ABSTRACT
Background
To investigate the possible associations between childhood noninfectious uveitis and cardio-respiratory fitness, physical activity, health related quality of life and fatigue.
Methods
Cross-sectional analysis of 23 patients with noninfectious uveitis, aged 8–18 years. BMI, exercise capacity, muscle strength and physical activity were measured. Health-related quality of life and fatigue were assessed. The results were compared to standardized values for age matched healthy children.
Results
Twenty-three patients were included. Children with uveitis had a higher bodyweight and body mass index. Children with uveitis had lower cardio-respiratory fitness and they were less physically active, but they experienced a normal quality of life and normal fatigue. Parents of children with uveitis reported a lower quality of life and more fatigue for their children than parents of healthy children.
Conclusion
Our study indicates that children with noninfectious uveitis are at risk of developing lower physical and psychosocial health.
Background
Uveitis is an inflammatory disorder of the eye, involving the uveal tract. In the western world the prevalence of pediatric uveitis is 30/100,000 and children account for 5–10% of the total uveitis population.Citation1 Uveitis may be caused by an infection, may be associated with a systemic auto-immune disease or may occur as an isolated auto-immune reaction without a known underlying cause.Citation2 In the developed countries, 87– 89% of the pediatric uveitis cases are noninfectious and the majority (41.5%) is related to juvenile idiopathic arthritis (JIA).1,Citation3
Patients with auto-immune diseases are more physically inactive compared to the general population.Citation4 Also, aerobic fitness in children with different types of chronic conditions is reduced and they report more fatigue.Citation5–7 In juvenile idiopathic arthritis (JIA), children are also found to be less physically active and have reduced physical fitness levelsCitation8 which does not restore after remission has been reached.Citation9,Citation10 The causes of these persistent impairments of physical fitness and physical activity are not known, but it has been suggested that a combination of disease-related factors, treatment (e.g., medication) and deconditioning could be involved.Citation5,Citation11,Citation12
The pathophysiology of noninfectious uveitis has not exactly been revealed.Citation13 It is not clear whether the inflammation in uveitis is really limited to the eye or may extend itself systemically.Citation14–17 A number of biomarkers have been identified in JIA-uveitisCitation18–20 and in auto-immune uveitis.Citation13 Also, in both idiopathic and JIA-uveitis a number of genetic predispositions have been found.Citation13,Citation21
Systemic treatment in children with idiopathic uveitis who do not respond sufficiently to topical therapy is comparable to that used in JIA. The first line of treatment in pediatric uveitis are local corticosteroids. If local corticosteroids are insufficient, a switch toward steroid sparing immunosuppressive therapy will be made in most cases. Systemic prednisone is started in case of severe uveitis and is given peri-operatively in case of intraocular surgery.
Because systemic inflammation can contribute to atherosclerosis,Citation22–24 there is concern that children with inflammatory disease are at higher risk for cardiovascular diseases. In addition to the inflammation itself, systemic corticosteroids have a negative impact on the cardiovascular risk profile. Well-known side effects are increased bodyweight, hypertension, and accelerated atherosclerosis.Citation25
In the literature, information on the physical and psychosocial health of children with uveitis is scarce.Citation26–28 A recent study on the quality of life (QoL) in children with JIA showed that children with uveitis had poorer vision-related QoL and function when compared to those without uveitis.Citation29 Also, in adolescents with noninfectious uveitis despite quiescence of disease and good visual function, certain factors, such as a high number of recurrences, chronicity of the uveitis and fear of blindness were correlated with a decreased health-related (HR)-QoL.Citation30,Citation31
In our clinical experience, fatigue is often reported by children with uveitis or by their parents. In adults, fatigue has been shown to be a barrier for being physically active.Citation32 Fatigue is highly present in patients with JIA and is related to many factors including physical activity, physical fitness and HR-QoL of which cause and effect are not exactly known.Citation33 Therefore, uveitis may have a large impact on a child’s life and can alter their QoL.Citation26,Citation27,Citation30,Citation34
To optimize treatment for children with uveitis it is of great importance to get insight in risk factors that have a negative impact on physical and psychosocial health. Regarding the possible negative effects of uveitis, we therefore studied levels of cardio-respiratory fitness, physical activity, muscle strength, HR-QoL and fatigue in pediatric noninfectious uveitis patients.
Patients and methods
The Medical Ethical Committee of the University Medical Center of Groningen (UMCG) approved the study. Patients were included from the departments of pediatric rheumatology and ophthalmology of the UMCG (the Netherlands) from July till December 2014. Patients aged 8–18 years, known with idiopathic or JIA-associated uveitis were eligible for this study. Patients with infectious uveitis were not included. Patients with co-morbidities, not related to the uveitis, that could influence the outcome of the exercise test, like pulmonary or cardiac diseases, were excluded from the study. All investigations were carried out on the same day following the regular visit. Informed consent was obtained from the parents and from the child if the child was ≥ 12 years old.
Patient characteristics
Information regarding patient characteristics (gender, age), disease characteristics (location of the uveitis, etiology, time since diagnosis, disease status), current treatment (medication, dose, route of administration), complications, and surgery was retrospectively gathered by consulting the medical charts of the patients. Median duration of active disease was recorded. Active disease was defined as observed cells in the anterior chamber or in the vitreous.Citation35 The diagnosis of posterior and panuveitis was made by fundoscopy and in some cases fluorescein angiography (FA) was performed.
