation75
According to Wang et al., an understanding of how HG results in NLRP3 activation as mediated by PGE2 stems from its interaction with the EP2R.Citation75 Upon activation by PGE2, the EP2R coupled cAMP/PKA signaling results in PKA entering the cell nucleus and subsequent phosphorylation of the transcriptional factor cAMP response element-binding protein.Citation75 The genomic modifications are thought to upregulate transcription and translation of both the NLRP3 and pro-IL-1β. To compound the effects, prolonged HG is suggested to increase both the expression and activation of the COX2 enzyme, which may further provoke the PGE2/EP2R-cAMP/PKA pathway of NLRP3 activation.Citation75 In fact, EP2R is not the only receptor implicated in DR. Studies antagonizing the EP4 receptor demonstrate significantly reduced PGE2 induced VEGF expression in Muller cells.Citation77,Citation78
Downstream Mediators of NLRP3
Interleukin-18
The retinal consequences of the dysregulated NLRP3 are primarily mediated by the proinflammatory cytokines IL-1β and IL-18. The ramifications of IL-18 are not particularly unified throughout the literature. Authors such as Shen et al. detail potential protective effects from IL-18 which are described as anti-angiogenic and anti-permeabilityCitation79; in essence, offsetting VEGF. Their studies exhibit a positive correlation between baseline levels of intraocular IL-18 and visual outcomes with VEGF antibodies, elevated IL-18 upon anti-VEGF treatment, and injections of IL-18 dampening the effects of VEGF such as vascular leakage and neovascularization.Citation79 It is proposed that IL-18 preserves the BRB by preventing a reduction in the tight junction protein, Claudin 5; the opposite is typically expected from dysregulated VEGF.Citation79 Nonetheless, it is worthwhile to note the previous study was not conducted on DR patients. Conversely, other studies with in vivo retinal injections of IL-18 did not statistically change choroidal neovascularization (CNV) volumes in age-related macular degeneration models.Citation80 What’s more, IL-18 may possibly be pro-angiogenic, as genetically deficient mice in IL-18 or its receptor Interleukin-18 Receptor 1 display a reduction in CNV volumes.Citation80 As such, the conflicting evidence does not paint a homogenous picture on the effects of IL-18 and epitomizes the need for further exploration.
Interleukin-1Beta
In contrast, the effects of IL-1β are congruent with the expectations from the inflammatory NLRP3. IL-1β and its parent enzyme caspase 1 are both heavily concentrated within Muller glia in the retina and affect neighboring cells in a paracrine manner.Citation42,Citation43 Upon binding the specific Interleukin-1 Receptor Type 1 (IL-1R1), IL-1β is able to exert the deleterious constellation of effects expected in DR.Citation35 Specifically, IL-1β contributes to retinal vessel permeability,Citation62 upregulation of adhesion molecules promoting occlusion of capillaries by leukocytes,Citation2,Citation43 and apoptosis of endothelial cells.Citation2 What is more, the evidence suggests that HG-induced IL-1β participates in a positive feedback loop by increasing the presence of its parent enzyme Caspase 1 in an autocrine manner.Citation35 Lastly, IL-1β contributes to an increase in the presence of pro-apoptotic Semaphorin-3A and caspase 3, suggesting that HG-induced apoptosis may in part be driven by an inflammatory process.Citation35,Citation42
Disrupting the NLRP3 Inflammasome Activation
Within the diabetic retina, there exist five material targets to avert the induction of the NLRP3. These include 1: blocking cell membrane receptors ex. Connexin43 hemichannels, 2: controlling cytoplasmic secondary messengers ex. ROS, 3: halting transcription ex. ChREBP, 4: preventing conjugation of the NLRP3 component proteins, and lastly, 5: antagonizing the Interleukins or their respective receptors.Citation70,Citation81 Although preventing the activation of the NLRP3 seems like a plausible therapeutic for DR, it is cautionary to note that complete systemic inhibition would not be ideal. The NLRP3 is involved in a host of homeostatic processes such as the elimination of infectious agents.Citation29 An alternative approach may be to target the reduction of NLRP3 activity only in the vitreous chamber and for controlled periods of time. Throughout the literature, halting the NLRP3 in the diabetic retina distills into either direct upstream inhibitors or antioxidants to alleviate oxidative stress. What is more, a promising niche of gene therapy is also currently being explored which will not be discussed in depth in this paper. With recombinant adeno-associated viral vectors, researchers are able to target IL-1 directly, TXNIP, or the M103 gene, which prevents proteolytic processing of caspase 1.Citation82–84
Direct and Upstream Inhibitors
