ABSTRACT
Purpose
To study the efficacy and safety of suprachoroidal CLS-TA (proprietary suspension of triamcinolone acetonide) in uveitic macular edema (UME) with and without concurrent systemic corticosteroid or steroid-sparing therapy (ST).
Methods
Post hoc analysis of the PEACHTREE phase 3 randomized trial.
Results
Among UME patients receiving no ST, at week 24, mean BCVA change was +15.6 letters in 68 CLS-TA patients versus +4.9 letters in 49 sham-control patients (p < .001), while mean CST change was −169.8 µm versus −10.3 µm, respectively (p < .001). Among patients receiving ST, at week 24, mean BCVA change was +9.4 letters in 28 CLS-TA patients versus −3.2 letters in 15 sham-control patients (p = .019), while mean CST change was −108.3 µm versus −43.5 µm, respectively (p = .190). No SAEs related to treatment were reported.
Conclusions
A clinically meaningful benefit of CLS-TA was noted in UME patients, regardless of concurrent ST usage.
Abbreviation and Acronyms
CST = central subfield thickness; BCVA = best corrected visual acuity; ME = macular edemaI; IVT = intravitreal; AE = adverse event; FA = fluocinolone acetonide; SD-OCT = spectral-domain optical coherence tomography; NIU = noninfectious uveitis; SAE = serious adverse event; TEAE = treatment emergent adverse event; ITT = intent to treat; CI = confidence interval
Uveitis, a heterogenous group of diseases characterized by intraocular inflammation, is a major cause of vision impairment, accounting for 10 to 15% of cases of blindness in the developed world.Citation1,Citation2 The leading cause of irreversible vision loss in uveitis is macular edema (ME), which is responsible for approximately 30% of cases of blindness and 40% of cases of visual impairment.Citation3 Currently available therapies for uveitis include systemic immunosuppression (corticosteroids or corticosteroid-sparing immunotherapies) and local corticosteroids, including topical ophthalmic medications, periocular injections, and intravitreal (IVT) injections. However, use of local steroid injections lead to ocular hypertension in 20–60% cases, mediated by corticosteroid-induced modulation of the aqueous fluid outflow system, which can lead to glaucoma or glaucoma progression. Use of local corticosteroid injections are also associated with cataract development or progression.Citation4–7 In preclinical studies, suprachoroidal administration of an investigational corticosteroid formulation (triamcinolone acetonide, CLS-TA) was shown to target affected posterior tissues, while limiting corticosteroid exposure to the anterior segment, suggesting the potential to decrease the adverse events (AEs) of ocular hypertension, glaucoma, and cataract.Citation8-11
Suprachoroidal injection of CLS-TA was assessed in the phase 3 PEACHTREE study (NCT02595398) in patients with noninfectious uveitis (NIU) complicated by ME.Citation12 PEACHTREE was a masked, randomized trial with 160 patients randomized in a 3:2 ratio to 4 mg (0.1 ml of 40 mg/ml) CLS-TA (‘Active arm’) or sham (‘Control arm’) with 2 administrations 12 weeks apart. In the Active arm, 46.9% of patients gained ≥15 Early Treatment Diabetic Retinopathy Study (ETDRS) letters in best corrected visual acuity (BCVA) versus 15.6% in the Control arm (p < .001) at Week 24, meeting the primary efficacy endpoint. Compared to baseline, ME, as measured by central subfield thickness (CST), improved by a mean of 152.6 µm versus 17.9 µm (p < .001) for the Active and Control arms, respectively. Adverse events of elevated intraocular pressure (IOP), not temporally related to the injection procedure, occurred in 11.5% of patients in the Active arm and 15.6% in the Control arm, likely reflecting the more frequent corticosteroid rescue in the Control arm. Incidence of cataract progression were comparable (7.3% and 6.3%) in the Active arm versus the Control arm.