Disease control was defined as an observable inactive disease in the affected eye for longer than 3 months without the use of systemic corticosteroids or local steroid injections (subtenon or subconjunctival).Citation35 During this period, local steroid medication such as eye drops or ointment were allowed in a maintenance dosage of maximum of 4 times a day.
Patients were examined by an ophthalmologist to determine the activity of the uveitis. The visual acuity was measured with a Snellen chart and was converted to LogMAR-acuity for calculation and statistical purposes.Citation35,Citation36 Visual field examination to assess the amount of glaucomatous damage, due to increased intraocular pressure, was only performed if the age of the child permitted perimetry. Unfortunately, because of the young age of our patients, measurement of optic disc cupping-changes and perimetry were not routinely performed. Blindness was defined as a visual acuity less than 0.01 (or LogMAR > 1.3) or a visual field ≤ 10º.Citation37 Visual impairment was defined as a visual acuity ≥ 0.05 (LogMAR ≤ 1.30) and < 0.3 (LogMAR > 0.50).Citation37
Disease activity of JIA was scored on a 0–10 Physician Global Assessment (PGA) scale by a pediatric rheumatologist. Height and bodyweight were measured and body mass index (BMI = bodyweight(kg)/heightCitation2 (m)) was calculated. These measurements were compared with the reference values of Dutch children.Citation38 Overweight was defined as ≥ 1SD above the mean reference BMI and obesity as >2SD above the mean reference BMI.Citation39
Physical fitness
Physical fitness was assessed by measuring exercise capacity and muscle strength. Exercise capacity was measured with a cardiopulmonary exercise test using an electronically braked cycle ergometer, and was expressed by peak oxygen consumption (VO2peak) and peak work rate (Wpeak). We used a ramp version of the Godfrey protocolCitation40 in which the work rate increased gradually over time with 10, 15 or 20 Watt/min depending on the body height of the patient, as described by Bongers et al.Citation41 All patients were verbally encouraged to cycle until exhaustion. Maximal exertion was defined as a heart rate of > 180 beats per minute and a respiratory exchange ratio of more than 1.0.Citation41 The absolute values obtained during the test were compared with the reference values of healthy Dutch children.Citation41 VO2peak - and Wpeak per kg bodyweight were calculated and these relative values were also compared with the reference values.Citation41
General muscle function was assessed by manual muscle testing using the scale of the Medical Research Council (MRC). This scale ranges from 0 till 5, in which 0 means no muscle contraction and 5 means normal muscle power.Citation42 Isometric muscle strength of four muscle groups was assessed bilaterally by hand-held dynamometry (HHD): the biceps, triceps, iliopsoas, and quadriceps muscles. The assessed values were converted to a total z-score of the four muscle groups and compared with the reference values of healthy children.Citation43
Physical activity
Physical activity (PA) was subsequently measured by an accelerometer (Actical, Philips respironics). The accelerometer was given on the day of the regular visit and research measurements. The Actical measures accelerations in any plane of movement which are translated into activity counts as a reflection of physical activity. Counts were summed in 1-minute periods. Cutoff points were used to categorize activities as sedentary, light physical activity (LPA), and moderate-to-vigorous physical activity (MVPA).Citation44 Patients were instructed to wear the accelerometer during 7 days, for all hours except during sleep and wet-activities (showering, swimming). Patients were also asked to record their physical activities in a diary during the same 7 days as they were wearing the accelerometer. In the diary, patients scored their dominant activity of each 15 minute period of every 24 hours of the day The parents were allowed to help the child with filling out the diary.Citation45 Patients were asked to register in the diary at which moment they put the accelerometer on and off. Because non-wearing time of the accelerometer can be mistakenly categorized into sedentary activity, we corrected non-wearing time with the information provided in the activity diary. Patients were included in the analysis if they had minimally 4 valid days of wearing the accelerometer. A valid day was defined as a wearing time of minimally 8 hours on a weekday or minimally 6 hours on a weekend day. Measurements were validated if there was concordance between the measurements of the accelerometer and diary. Mean daily counts were determined by the sum of the total daily counts divided by the number of valid days. The mean amount of time spent in the four different categories of physical activity per day was compared to the values of healthy Canadian youth.Citation46
Functional ability
Functional ability was assessed by using the Child Health Assessment Questionnaire (CHAQ38). Functional ability was expressed in the disability index (DI) which was calculated as the mean of the maximum scores of all domains. A higher score suggests more disability (range 0– 3). The DI of the patients was compared to the DI of healthy Dutch children.Citation47,Citation48
Health related quality of life
Health related quality of life (HR-QoL) was evaluated with the Pediatric Quality of Life Inventatory (PedsQL 4.0). The PedsQL measures HR-QoL in four domains: physical, emotional, social and school functioning.Citation49 The questionnaire consists of a child self-report and a parent proxy report part and was completed by the child and the parent. A higher score (range 0–100) represents a higher quality of life. The scores of the patients were compared to the scores of healthy children.Citation49,Citation50
Fatigue
The level of fatigue in the patients was measured by the PedsQL Multidimensional Fatigue Scale, which measured fatigue in three domains: general fatigue, sleep/rest fatigue and cognitive fatigue.Citation50 The questionnaire consists of a child self-report and a parent proxy report part and was completed by the child and the parent. A higher score (range 0–100) indicates less fatigue. The scores of the patients were compared to the scores of healthy children.Citation49,Citation51
Statistical analysis
Statistical analyses were performed by using SPSS software (version 22). Descriptive statistics were used to present mean and standard deviation (SD) or median and range if data were abnormally distributed. The variables of the children were compared to the reference values of healthy children. Z-scores were calculated for age and gender dependent outcome measures as length, weight, BMI, peak oxygen consumption, peak work rate, and muscle strength. A z-score represents the amount of standard deviations the value differs from the age and gender specific reference value. A z-score above 0 means that the value measured in the study group is higher than in the reference group. A z-score below 0 is the other way around. The one sample t-test was used to compare the normally distributed outcomes of the patients with healthy controls, in case of abnormal distribution of the outcome parameters the one-sample Wilcoxon Signed Rank Test was used. To examine the possible relations between the outcome measurements, we analyzed which measurements were correlated to VO2peak, muscle strength, and quality of life. In all analyses a P < .05 was considered statistically significant.