The small molecule, MCC950, is an NLRP3 inhibitor that prevents both canonical and non-canonical activation presumablyCitation22 by preventing the interaction of the NLRP3 with NEK7; a kinase NLRP3 activator that coordinates the oligomerization of the NLRP3 and ASC.Citation22,Citation85 For DR, HG exposure studies exhibit MCC950 reducing the levels of IL-1β, IL-18, and cellular apoptosis.Citation22,Citation86,Citation87 Besides DR, remarkably the MCC950 yields additional aid to diabetics in the form of dampened diabetic encephalopathy and attenuated hippocampal NLRP3 activity manifesting as reduced anxiety and depression.Citation88 Lastly, the MCC950 is structurally a sulfonylurea molecule, a traditional diabetes pharmaceutical employed to enhance insulin tolerance and secretion from pancreatic beta cells.Citation88,Citation89 This dual nature proposes the possibility of a uniquely engineered molecule that may inhibit both the NLRP3 and act as a potent insulin secretagogue. For instance, the conventional diabetes therapeutic, Glyburide, can also halt the induction of the NLRP3.Citation61
Additional upstream inhibitors include Compound 49B, a beta adrenergic receptor agonist which exerts its effects by reducing TLR4 signaling.Citation90 Specifically, it is proposed that beta adrenergic receptors signal through cAMP leading to a spike in exchange protein activated by cAMP 1 (EPAC1) which can subsequently regulate the levels of pro-inflammatory cytokines.Citation91 Jiang et al. propose that Compound 49B reduces TLR4 signaling which in turn signals via HMGB1 to initiate the NLRP3.Citation92 However, this is in stark contrast to both Kim et al. and Van Beijnum JR et al., who propose instead it is HMGB1 signaling via TLR4 to activate the NLRP3.Citation93,Citation94 Moreover, Resolvin D1 acts as an upstream mediator of the NLRP3 likely by preventing the initial transcription of the component proteins.Citation41 Lastly, Peptide 7, a Connexin43 hemichannel blockade, prevents HG and cytokine driven ATP efflux and thus, autocrine NLRP3 activation.Citation48
Antioxidants
An emerging theme in the inhibition of the NLRP3 is the use of antioxidants to reduce ROS. The average diabetic patient, especially with PDR, tends to be deficient in Vitamin D3,Citation95 a multifunctional hormone possessing potent anti-oxidative and anti-inflammatory capabilities.Citation96 The evidence implies Vitamin D3 dampens NLRP3 activity by reducing ROS and thus preventing the dissociation of TXNIP from TRX.Citation96 In the diabetic retina, Vitamin D3 is able to inhibit the elevation of TXNIP due to ROS at a level comparable to ROS scavengers, reduce VEGF secretion, and thwart the change in density and disorganization of the retina.Citation96 Additionally, the application of Vitamin D3 attenuates the apoptosis of various cells exposed to HG likely as byproduct of preventing IL-1β induced mitochondrial dysfunctionCitation96,Citation97 or possibly due to reduced ROS inflicted mitochondrial damage and subsequent homeostatic apoptosis.Citation44
Nuclear Erythroid Type 2 Factor (NRF2) is a transcriptional factor that binds the antioxidant response element to regulate the transcription of anti-oxidants such as HO1 and NQO1.Citation43,Citation98 The antioxidants subsequently reduce ROS and thus, NLRP3 activation.Citation98 For DR patients, there exist two molecules that mediate their effects by upregulating NRF2. Firstly, Sulforaphane (SFN), an isothiocyanate found in cruciferous vegetables demonstrates reduced retinal thickness and pro-inflammatory cytokines such IL-1β in a dose-dependent manner.Citation98 Similarly, the widely employed triglyceride lowering drug, Fenofibrate, attenuates the generation ROS via stimulating NRF2 signaling.Citation43 This peroxisome proliferator-activated receptor alpha agonist has affirmed its ability to delay the progression of DR in both the ACCORD and FIELD trials, reducing the need for PRP by up to 32% in diabetics.Citation99,Citation100
Other antioxidants include Methylene Blue, which reduces canonical NLRP3 activation with secondary effects of reducing glycated hemoglobin.Citation101 Methylene Blue is assumed to be either directly inhibiting TXNIP or preventing its association with the NLRP3.Citation101 Lastly, Minocycline, a tetracycline antibiotic, possesses similar anti-oxidative effects conceivably through the reduction of ROS induced TXNIP activation of the NLRP3.Citation36 Additional effects include a reduction in cellular apoptosis in HG environments,Citation35 dampened diabetic retinal microglia activation,Citation102 and halting of the transcription of NLRP3 proteins.Citation36 Nonetheless, an obvious impediment exists in that systemic application of a tetracycline antibiotic is not warranted as a long-term solution; rather, controlled doses within the vitreous may be of relevance to DR patients.