In clinical practice, treatment of uveitis and/or uveitic ME may require a combination of systemic and local therapies. For example, in the HURON registration trial of the 0.7 mg IVT dexamethasone implant for NIU, 26% of Active and 24% of Sham Control patients received baseline systemic anti-inflammatory or immunosuppressant medication, but subgroup analysis was not reported.Citation13 In the registration trial of the 0.18 mg IVT fluocinolone acetonide (FA) insert for chronic NIU, 50.6% of Active and 50% of Sham Control patients received baseline systemic corticosteroid or immunosuppressive therapy. Post hoc analysis of data from that study showed a significant difference favoring the FA insert over sham in those patients also receiving systemic therapies at baseline.Citation14 In contrast, the same analysis showed no significant difference between the FA insert and sham in those patients not receiving systemic therapy at baseline. In the PeriOcular and INTravitreal Corticosteroids for Uveitic Macular Edema Trial (POINT) study, systemic therapies were administered at baseline in 37%, 37% and 36% of those patients randomized to 40 mg periocular triamcinolone acetonide (TA), 4 mg IVT TA, or the 0.7 mg IVT dexamethasone implant respectively; however, subgroup analysis was not reported.Citation15 Similarly, in PEACHTREE, patients with uveitic ME were eligible for enrollment if on a low dose corticosteroid or stable dose of immunomodulatory therapy for at least 2 weeks prior to Baseline with no anticipated increase in dosing during the study. Given the limited literature on combination therapy for uveitic ME, this post hoc analysis explored the efficacy and safety of suprachoroidal CLS-TA in PEACHTREE for patients receiving and not receiving concurrent systemic therapy.
Methods
Study participants
The PEACHTREE study design and main findings have been reported previously.Citation12 Patients were eligible if they had a diagnosis of NIU of any etiology and had ME secondary to uveitis, defined as having intraretinal or subretinal fluid along with a retinal thickness of ≥300 µm in the central subfield as measured by spectral-domain Optical Coherence Tomography (SD-OCT). Patients were required to have a BCVA score of ≥5 ETDRS letters (20/800 Snellen equivalent) and ≤70 letters (20/40 Snellen equivalent) in the study eye.
The protocol excluded all patients receiving systemic corticosteroid at doses greater than 20 mg per day for oral prednisone (or equivalent for other corticosteroid) in the 2 weeks prior to Visit 2 (Baseline); patients on 20 mg or less per day could be enrolled if no increase in dosing was anticipated for the duration of the study. Also excluded from participation were all patients receiving prescription steroid-sparing immunomodulatory therapy, unless on a stable dose of these agents for at least 2 weeks prior to randomization and not expected to require an escalation of systemic therapy during the study. Decreases and termination of dose were allowed during the study.
Study design
PEACHTREE was a phase 3, multicenter study to assess the safety and efficacy of 4 mg of CLS-TA administered via suprachoroidal injection compared to a sham procedure for the treatment of patients with ME associated with NIUCitation12. Administrations occurred at Day 0 and Week 12. The trial protocol was approved by an independent ethics committee or institutional review board at each study site, and the trial was performed in compliance with the provisions of the Declaration of Helsinki, International Conference on Harmonization Good Clinical Practice guidelines, and applicable local regulations. Patients provided written informed consent prior to enrollment into the study.
In PEACHTREE, pre-defined criteria were established for the administration of rescue therapy, with the timing and type of rescue therapy administered at the investigator’s discretion.
Efficacy and safety endpoint assessments
Key efficacy endpoints in PEACHTREE included: ≥15-letter improvement from baseline in BCVA, change from baseline in BCVA letter score, change from baseline in CST, retinal thickness < 300 microns (all at 24 weeks) and proportion of patients requiring rescue therapy. Safety endpoints included the incidences of serious adverse events (SAEs) and treatment-emergent adverse events (TEAEs) reported during the entire 24 week study period, mean change from baseline in IOP, and the incidences of IOP-related events (IOP≥30 mm Hg, IOP increases ≥10 mm Hg, and use of IOP-lowering medications and procedures) and cataract.