Results
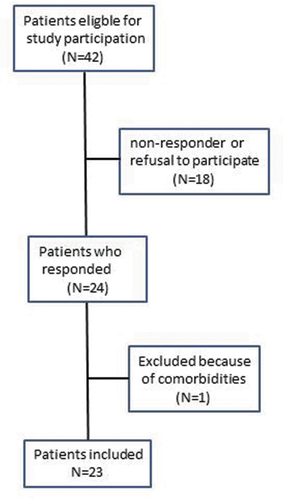
Forty-two patients were eligible for the study, 24 of whom (57.1%) were willing to participate (). One patient was excluded because of pulmonary comorbidities. Thirteen patients (56.5%) had idiopathic uveitis and the other 10 patients (43.5%) had JIA-associated uveitis. Patients with JIA had no clinically important systemic disease activity at the time of study participation (median PGA 0.0, range 0.0– 0.5).
At the time of study participation 20 patients (87.0%) had disease control on medication with regard to their uveitis. Three patients, 2 with JIA-uveitis and 1 with idiopathic uveitis, had mild uveitis activity. Eighteen patients (78.6%) used eye drops, 8 of these used steroid eye drops, one patient used anti-glaucoma medication and 9 patients used a combination of steroid eye drops and anti-glaucoma medication () . Fourteen (60.9%) patients used systemic medication, 7 of whom used methotrexate (MTX), 6 were treated with a combination of adalimumab and MTX and 1 patient used 5 mg systemic corticosteroids daily next to MTX (). The majority (n = 20) of the patients were treated with systemic corticosteroids in the past. On average, these patients were treated with > 0.5 mg/kg systemic corticosteroids for 16.3 (range 0– 77) weeks. At the moment of investigation high dose corticosteroids (> 0.5/mg/kg) were ceased for a median of 243.3 (range 0– 464) weeks.
Table 1. Patient characteristics and outcome measurements compared to reference values.
Table 2. Ocular features.
In total 8 patients developed visual loss. Three patients experienced visual loss due to the uveitis, two of whom (8.7%) had unilateral visual impairment and one (4.3%) unilateral blindness. Visual field loss due to glaucomatous optic nerve damage caused by elevated intraocular pressure was found in 7 patients, 2 of whom had already lost visual acuity. Because of the complications, 9 patients (39.1% of total study population) had undergone surgery; seven of whom (30.4% of total study population) had needed re-surgery ().
Mean weight and body mass index of the patients were statistically significantly higher when compared to the reference population (, ). Nine patients had higher BMI than the reference population, three of whom (13.0%) were obese and six were (26.1%) overweight. Patients with JIA-associated uveitis had a significantly higher BMI z-score than patients with idiopathic uveitis (z-score 1.26 vs 0.22, p =.02).
Figure 2. Z-scores. The z-score represents the amount of standard deviations the value differs from the age and gender specific reference value. Values are presented as mean with 95% confidence interval.
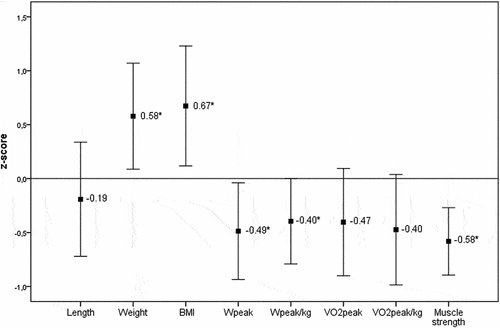
At the cardiopulmonary exercise test, patients reached a mean peak heart rate (HRmax) of 191 (±11) beats per minute. At maximal exertion, four patients did not reach a heart rate of > 180 beats per minute, but all patients reached a respiratory exchange ratio of more than 1.0, meaning that the exercise is intense because carbon dioxide (CO2) production by the working muscles becomes greater and more of the inhaled oxygen (O2)gets used rather than being expelled. Median VO2peak was comparable to VO2peak of healthy children. Mean VO2peak per kilogram bodyweight, median Wpeak, and mean Wpeak per kilogram bodyweight were all significantly lower than the reference value of healthy children (p < .05) (, ).