Conclusion
Diabetic Retinopathy is a neurovascular retinal disease which in it’s late stage is characterized by compromised visual quality secondary macular edema driven by chronic HG insult. With the accelerating global prevalence of diabetes, exploration into the complex pathophysiology of diabetic retinopathy is absolutely necessary in order to promote primary prevention and relieve the costly burden of chronic management. In this review, we present evidence for the dysregulation of the NLRP3 Inflammasome as a contributing factor to the constellation of tissue insults evident in the diabetic retina. As suggested by experimental studies, multiple concurrent instigators such as reactive oxygen species, adenosine triphosphate, lysosomal damage, and prostaglandins may provoke the dysregulated activation of the NLRP3 Inflammasome and it’s damaging pro-inflammatory cytokines IL-1β and IL-18. While this understanding presents various potential checkpoints to control inflammation, it also suggests that a more efficient approach is to focus on the common denominator with direct inhibition of the NLRP3 Inflammasome. Though direct inhibition of the NLRP3 Inflammasome is feasible, we are currently lacking substantial in vivo studies. We, therefore, advocate for a heightened focus in this particular area of inflammation and are hopeful that local inhibition of the NLRP3 Inflammasome may reduce the disease burden of Diabetic Retinopathy.
Abbreviations
| BRB | = | Blood-retina-barrier |
| ChREBP | = | Carbohydrate response element binding |
| CARDS | = | Caspase activation and recruitment domain |
| CNV | = | Choroidal neovascularization |
| COX2 | = | Cyclooxygenase 2 |
| DME | = | Diabetic Macular Edema |
| HRMEC | = | Human retinal microvascular endothelial cells |
| IL-1 | = | Interleukin-1 |
| IL-18 | = | Interleukin-18 |
| IL-1β | = | Interleukin-1Beta |
| IL-1R1 | = | Interleukin-1 Receptor Ty[e 1 |
| LRRs | = | Leucine rich repeats |
| MSU | = | Monosodium urate |
| NLRP3 | = | NLR Family Pyrin Domain Containing 3 Inflammasome |
| NLRs | = | Nod like receptors |
| NPDR | = | Non-Proliferative Diabetic Retinopathy |
| NACHT | = | Nucleotide binding and oligomerization domain |
| PDR | = | Proliferative Diabetic Retinopathy |
| PGE2 | = | Prostaglandin E2 |
| ROS | = | Reactive oxygen species |
| RPE | = | Retinal pigment epithelium |
| STZ | = | Streptozotocin |
| SFN | = | Sulforaphane |
| TRX | = | Thioredoxin |
| TXNIP | = | Thioredoxin Interacting Protein |
| VEGF | = | Vascular Endothelial Growth Factor A |
Author’s contributions
KSR sourced the referenced literature, analyzed the literature, and subsequently wrote the manuscript. JAM was a significant contributor in refining the topic of the review and in reviewing the drafts of the manuscript. All authors read and approved the final manuscript.
Availability of data and material
All data generated or analyzed in this review are publicly available and referenced within the text.
Declaration of interests
The authors declare that they have no competing interests.
Additional information
Funding
References
- Bourne RRA, Stevens GA, White RA, et al. Causes of vision loss worldwide, 1990-2010: a systematic analysis. Lancet Glob Health. 2013;1:e339–49. doi:https://doi.org/10.1016/S2214-109X(13)70113-X.
- Homme RP, Singh M, Majumder A, et al. Remodeling of retinal architecture in diabetic retinopathy: disruption of ocular physiology and visual functions by inflammatory gene products and pyroptosis. Front Physiol. 2018;9:1268. doi:https://doi.org/10.3389/fphys.2018.01268.
- Kusuhara S, Fukushima Y, Ogura S, Inoue N, Uemura A. Pathophysiology of diabetic retinopathy: the old and the new. Diabetes Metab J. 2018;42:364–376. doi:https://doi.org/10.4093/dmj.2018.0182.
- Sun Y, Smith LEH. Retinal vasculature in development and diseases. Annu Rev Vis Sci. 2018;4:101–122. doi:https://doi.org/10.1146/annurev-vision-091517-034018.
- Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–136. doi:https://doi.org/10.1016/S0140-6736(09)62124-3.