Statistical analysis
In this post hoc study, analyses were performed to evaluate the efficacy and safety findings in the subgroups of patients receiving systemic corticosteroid and/or steroid-sparing therapy at baseline, and in patients not receiving systemic therapies at baseline. The intent-to-treat (ITT) population was used for all efficacy analyses and consisted of all randomized patients. The last observation carried forward method was used to impute values for all data points after rescue medication had been administered or to impute values for missing data. Summaries of safety were based on the safety population, defined as all patients receiving at least a single dose of study drug. Descriptive statistics were used to summarize continuous variables, while counts and percentages were used to summarize categorical variables. Demographic and disease characteristics were evaluated to assess for clinically important imbalances at baseline.
The number and percentage of patients with an improvement from baseline of 15 letters or more in BCVA or a CST less than 300 µm were summarized. Differences between treatments in the number of patients receiving rescue therapy were tested using a Cochran-Mantel-Haenszel chi-square test stratified by country. For mean changes from baseline in BCVA and CST the estimate of the between-treatment difference, 95% confidence interval and p-value were calculated based on an analysis of variance model with fixed effects for treatment group and country.
Statistical testing was performed in a pair-wise fashion in which comparisons between the two treatment groups (CLS-TA vs Control/sham) were made within each systemic therapy subgroup (those with and those without concurrent systemic treatment).
The summary of safety included the incidences of TEAEs and SAEs, mean change from baseline in IOP, and the incidences of IOP- and cataract-related events. Treatment-emergent adverse events were defined as an event that emerged during treatment having been absent pre-treatment or worsening relative to the pre-treatment state.
An alpha level of 0.050 was used for declaring statistical significance. No adjustments were made to the type 1 error rate to account for multiple comparisons.
Results
Baseline characteristics
PEACHTREE randomized a total of 160 patients (96, Active; 64, Control).Citation12 Following a medical review of all non-ocular medications administered prior to and during the study, the following medications were identified as a ‘systemic corticosteroid or corticosteroid-sparing medication’: adalimumab, celecoxib, cyclosporin, diclofenac potassium, etanercept, ibuprofen, infliximab, meloxicam, methotrexate, mycophenolate mofetil, Napra-D (Domperidone with Naproxen), naproxen, prednisone, and oral prednisolone.
In this post-hoc analyses, 68 (70.8%) patients in the Active arm and 49 (76.5%) in the Control were not receiving any systemic corticosteroid and/or steroid-sparing therapy. Mean BCVA at baseline was well balanced among patients not receiving systemic therapy, measuring 54.9 and 54.6 letters read, respectively. Mean CST at baseline were 483.6 and 524.6 µm, respectively. A smaller subgroup, 28 (29.2%) patients in the Active arm and 15 (23.4%) in the Control arm were receiving systemic corticosteroid and/or steroid-sparing therapy at baseline. Mean BCVA at baseline among those patients was 54.0 and 50.1 letters, respectively, while, mean CST was 474.4 and 528.2 µm, respectively.
Imbalances were noted in certain demographic and uveitic disease characteristics at baseline. Caucasians were most highly represented among those patients receiving systemic therapy (57.1% and 60.0% in the Active and Control arms, respectively), while Asians (predominantly from sites in India) were most highly represented among those patients not receiving any systemic therapies (58.8% and 53.1% in the Active and Control arms, respectively). The mean time since receiving a diagnosis of uveitis was longer among those receiving systemic therapy, compared to those not receiving systemic therapy in both the Active arm (257.7 versus 144.4 weeks, respectively) and the Control arm (168.0 versus 88.5 weeks, respectively). Similarly, the mean time since receiving a diagnosis of ME was longer in patients receiving systemic therapies, compared to those not receiving systemic therapies in both the Active arm (141.7 versus 42.0 weeks, respectively) and the Control arm (122.7 versus 51.0 weeks, respectively). Patients receiving systemic therapies were predominantly diagnosed with panuveitis (53.6% and 66.7% in the Active and Control arms, respectively), whereas patients not receiving any systemic therapies were mainly diagnosed with intermediate uveitis (45.6% and 40.8% in the Active and Control arms, respectively).