All patients had a normal general muscle power (MRC-scale 5). However, in comparison to healthy children of the same age maximal isometric muscle strength was significantly reduced in patients (p <.01).Citation43 There was no difference in physical fitness (VO2peak, Wpeak, and muscle strength) between patients with JIA-associated uveitis and patients with idiopathic uveitis.
Measurement of physical activity by the accelerometer was valid in 21 children (91.3%). Patients were physically active during 182 (light) and 36 minutes (moderate-to-vigorously) per day, respectively. This is significantly lower than in healthy Canadian children (p < .001) ().Citation30 There was no difference in the amount of moderate-to-vigorous physical activity (MVPA) between patients with JIA-associated uveitis and patients with idiopathic uveitis.
Parents indicated that their children had a lower quality of life compared to a reference group of parents of healthy children. Children themselves reported an equal HR-QoL compared to their healthy peers(). Children with uveitis did not experience more fatigue than healthy children, but their parents judged their children were more fatigued compared to parents of healthy children. Patient and parent scores on HR-QoL and fatigue did not differ between patients with JIA-associated and idiopathic uveitis.
The correlation-coefficient between VO2peak and Wpeak was 0.94 (P = < 0.001), VO2peak was therefore used and interpreted as a measure for exercise capacity (). Muscle strength (HDD) was correlated with higher VO2peak. Older age and higher BMI were correlated with higher muscle strength. Higher BMI was not correlated with previous corticosteroid use. Higher child reported HR-QoL was correlated with higher muscle strength and less fatigue. Higher disability was correlated with lower HR-QoL. Longer duration of active disease was correlated with lower HR-QoL reported by the parents about their child. Less fatigue was associated with a higher HR-QoL reported by the parents about their children. None of the outcome parameters correlated with the visual loss found in 8 patients.
Table 3. Correlations.
Discussion
Patients with uveitis have higher BMI compared to healthy children, they are at risk for reduced physical fitness levels as indicated by a lower aerobic exercise capacity and reduced muscle strength when compared to the healthy pediatric population. Also, children with uveitis are less physically active (PA), and their parents report a lower quality of life (HR-QoL) and more fatigue for their children when compared to parents of healthy children. In contrast, the children themselves report a normal HR-QoL and fatigue. The children with JIA-uveitis have a statistically significantly higher BMI than the children with idiopathic uveitis. No differences are found between JIA and idiopathic uveitis patients in physical fitness levels.
We found a significantly higher percentage of overweight (26%) and obese (14%) patients compared to the Dutch population, 13–15% and 2.2%, respectively.Citation38 In patients with JIA-uveitis BMI was significantly higher compared to non JIA uveitis. Corticosteroids are a well-known cause of weight gain,Citation12 however in our study only one patient used low dose (5 mg) systemic corticosteroids and most of the patients had not used systemic steroids for a median of 243.3 (range 0– 464) weeks . Also, no correlation was found between previous systemic corticosteroids and higher BMI. In JIA, contradictory results concerning obesity have been found and the cause has not been revealed yet.Citation53,Citation54 A possible explanation is a more sedentary lifestyle which we also found in this study. There are indications that obesity in JIA can result in higher inflammatory markers and an increased risk of atherosclerosis.Citation11,Citation12,Citation52 It is reasonable to assume that this risk is comparable in patients with uveitis, so healthcare professionals and carers should be aware of weight gain in patients with uveitis.
Children with uveitis have lower aerobic exercise capacity levels than their healthy peers, but relatively well preserved levels when compared to children with other chronic conditions,Citation5,Citation6 such as children with end stage renal disease, spina bifida, achondroplasia, acute lymphoblastic leukemia, osteogenesis imperfecta, cystic fibrosis and cerebral palsy.Citation5 When comparing the exercise capacity found in our study to the reported exercise capacity of children with JIA without uveitis in the literature,Citation5 the children in our study perform relatively well.
Interestingly, we found no differences in aerobic exercise capacity between JIA and idiopathic uveitis patients. The arthritis of the ten patients with JIA uveitis was in remission. It is known that the aerobic exercise capacity in patients with JIA does not restore after remission has been reached.Citation9 We assume that comparable underlying mechanisms could play a causative role in uveitis but their nature has not yet been revealed . The general assumption is that reduced levels of aerobic fitness are caused by a combination of disease-related pathophysiology, treatment (e.g., medication), hypo-activity, and deconditioning.Citation5,Citation11,Citation12
Patients with uveitis have decreased muscle strength that is possibly caused by the same combination of mechanisms that are responsible for the reduced exercise capacity.
From the literature it is known that low exercise capacity, decreased muscle strength, the inflammation itself, circulating cytokines and the use of systemic corticosteroids are correlated with an increased risk of cardiovascular diseases.Citation11,Citation12,Citation55,Citation56 In children with uveitis, these factors are present. Therefore, physicians should be alert and try to eliminate extra cardiovascular risk factors.