- Adamis AP, Aiello LP, D’Amato RA. Angiogenesis and ophthalmic disease. Angiogenesis. 1999;3:9–14. doi:https://doi.org/10.1023/a:1009071601454.
- Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. 2018;19. doi:https://doi.org/10.3390/ijms19061816.
- Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology. 2015;122:1375–1394. doi:https://doi.org/10.1016/j.ophtha.2015.03.024.
- Cogan DG, Toussaint D, Kuwabara T. Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol. 1961;66:366–378. doi:https://doi.org/10.1001/archopht.1961.00960010368014.
- Barouch FC, Miyamoto K, Allport JR, et al. Integrin-mediated neutrophil adhesion and retinal leukostasis in diabetes. Invest Ophthalmol Vis Sci. 2000;41:1153–1158. https://www.ncbi.nlm.nih.gov/pubmed/10752954.
- Chaurasia SS, Lim RR, Parikh BH, et al. The NLRP3 inflammasome may contribute to pathologic neovascularization in the advanced stages of diabetic retinopathy. Sci Rep. 2018;8:2847. doi:https://doi.org/10.1038/s41598-018-21198-z.
- Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17:611–625. doi:https://doi.org/10.1038/nrm.2016.87.
- Kinnunen K, Piippo N, Loukovaara S, Hytti M, Kaarniranta K, Kauppinen A. Lysosomal destabilization activates the NLRP3 inflammasome in human umbilical vein endothelial cells (JHUVECs) cell commun. Signal. 2017;11:275–279. doi:https://doi.org/10.1007/s12079-017-0396-4.
- Hu L, Yang H, Ai M, Jiang S. Inhibition of TLR4 alleviates the inflammation and apoptosis of retinal ganglion cells in high glucose. Graefes Arch Clin Exp Ophthalmol. 2017;255:2199–2210. doi:https://doi.org/10.1007/s00417-017-3772-0.
- Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–1203. doi:https://doi.org/10.1056/NEJMoa1414264.
- Grunwald JE, Daniel E, Huang J, et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121:150–161. doi:https://doi.org/10.1016/j.ophtha.2013.08.015.
- Ogata N, Tombran-Tink J, Jo N, Mrazek D, Matsumura M. Upregulation of pigment epithelium-derived factor after laser photocoagulation. Am J Ophthalmol. 2001;132:427–429. doi:https://doi.org/10.1016/s0002-9394(01)01021-2.
- Arnarsson A, Stefánsson E. Laser treatment and the mechanism of edema reduction in branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 2000;41:877–879. https://www.ncbi.nlm.nih.gov/pubmed/10711707.
- Wong TY, Sun J, Kawasaki R, et al. Guidelines on diabetic eye care: the international council of ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology. 2018;125:1608–1622. doi:https://doi.org/10.1016/j.ophtha.2018.04.007.
- Campochiaro PA, Brown DM, Pearson A, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118:626–635.e2. doi:https://doi.org/10.1016/j.ophtha.2010.12.028.
- Diabetic Retinopathy Clinical Research Network, Elman MJ, Aiello LP, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–1077.e35. doi:https://doi.org/10.1016/j.ophtha.2010.02.031.
- Yang Y, Wang H, Kouadir M, Song H, Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10:128. doi:https://doi.org/10.1038/s41419-019-1413-8.
- Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. doi:https://doi.org/10.1038/nri2725.
- Maslanik T, Mahaffey L, Tannura K, Beninson L, Greenwood BN, Fleshner M. The inflammasome and danger associated molecular patterns (DAMPs) are implicated in cytokine and chemokine responses following stressor exposure. Brain Behav Immun. 2013;28:54–62. doi:https://doi.org/10.1016/j.bbi.2012.10.014.
- Savage CD, Lopez-Castejon G, Denes A, Brough D. NLRP3-inflammasome activating DAMPs stimulate an inflammatory response in Glia in the absence of priming which contributes to brain inflammation after injury. Front Immunol. 2012;3:288. doi:https://doi.org/10.3389/fimmu.2012.00288.
- Davis BK, Wen H, Ting JP-Y. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–735. doi:https://doi.org/10.1146/annurev-immunol-031210-101405.
- Yerramothu P, Vijay AK, Willcox MDP. Inflammasomes, the eye and anti-inflammasome therapy. Eye. 2018;32:491–505. doi:https://doi.org/10.1038/eye.2017.241.
- Kim YK, Shin JS, Nahm MH. NOD-like receptors in infection, immunity, and diseases. Yonsei Med J. 2016;57:5–14. doi:https://doi.org/10.3349/ymj.2016.57.1.5.