All other demographic () and baseline ocular and disease characteristics () were considered balanced within and between each systemic therapy subgroup.
Table 1. Demographics Summary Among Patients Receiving and Not Receiving Systemic Corticosteroid and/or Steroid-Sparing Therapy at Baseline.
Table 2. Baseline Disease Characteristics in the Study Eye Among Patients Receiving and Not Receiving Systemic Corticosteroid and/or Steroid-Sparing Therapy at Baseline.
Efficacy
Among those 68 Active and 49 Control patients not receiving any systemic corticosteroid and/or steroid-sparing therapy at baseline, 48.5% of the Active arm and 16.3% of the Control arm experienced an improvement in BCVA of 15 letters or more at 24 weeks (95% CI for difference: 16.4%, 48.0%; p < .001) (). In the subgroups of 28 Active and 15 Control patients who were receiving systemic therapy at baseline, 42.9% of the Active arm and 13.3% of the Control arm experienced an improvement in BCVA of 15 letters or more at 24 weeks (95% CI for difference: 4.4%, 54.7%; p = .052).
Figure 1. Efficacy Summary Among Patients Receiving and Not Receiving Systemic Corticosteroid and/or Steroid-Sparing Therapy at Baseline.
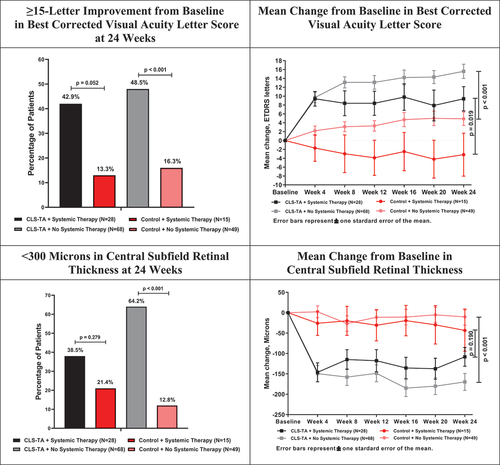
In the 68 Active and 49 Control patients who received no additional systemic therapies at baseline, the increase in BCVA at week 24 was 15.6 letters versus 4.9 letters (95% CI for difference: 6.2, 15.2 letters; p < .001), while in the 28 Active and 15 Control patients who received systemic therapy, the change in BCVA at week 24 was an increase of 9.4 letters versus a decrease of 3.2 letters (95% CI for difference: 2.2, 23.0 letters; p = .019).
Mean reductions in CST of 169.8 µm and 10.3 µm were observed at 24 weeks in the Active and Control arms, respectively, in those patients not receiving systemic therapies at baseline (95% CI for difference: −219.1, −99.9 µm; p < .001). In the patients receiving systemic therapies at baseline, a smaller difference was noted between the Active and Control arms, with 108.3 µm and 43.5 µm reductions in CST at 24 weeks, respectively (95% CI for difference: −163.2, 33.6 µm; p = .190). Similarly, in those patients not receiving any systemic therapies at baseline, the percentage of patients in the Active arm (64.2%) with a CST < 300 µm at 24 weeks was significantly higher than in the Control arm (12.8%) (95% CI for difference: 36.5%, 66.3%; p < .001). In the subgroup of patients receiving systemic therapies at baseline, 38.5% of patients in the Active arm had CST < 300 µm compared to 21.4% of patients in the Control arm (95% CI for difference: −11.5%, 45.5%; p = .279) ().