Our patients report 32 minutes of moderate-to-vigorous physical activity (MVPA) per day which is considerably less than the 60 minutes of daily MVPA as recommended by the WHO and the MVPA of the reference group.Citation46,Citation57 Similar results have been found for adolescents with JIA.Citation8,Citation9 Hypoactive children are often at greater risk of preventable health problems, such as obesity and cardio-metabolic diseases.Citation5,Citation52 Cardiovascular health in children can be improved by sufficient physical activity (PA) and physical fitness,Citation58 whereas PA also has a beneficial effect on HR-QoL.Citation4 In several auto-immune diseases, PA has been shown to be safe, to improve HR-QoL and to reduce fatigue.Citation4
The parents of our patients score a lower quality of life and higher levels of fatigue for their children than parents of healthy children, whereas the children themselves report outcomes comparable to those of their healthy peers on both questionnaires. This difference is probably due the proxy-problem, a known variation in patient and parent-report.Citation59 In the measurement of quality of life, parents tend to score a lower quality of life for their chronically ill children than the children themselves. This is possibly due to the differences in adaptation to a chronic disease in child and parent. Parents are possibly more aware of the health risks and have a broader perspective than children.Citation59,Citation60 Also, it is likely that the parent- reported HR-QoL and fatigue are influenced by their frequent visits to the hospital and their efforts associated with the medical treatment of their child.
The positive correlations in our study between exercise capacity, muscle strength and BMI are not supported in the literature.Citation61 Also, the reported loss of HR-QoLCitation49,Citation62 and increase in fatigue in children with overweight is not found in our results. We cannot explain these findings. Perhaps the significantly lower PA combined with adaptation in coping strategies by the children are responsible for these contradictory results. We did not investigate body composition, so we cannot comment on the influence of differences between muscle and fat mass on measured BMI in relation the muscle strength and exercise capacity. The negative correlations between lower HR-QoL (reported by children) and loss of functional ability and between lower HR-QoL (reported by parents) and longer disease duration are in line with the literature.Citation48–51,Citation63,Citation64
Limitations of the study
We performed this study as a pilot with a small number of patients. Next to that, the study-design is cross sectional, data was collected retrospectively and most patients had a long disease duration and had disease control for a relatively long time. Patients in other phases of the disease may have different results. A prospective case-control study with a group of healthy children from the same region as controls would have improved the power and interpretation of the results. Also, there is an unknown selection bias, because - for unknown reasons - not all eligible patients participated. Furthermore, visual field examination was not possible in all patients. Therefore, we could not investigate nor comment on the likely impact of visual field loss on the level of activity, functioning and HR-QoL. Measurements of physical activity by the accelerometer where verified by filling in a diary which is subject to interpretation and therefore a possible cause of bias.Citation65
Conclusion
This pilot-study investigated the physical and psychosocial consequences of uveitis in childhood. We showed that patients with noninfectious uveitis are at risk of developing cardiovascular risk factors early in life. Children with uveitis have a higher BMI, lower cardio-respiratory fitness and are less physically active when compared to healthy peers. Furthermore, their parents report a lower quality of life and more fatigue for their children compared to the parents of healthy children. To optimize the treatment for children with uveitis, treatment should be aimed at improving the physical and psychosocial health and reducing cardiovascular risk factors in addition to maintaining and preserving vision. Therefore, clinicians should discuss the importance of sufficient levels of physical fitness and PA with patients and their parents during outpatient visits. Also, close monitoring of body weight should be performed and the prevention of overweight should be a treatment goal.
Authors contributions
WW and RB designed the study, recruited the patients, performed patients testing, performed statistical analysis and wrote the manuscript. LL designed the study and was major contributor in writing the manuscript. OL designed the study, performed patients testing and was major contributor in writing the manuscript. WA designed the study, performed patients testing and was major contributor in writing the manuscript. All authors have read the manuscript, have approved the paper and agree to it being submitted for publication. All authors meet the Uniform Requirements for Manuscripts Submitted to Biomedical Journals criteria for authorship.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Consent for publication
Written consent was collected from parents and patients to collect data including permission to publish the results.
Disclosure of potential conflicts of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The Medical Ethical Committee of the University Medical Center of Groningen (UMCG) approved the conduction of the study. Consent to participate was obtained from the parents and from the child if the child was ≥ 12 years old.
References
- Päivönsalo-Hietanen T, Tuominen J, Saari KM. Uveitis in Children: population-based Study in Finland. Acta Ophthalmol Scand. 2000;78(1):84–88. doi:10.1034/j.1600-0420.2000.078001084.x.
- Zierhut M, Doycheva D, Biester S, Stübiger N, Kümmerle-Deschner J, Deuter C. Therapy of uveitis in Children. Int Ophthalmol Clin. 2008;48(3):131–152. doi:10.1097/IIO.0b013e31817d7107.
- Mehta PJ, Alexander JL, Sen HN. Pediatric uveitis: new and future treatments. Curr Opin Ophthalmol. 2013;24(5):453–462. doi:10.1097/ICU.0b013e3283641ede.
- Sharif K, Watad A, Bragazzi NL, Lichtbroun M, Amital H, Shoenfeld Y. Physical activity and autoimmune diseases: get moving and manage the disease. Autoimmun Rev. 2018;17:53–72.