- Sepehri Z, Kiani Z, Afshari M, Kohan F, Dalvand A, Ghavami S. Inflammasomes and type 2 diabetes: an updated systematic review. Immunol Lett. 2017;192:97–103. doi:https://doi.org/10.1016/j.imlet.2017.10.010.
- Hornung V, Latz E. Critical functions of priming and lysosomal damage for NLRP3 activation. Eur J Immunol. 2010;40:620–623. doi:https://doi.org/10.1002/eji.200940185.
- Jo E-K, Kim JK, Shin D-M, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Immunol. 2016;13:148–159.
- Mandrup-Poulsen T. The role of interleukin-1 in the pathogenesis of IDDM. Diabetologia. 1996;39:1005–1029. https://link.springer.com/article/10.1007/BF00400649.
- Lee H-M, Kim -J-J, Kim HJ, Shong M, Ku BJ, Jo E-K. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62:194–204. doi:https://doi.org/10.2337/db12-0420.
- Maedler K, Sergeev P, Ris F, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–860. doi:https://doi.org/10.1172/JCI15318.
- Vincent JA, Mohr S. Inhibition of caspase-1/interleukin-1beta signaling prevents degeneration of retinal capillaries in diabetes and galactosemia. Diabetes. 2007;56:224–230. doi:https://doi.org/10.2337/db06-0427.
- Chen W, Zhao M, Zhao S, et al. Activation of the TXNIP/NLRP3 inflammasome pathway contributes to inflammation in diabetic retinopathy: a novel inhibitory effect of minocycline. Inflamm Res. 2017;66:157–166. doi:https://doi.org/10.1007/s00011-016-1002-6.
- Lebreton F, Berishvili E, Parnaud G, et al. NLRP3 inflammasome is expressed and regulated in human islets. Cell Death Dis. 2018;9:726. doi:https://doi.org/10.1038/s41419-018-0764-x.
- Loukovaara S, Piippo N, Kinnunen K, Hytti M, Kaarniranta K, Kauppinen A. NLRP3 inflammasome activation is associated with proliferative diabetic retinopathy. Acta Ophthalmol. 2017;95:803–808. doi:https://doi.org/10.1111/aos.13427.
- Chen H, Zhang X, Liao N, et al. Enhanced expression of NLRP3 inflammasome-related inflammation in diabetic retinopathy. Science. 2018;59:978–985. doi:https://doi.org/10.1167/iovs.17-22816.
- Song Z, Sun M, Zhou F, Huang F, Qu J, Chen D. Increased intravitreous interleukin-18 correlated to vascular endothelial growth factor in patients with active proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2014;252:1229–1234. doi:https://doi.org/10.1007/s00417-014-2586-6.
- Yin Y, Chen F, Wang W, Wang H, Zhang X. Resolvin D1 inhibits inflammatory response in STZ-induced diabetic retinopathy rats: possible involvement of NLRP3 inflammasome and NF-κB signaling pathway. Mol Vis. 2017;23:242–250. https://www.ncbi.nlm.nih.gov/pubmed/28465656.
- Rivera JC, Sitaras N, Noueihed B, et al. Microglia and interleukin-1β in ischemic retinopathy elicit microvascular degeneration through neuronal semaphorin-3A. Arterioscler Thromb Vasc Biol. 2013;33:1881–1891. doi:https://doi.org/10.1161/ATVBAHA.113.301331.
- Liu Q, Zhang F, Zhang X, et al. Fenofibrate ameliorates diabetic retinopathy by modulating Nrf2 signaling and NLRP3 inflammasome activation. Mol Cell Biochem. 2018;445:105–115. doi:https://doi.org/10.1007/s11010-017-3256-x.
- Devi TS, Lee I, Hüttemann M, Kumar A, Nantwi KD, Singh LP. TXNIP links innate host defense mechanisms to oxidative stress and inflammation in retinal Muller glia under chronic hyperglycemia: implications for diabetic retinopathy. Exp Diabetes Res. 2012;2012:438238. doi:https://doi.org/10.1155/2012/438238.
- Shi H, Zhang Z, Wang X, et al. Inhibition of autophagy induces IL-1β release from ARPE-19 cells via ROS mediated NLRP3 inflammasome activation under high glucose stress. Biochem Biophys Res Commun. 2015;463:1071–1076. doi:https://doi.org/10.1016/j.bbrc.2015.06.060.