- Takken T, Bongers BC, van Brussel M, Haapala EA, Hulzebos EHJ. Cardiopulmonary exercise testing in pediatrics. Ann Am Thorac Soc. 2017;14(Supplement 1):S123–S128. doi:10.1513/AnnalsATS.201611-912FR.
- van Brussel M, van der Net J, Hulzebos E, Helders PJ, Takken T. The Utrecht approach to exercise in chronic childhood conditions: the decade in review. Pediatr Phys Ther. 2011;23(1):2–14. doi:10.1097/PEP.0b013e318208cb22.
- Gualano B, Bonfa E, Pereira RMR, Silva CA. Physical activity for paediatric rheumatic diseases: standing up against old paradigms. Nat Rev Rheumatol. 2017;13(6):368–379. doi:10.1038/nrrheum.2017.75.
- Lelieveld OT, Armbrust W, van Leeuwen M a, et al. Physical activity in adolescents with Juvenile Idiopathic Arthritis. Arthritis Rheum. 2008;59(10):1379–1384. doi:10.1002/art.24102.
- van Brussel M, Lelieveld OTHM, van der Net J, Engelbert RHH, Helders PJM, Takken TT. Aerobic and anaerobic exercise capacity in children with juvenile idiopathic arthritis. Arthritis Rheum. 2007;57(6):891–897. doi:10.1002/art.22893.
- Ploeger HE, Takken T, Wilk B, et al. Exercise capacity in pediatric patients with inflammatory bowel disease. J Pediatr. 2011;158(5):814–819. doi:10.1016/j.jpeds.2010.10.020.
- Roubenoff R. Exercise and inflammatory disease. Arthritis Care Res (Hoboken). 2003;49(2):263. doi:10.1002/art.11008.
- Gupta Y, Gupta A. Glucocorticoid-induced myopathy: pathophysiology, diagnosis, and treatment. Indian J Endocrinol Metab. 2013;17(5):913–916. doi:10.4103/2230-8210.117215.
- Angeles-Han ST, Rabinovich CE. Uveitis in children. Curr Opin Rheumatol. 2016 Sep;28(5):544–549. doi:10.1097/BOR.0000000000000316.
- Lee RW, Nicholson LB, Sen HN, et al. Autoimmune and auto-inflammatory mechanisms in Uveitis. Semin Immunopathol. 2014;36(5):581–594. doi:10.1007/s00281-014-0433-9.
- Dana MR, Merayo-Lloves J, Schaumberg DA, Foster CS. Visual outcomes prognosticators in juvenile rheumatoid arthritis-associated uveitis. Ophthalmology. 1997;104(2):236–244. doi:10.1016/S0161-6420(97)30329-7.
- Kalinina Ayuso V, Makhotkina N, van Tent-Hoeve M, et al. Pathogenesis of juvenile idiopathic arthritis associated uveitis: the known and unknown. Surv.Ophthalmol 2014;59(5):517–531. doi:10.1016/j.survophthal.2014.03.002.
- Haasnoot AM, Kuiper JJ, Hiddingh S, et al. Ocular fluid analysis in children reveals Interleukin-29/Interferon-lambda1 as a biomarker for Juvenile Idiopathic Arthritis-Associated Uveitis. Arthritis Rheumatol. 2016;68(7):1769–1779. doi:10.1002/art.39621.
- Haasnoot AJ, van Tent-Hoeve M, Wulffraat NM, et al. Erythrocyte sedimentation rate as baseline predictor for the development of uveitis in children with juvenile idiopathic arthritis. Am J Ophthalmol. 2015 Feb;159(2):372–377. doi:10.1016/j.ajo.2014.11.007.
- Pelegrin L, Casaroli-Marano R, Anton J, et al. Predictive value of selected biomarkers, polymorphisms, and clinical features for oligoarticular juvenile idiopathic arthritis-associated uveitis. Ocul Immunol Inflamm. 2014;22(3):208–212. doi:10.3109/09273948.2013.841495.
- Walscheid K, Heiligenhaus A, Holzinger D, et al. Elevated S100A8/A9 and S100A12 serum levels reflect intraocular inflammation in Juvenile Idiopathic Arthritis-Associated Uveitis: results from a pilot study. Invest Ophthalmol Vis Sci. 2015 Dec;56(13):7653–7656. doi:10.1167/iovs.15-17066.
- Haasnoot AJW, Schilham MW, Kamphuis S, et al. Identification of an Amino Acid Motif in HLA-DRβ1 that distinguishes uveitis in patients with Juvenile Idiopathic Arthritis. Arthritis Rheumatol. 2018 Jul;707:1155–1165. doi:10.1002/art.40484.
- Coulson EJ, Ng W-F, Goff I, Foster HE. Cardiovascular risk in Juvenile Idiopathic Arthritis. Rheumatology. 2013;52(7):1163–1171. doi:10.1093/rheumatology/ket106.
- Barsalou J, Bradley TJ, Silverman ED. Cardiovascular risk in pediatric-onset rheumatological diseases. Arthritis Res Ther. 2013;15:212. doi:10.1186/ar4212.