- Liu J, Copland DA, Theodoropoulou S, et al. Impairing autophagy in retinal pigment epithelium leads to inflammasome activation and enhanced macrophage-mediated angiogenesis. Sci Rep. 2016;6:20639. doi:https://doi.org/10.1038/srep20639.
- Gao J, Cui JZ, To E, Cao S, Matsubara JA. Evidence for the activation of pyroptotic and apoptotic pathways in RPE cells associated with NLRP3 inflammasome in the rodent eye. J Neuroinflammation. 2018;15:15. doi:https://doi.org/10.1186/s12974-018-1062-3.
- Mugisho OO, Green CR, Kho DT, et al. The inflammasome pathway is amplified and perpetuated in an autocrine manner through connexin43 hemichannel mediated ATP release. Biochim Biophys Acta Gen Subj. 2018;1862:385–393. doi:https://doi.org/10.1016/j.bbagen.2017.11.015.
- Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi:https://doi.org/10.1016/j.immuni.2013.05.016.
- Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–420. doi:https://doi.org/10.1038/nri.2016.58.
- Mugisho OO, Green CR, Zhang J, Acosta ML, Rupenthal ID. Connexin43 hemichannels: a potential drug target for the treatment of diabetic retinopathy. Drug Discov Today. 2019;24:1627–1636. doi:https://doi.org/10.1016/j.drudis.2019.01.011.
- Contreras JE, Sáez JC, Bukauskas FF, Bennett MVL. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci USA. 2003;100:11388–11393. doi:https://doi.org/10.1073/pnas.1434298100.
- Giaume C, Leybaert L, Naus CC, Sáez JC. Connexin and pannexin hemichannels in brain glial cells: properties, pharmacology, and roles. Front Pharmacol. 2013;4. doi:https://doi.org/10.3389/fphar.2013.00088.
- Green CR, Nor MNM, Mugisho OO, Rupenthal ID, Squirrell DM, Acosta ML. Connexin hemichannel block shuts down inflammation in an animal model of chronic diabetic retinopathy to improve structural and functional outcomes. Invest Ophthalmol Vis Sci. 2019;60:2784.
- Wang S, Ji L-Y, Li L, Li J-M. Oxidative stress, autophagy and pyroptosis in the neovascularization of oxygen-induced retinopathy in mice. Mol Med Rep. 2019;19:927–934. doi:https://doi.org/10.3892/mmr.2018.9759.
- Chen Y, Wang L, Pitzer AL, Li X, P-L L, Zhang Y. Contribution of redox-dependent activation of endothelial NLRP3 inflammasomes to hyperglycemia-induced endothelial dysfunction. J Mol Med. 2016;94:1335–1347. doi:https://doi.org/10.1007/s00109-016-1481-5.
- Calderon GD, Juarez OH, Hernandez GE, Punzo SM, De la Cruz ZD. Oxidative stress and diabetic retinopathy: development and treatment. Eye. 2017;31:1122–1130. doi:https://doi.org/10.1038/eye.2017.64.
- Nakahira K, Haspel JA, Rathinam VAK, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi:https://doi.org/10.1038/ni.1980.
- Kumar A, Mittal R. Mapping Txnip: key connexions in progression of diabetic nephropathy. Pharmacol Rep. 2018;70:614–622. doi:https://doi.org/10.1016/j.pharep.2017.12.008.
- Hwang J, Suh H-W, Jeon YH, et al. The structural basis for the negative regulation of thioredoxin by thioredoxin-interacting protein. Nat Commun. 2014;5:2958. doi:https://doi.org/10.1038/ncomms3958.
- Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi:https://doi.org/10.1038/ni.1831.
- Singh LP, Devi TS, Yumnamcha T. The role of txnip in mitophagy dysregulation and inflammasome activation in diabetic retinopathy: a new perspective. JOJ Ophthalmol. 2017;4. doi:https://doi.org/10.19080/jojo.2017.04.555643.
- Perrone L, Devi TS, Hosoya K-I, Terasaki T, Singh LP. Inhibition of TXNIP expression in vivo blocks early pathologies of diabetic retinopathy. Cell Death Dis. 2010;1:e65. doi:https://doi.org/10.1038/cddis.2010.42.
- Shalev A. Minireview: thioredoxin-interacting protein: regulation and function in the pancreatic β-celll. Mo Endocrinol. 2014;28:1211–1220. doi:https://doi.org/10.1210/me.2014-1095.
- El-Osta A, Brasacchio D, Yao D, et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205:2409–2417. doi:https://doi.org/10.1084/jem.20081188.