- Libby P. Role of Inflammation in Atherosclerosis associated with Rheumatoid Arthritis. Am J Med. 2008;121(10 Suppl 1):S21–S31. doi:10.1016/j.amjmed.2008.06.014.
- Gedalia A, Shetty AK. Chronic steroid and immunosuppressant therapy in Children. Pediatr Rev. 2004;25(12):425–434. doi:10.1542/pir.25-12-425.
- Angeles-Han S, Griffin K, Lehman T, et al. The importance of visual function in the quality of life of children with uveitis. J Am Assoc Pediatr Ophthalmol Strabismus. 2010;12(2):163–168. doi:10.1016/j.jaapos.2009.12.160.
- Angeles-Han ST. Quality-of-life metrics in pediatric uveitis. Int Ophthalmol Clin. 2015;55(2):93–101. doi:10.1097/IIO.0000000000000067.
- Angeles-Han ST, Griffin KW, Harrison MJ, et al. Development of a vision-related quality of life instrument for children ages 8-18 years for use in juvenile idiopathic arthritis-associated uveitis. Arthritis Care Res. 2011; Hoboken 63:(9):1254–1261. doi:10.1002/acr.20524.
- Angeles-Han ST, McCracken C, Yeh S, et al. Characteristics of a cohort of children with Juvenile Idiopathic Arthritis and JIA-associated Uveitis. Pediatr Rheumatol Online J. Jun 2015;2(13):19.
- Maca SM, Amirian A, Prause C, Gruber K, Mejdoubi L, Barisani-Asenbauer T. Understanding the impact of Uveitis on health-related quality of life in adolescents. Acta Ophthalmol. 2013;91(3):219–224. doi:10.1111/aos.12016.
- Tan P, Koh YT, Wong PY, Teoh SC. Evaluation of the Impact of Uveitis on visual-related quality of life. Ocul Immunol Inflamm. 2012;20(6):453–459. doi:10.3109/09273948.2012.723781.
- Reichert FF, Barros AJD, Domingues MR, Hallal PC. The role of perceived personal barriers to engagement in leisure-time physical activity. Am J Public Health. 2007;97(3):515–519. doi:10.2105/AJPH.2005.070144.
- Armbrust W, Lelieveld OH, Tuinstra J, et al. Fatigue in patients with Juvenile Idiopathic Arthritis: relationship to perceived health, physical health, self-efficacy, and participation. Pediatr Rheumatol Online J. 2016 Dec 6;14(1):65. doi:10.1186/s12969-016-0125-1.
- Sen ES, Morgan MJ, MacLeod R, et al. Cross sectional, qualitative thematic analysis of patient perspectives of disease impact in juvenile idiopathic arthritis-associated uveitis. Pediatr Rheumatol Online J. 2017;15(1):58. Accessed 2017 Aug 4. doi:10.1186/s12969-017-0189-6.
- Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. Sep 2005;140(3):509–516.
- Holladay JT. Proper method for calculating average visual acuity. J Refract Surg. 1997 Jul-Aug;13(4):388–391. doi:10.3928/1081-597X-19970701-16.
- World Health Organization. Universal eye health: a global action plan 2014–2019. [Internet].p 7–8. Accessed 2018 July 8th. http://www.who.int/blindness/AP2014_19_English.pdf?ua=1
- TNO. Vijfde Landelijke Groeistudie: Groeidiagrammen [Internet]. 2010. Accessed 2018 july 8th Oct 20]. https://www.tno.nl/nl/aandachtsgebieden/gezond-leven/prevention-work-health/lang-gezond-en-actief-leven/pdf-groeidiagrammen/
- Cole TJ, Lobstein T. Extended International (IOTF) Body Mass Index Cut-offs for Thinness, Overweight and Obesity. Pediatr Obes. 2012;7(4):284–294. doi:10.1111/j.2047-6310.2012.00064.x.
- Godfrey S. Methods of measuring the response to exercise in children. In: Godfrey S. editor, Exercise Testing in Children: Applications in Health and Disease. London: W.B. Saunders Company Ltd; 1974:12–41.
- Bongers BC, Hulzebos EHJ, van Brussel M, Takken T. Pediatric norms for cardiopulmonary exercise testing: in relation to gender and age. Second revised edition. ’s-Hertogenbosch Uitg BOXPRESS. 2014:129.
- Compston A. From the archive: aids to the investigation of peripheral nerve injuries: Medical Research Council 1942. Brain. 2010 Sep 29;133(10):2838–2844. doi:10.1093/brain/awq270.
- Beenakker E a, van der Hoeven JH, Fock JM, Maurits NM. Reference values of maximum isometric muscle force obtained in 270 children aged 4-16 years by hand-held dynamometry. Neuromuscul Disord. 2001 July;11(5):441–643. Schiffman RM, Jacobsen G, Whitcup SM.Visual functioning and general health status in patients with uveitis. Arch Ophthalmol. 2001 Jun;119(6):841-9.
- Puyau MR, Adolph AL, Vohra FA, Zakeri I, Butte NF. Prediction of activity energy expenditure using accelerometers in Children. Med Sci Sports Exerc. 2004;36:1625–1631.
- Bratteby L, Sandhagen B, Fan H, Samuelson G. A 7-day activity diary for assessment of daily energy expenditure validated by the doubly labelled water method in adolescents. Eur J Clin Nutr. 1997;51:585–591. doi:10.1038/sj.ejcn.1600449.