- Devi TS, Somayajulu M, Kowluru RA, Singh LP. TXNIP regulates mitophagy in retinal Müller cells under high-glucose conditions: implications for diabetic retinopathy. Cell Death Dis. 2017;8:e2777. doi:https://doi.org/10.1038/cddis.2017.190.
- Okada M, Matsuzawa A, Yoshimura A, Ichijo H. The lysosome rupture-activated TAK1-JNK pathway regulates NLRP3 inflammasome activation. J Biol Chem. 2014;289:32926–32936. doi:https://doi.org/10.1074/jbc.M114.579961.
- Piippo N, Korkmaz A, Hytti M, et al. Decline in cellular clearance systems induces inflammasome signaling in human ARPE-19 cells. Biochim Biophys Acta. 2014;1843:3038–3046. doi:https://doi.org/10.1016/j.bbamcr.2014.09.015.
- Thounaojam MC, Montemari A, Powell FL, et al. Monosodium urate contributes to retinal inflammation and progression of diabetic retinopathy. Diabetes. 2019;68:1014–1025. doi:https://doi.org/10.2337/db18-0912.
- Gao J, Liu RT, Cao S, et al. NLRP3 inflammasome: activation and regulation in age-related macular degeneration. Mediators Inflamm. 2015;2015:690243. doi:https://doi.org/10.1155/2015/690243.
- Kono H, Chen C-J, Ontiveros F, Rock KL. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J Clin Invest. 2010;120:1939–1949. doi:https://doi.org/10.1172/JCI40124.
- Schoenberger SD, Kim SJ, Sheng J, Rezaei KA, Lalezary M, Cherney E. Increased prostaglandin E2 (PGE2) levels in proliferative diabetic retinopathy, and correlation with VEGF and inflammatory cytokines. Inv Ophthalmol Visual Sci. 2012;53:5906–5911. doi:https://doi.org/10.1167/iovs.12-10410.
- Schütt F, Bergmann M, Holz FG, Kopitz J. Isolation of intact lysosomes from human RPE cells and effects of A2-E on the integrity of the lysosomal and other cellular membranes. Graefes Arch Clin Exp Ophthalmol. 2002;240:983–988.
- Wang Y, Tao J, Yao Y. Prostaglandin E2 activates NLRP3 inflammasome in endothelial cells to promote diabetic retinopathy. Horm Metab Res. 2018;50:704–710. doi:https://doi.org/10.1055/a-0664-0699.
- Wang M, Wang Y, Xie T, et al. Prostaglandin E2/EP2 receptor signalling pathway promotes diabetic retinopathy in a rat model of diabetes. Diabetologia. 2019;62:335–348. doi:https://doi.org/10.1007/s00125-018-4755-3.
- Johnson EI, Dunlop ME, Larkins RG. Increased vasodilatory prostaglandin production in the diabetic rat retinal vasculature. Curr Eye Res. 1999;18:79–82. doi:https://doi.org/10.1076/ceyr.18.2.79.5386.
- Yanni SE, Barnett JM, Clark ML, Penn JS. The role of PGE2 receptor EP4 in pathologic ocular angiogenesis. Invest Ophthalmol Vis Sci. 2009;50:5479–5486. doi:https://doi.org/10.1167/iovs.09-3652.
- Cheng T, Cao W, Wen R, Steinberg RH, LaVail MM. Prostaglandin E2 induces vascular endothelial growth factor and basic fibroblast growth factor mRNA expression in cultured rat Müller cells. Invest Ophthalmol Vis Sci. 1998;39:581–591. https://www.ncbi.nlm.nih.gov/pubmed/9501870.
- Shen J, Choy DF, Yoshida T, et al. Interleukin-18 has antipermeablity and antiangiogenic activities in the eye: reciprocal suppression with VEGF. J Cell Physiol. 2014;229:974–983. doi:https://doi.org/10.1002/jcp.24575.
- Hirano Y, Yasuma T, Mizutani T, et al. IL-18 is not therapeutic for neovascular age-related macular degeneration. Nat Med. 2014;20:1372–1375. doi:https://doi.org/10.1038/nm.3671.
- Di Virgilio F. The therapeutic potential of modifying inflammasomes and NOD-like receptors. Pharmacol Rev. 2013;65:872–905. doi:https://doi.org/10.1124/pr.112.006171.
- Ildefonso CJ, Jaime H, Rahman MM, et al. Gene delivery of a viral anti-inflammatory protein to combat ocular inflammation. Hum Gene Ther. 2015;26:59–68.
- Fabiani C, Sota J, Tosi GM, et al. The emerging role of interleukin (IL)-1 in the pathogenesis and treatment of inflammatory and degenerative eye diseases. Clin Rheumatol. 2017;36:2307–2318. doi:https://doi.org/10.1007/s10067-016-3527-z.
- Johnston JB, Barrett JW, Nazarian SH, et al. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity. 2005;23:587–598. doi:https://doi.org/10.1016/j.immuni.2005.10.003.
- Shi H, Wang Y, Li X, et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol. 2016;17:250–258. doi:https://doi.org/10.1038/ni.3333.
- Zhang Y, Lv X, Hu Z, et al. Protection of Mcc950 against high-glucose-induced human retinal endothelial cell dysfunction. Cell Death Dis. 2017;8:e2941. doi:https://doi.org/10.1038/cddis.2017.308.
- Coll RC, Robertson AAB, Chae JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–255. doi:https://doi.org/10.1038/nm.3806.
- Zhai Y, Meng X, Ye T, Xie W, Sun G, Sun X. Inhibiting the NLRP3 inflammasome activation with MCC950 ameliorates diabetic encephalopathy in db/db mice. Molecules. 2018;23. doi:https://doi.org/10.3390/molecules23030522.
- Hill JR, Coll RC, Sue N, et al. Sulfonylureas as concomitant insulin secretagogues and NLRP3 inflammasome inhibitors. ChemMedChem. 2017;12:1449–1457. doi:https://doi.org/10.1002/cmdc.201700270.
- Berger EA, Carion TW, Jiang Y, et al. β-adrenergic receptor agonist, compound 49b, inhibits TLR4 signaling pathway in diabetic retina. Immunol Cell Biol. 2016;94:656–661. doi:https://doi.org/10.1038/icb.2016.21.
- Jiang Y, Liu L, Steinle JJ. Compound 49b regulates ZO-1 and occludin levels in human retinal endothelial cells and in mouse retinal vasculature. Invest Ophthalmol Vis Sci. 2017;58:185–189. doi:https://doi.org/10.1167/iovs.16-20412.
- Jiang Y, Liu L, Curtiss E, Steinle JJ. Epac1 blocks NLRP3 inflammasome to reduce IL-1β in retinal endothelial cells and mouse retinal vasculature. Mediators Inflamm. 2017;2017:2860956. doi:https://doi.org/10.1155/2017/2860956.
- Kim EJ, Park SY, Baek SE, et al. HMGB1 increases IL-1β production in vascular smooth muscle cells via NLRP3 inflammasome. Front Physiol. 2018;9:313. doi:https://doi.org/10.3389/fphys.2018.00313.
- van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis. 2008;11:91–99. doi:https://doi.org/10.1007/s10456-008-9093-5.
- Payne JF, Ray R, Watson DG, et al. Vitamin D insufficiency in diabetic retinopathy. Endocr Pract. 2012;18:185–193. doi:https://doi.org/10.4158/EP11147.OR.
- Lu L, Lu Q, Chen W, Li J, Li C, Zheng Z. Vitamin D3 protects against diabetic retinopathy by inhibiting high-glucose-induced activation of the ROS/TXNIP/NLRP3 inflammasome pathway. J Diabetes Res. 2018;2018:8193523. doi:https://doi.org/10.1155/2018/8193523.
- Kowluru RA, Mohammad G, Santos JM, Tewari S, Zhong Q. Interleukin-1β and mitochondria damage, and the development of diabetic retinopathy. J Ocul Biol Dis Infor. 2011;4:3–9.
- Li S, Yang H, Chen X. Protective effects of sulforaphane on diabetic retinopathy: activation of the NRF2 pathway and inhibition of NLRP3 inflammasome formation. Exp Anim. 2019;68:221–231. doi:https://doi.org/10.1538/expanim.18-0146.
- Keech AC, Mitchell P, Summanen PA, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687–1697. doi:https://doi.org/10.1016/S0140-6736(07)61607-9.
- ACCORD Study Group, ACCORD Eye Study Group, Chew EY, Ambrosius WT, Davis MD, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244. doi:https://doi.org/10.1056/NEJMoa1001288.
- Hao J, Zhang H, Yu J, Chen X, Yang L. Methylene blue attenuates diabetic retinopathy by inhibiting NLRP3 inflammasome activation in STZ-induced diabetic rats. Ocul Immunol Inflamm. 2019;27:836–843. doi:https://doi.org/10.1080/09273948.2018.1450516.
- Krady JK, Basu A, Allen CM, et al. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005;54:1559–1565. doi:https://doi.org/10.2337/diabetes.54.5.1559.