- Colley RC, Garriguet D, Janssen I, Craig CL, Clarke J, Tremblay MS. Physical activity of Canadian Children and Youth: accelerometer results from the 2007 to 2009 Canadian Health Measures survey. Heal Rep. 2011;22(1):1–9.Accessed 2018 july 8th. http://www.statcan.gc.ca/pub/82-003-x/2011001/article/11396-eng.htm
- Wulffraat N, van der Net JJ, Ruperto N, et al. The Dutch version of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ). Clin Exp Rheumatol. 2001;19(Suppl 23):S111–S115.
- Ouwerkerk JW, van Pelt PA, Takken T, Helders PJ, Net J. Evaluating score distributions in the revised Dutch version of the Childhood Health Assessment Questionnaire. Pediatr Rheumatol Online J. 2008 Sep;11(6):14. doi:10.1186/1546-0096-6-14.
- Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQLTM 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3(6):329–341. doi:10.1367/1539-4409(2003)003<0329:TPAAPP>2.0.CO;2.
- Varni JW, Burwinkle TM, Szer IS. The PedsQL TM multidimensional fatigue scale in pediatric rheumatology: reliability and validity. J Rheumatol. 2004;31:2494–2500.
- Gordijn M, Cremers EM, Kaspers GJ, Gemke RJ. Fatigue in children: reliability and validity of the Dutch PedsQL™ Multidimensional Fatigue Scale. Qual Life Res. 2011 Sep;20(7):1103–1844. Hoeksema L, Los LI. Vision-related quality of life in herpetic anterior uveitis patients. PLoS One. 2014 Jan 2; 9(1).
- Zoico E, Roubenoff R. The role of cytokines in regulating protein metabolism and muscle function. Nutr Rev. 2002;60(2):39–51. doi:10.1301/00296640260085949.
- Pelajo CF, Lopez-Benitez JM, Miller LC. Obesity and disease activity in juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2012;10:3. doi:10.1186/1546-0096-10-3.
- Schenck S1, Niewerth M, Sengler C, et al. Prevalence of overweight in children and adolescents with juvenile idiopathic arthritis. Scand J Rheumatol. 2015;44(4):288–295. doi:10.3109/03009742.2014.999351.
- Carnethon M, Gidding S, Nehgme R, Sidney S, Jacobs D, Liu K. Cardiorespiratory fitness in young adulthood and the development of cardiovascular diseases risk factors. JAMA. 2003;290(23):3092–3100. doi:10.1001/jama.290.23.3092.
- Steene-Johannessen J, Anderssen S a, Kolle E, Andersen LB. Low muscle fitness is associated with metabolic risk in youth. Med Sci Sports Exerc. 2009 Jul;41(7):1361–1367. doi:10.1249/MSS.0b013e31819aaae5.
- WorldHealthOrganization. Global recommendations on physical activity for health. 2010; Accessed 2018 july 8th]. http://www.who.int/dietphysicalactivity/publications/9789241599979/en/
- Strong WB, Malina RM, Blimkie CJR, et al. Evidence based physical activity for school-age youth. J Pediatr. 2005;146(6):732–737. doi:10.1016/j.jpeds.2005.01.055.
- Sattoe JNT, van Staa A, Moll HA. The proxy problem anatomized: child-parent disagreement in health related quality of life reports of chronically ill adolescents. Health Qual Life Outcomes. 2012;10(1):10. doi:10.1186/1477-7525-10-10.
- Jardine J, Glinianaia SV, McConachie H, Embleton ND, Rankin J. Self-reported quality of life of young children with conditions from early infancy: a systematic review. Pediatrics. 2014;134(4):e1129–e1148. doi:10.1542/peds.2014-0352.
- Rauner A, Mess F, Woll A. The relationship between physical activity, physical fitness and overweight in adolescents: a systematic review of studies published in or after 2000. BMC Pediatr. 2013;13(1):19. doi:10.1186/1471-2431-13-19.
- Keating CL, Moodie ML, Swinburn B a. The health-related quality of life of overweight and obese adolescents: a study measuring body mass index and adolescent-reported perceptions. Int J Pediatr Obes. 2011 Oct;6(5–6):434–441. doi:10.3109/17477166.2011.590197.
- Miserocchi E, Modorati G, Mosconi P, Colucci A, Bandello F. Quality of life in patients with Uveitis on chronic systemic immunosuppressive treatment. Ocul Immunol Inflamm. 2010;18(4):297–304. doi:10.3109/09273941003637510.
- Haverman L, Grootenhuis MA, van den Berg JM, et al. Predictors of health-related quality of life in children and adolescents with juvenile idiopathic arthritis: results from a web-based survey. Arthritis Care Res (Hoboken). 2012 May;64(5):694–703. doi:10.1002/acr.21609.
- Armbrust W, Bos GJFJ, Geertzen JHB, Sauer PJJ, Dijkstra PU, Lelieveld OTHM. Measuring physical activity in Juvenile Idiopathic Arthritis: activity diary versus accelerometer. J Rheumatol. 2017;44(8):1249–1256. doi:10.3899/jrheum.160671.