The investigators were instructed to administer rescue therapy provided that pre-specified vision and anatomical criteria were met, with the therapy being implemented left to the discretion of the investigator. During the course of the study, 14.7% of patients in the Active arm who did not receive any systemic therapies at baseline received rescue therapy compared to 69.4% of patients in the Control arm (95% CI for difference: −70.1%, −39.3%; p < .001). Of patients receiving systemic therapy at baseline, 10.7% of patients in the Active arm and 80.0% of patients in the Control arm received rescue therapy during the course of the study (95% CI for difference: −92.5%, −46.0%; p < .001).
Safety
As reported in PEACHTREE, there were 3 SAEs reported during the study in three patients including retinal detachment in the study eye, sialoadenitis and lumbar vertebral fracture. All three patients were in the Active arm and not receiving any systemic therapy at baseline. All three SAEs were considered unrelated to study drug according to investigator determination.
Ocular adverse events that are relevant to corticosteroids are summarized in . Given the small sample sizes of each subgroup, there were no meaningful imbalances with respect to TEAEs between those receiving and not receiving baseline systemic therapy within the Active or Control arms. Seven patients out of 68 in the Active arm (10.3%) and not receiving systemic corticosteroid and/or steroid-sparing therapy at baseline showed progression of cataracts compared to 3 patients out of 49 in the Control arm (6.1%) who were not receiving systemic therapy. Of patients receiving systemic therapies at baseline, one patient out of 15 in the Control arm (6.7%) experienced cataract progression compared to none of the 28 patients in the Active arm who were receiving therapy. Two patients in the Control arm underwent surgery to remove the cataract, one in each systemic therapy subgroup.
Table 3. Ocular Adverse Events Among Patients Receiving and Not Receiving Systemic Corticosteroid and/or Steroid-Sparing Therapy at Baseline.
Intraocular pressure is summarized in . At 24 weeks, patients not receiving any systemic therapies at baseline showed a mean IOP of 15.2 and 14.3 mm Hg in the Active and Control arms, respectively, representing mean increases of 2.0 and 0.9 mm Hg. Patients in the Active and Control arms receiving systemic therapies showed mean IOP of 14.6 and 15.9 mm Hg, respectively, reflecting mean increases of 1.1 and 2.1 mm Hg.
Figure 2. Intraocular Pressure Among Patients Receiving and Not Receiving Systemic Corticosteroid and/or Steroid-Sparing Therapy at Baseline.
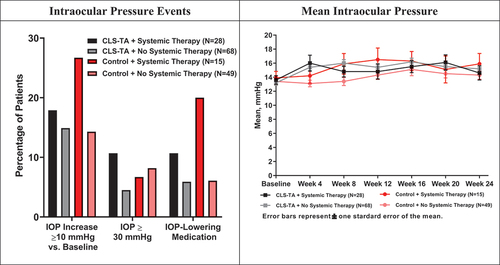
Excluding volume-related IOP elevations acutely following the injection procedure, patients in the Active and Control arms, respectively, experienced an IOP rise from baseline of 10 mm Hg or more in 14.9% and 14.3% of patients not receiving systemic therapy and in 17.9% and 26.7% of patients receiving systemic therapies at baseline. Patients in the Active arm (4.5%) not receiving any systemic therapies at baseline tended toward a lower rate of IOP ≥ 30 mm Hg at any time during the study compared to the Control arm (8.2%). Conversely, patients in the Control arm (6.7%) receiving systemic therapies at baseline tended toward a lower incidence compared to patients in the Active arm (10.7%). In patients not receiving systemic therapies, the use of IOP-lowering medications was similar in both treatment arms (Active: 5.9%, Control: 6.1%), whereas the use of these medications was nearly double in the Control arm (20.0%) compared to the Active arm (10.7%) in patients receiving systemic therapies at baseline.
Discussion
The Multicenter Uveitis Steroid Treatment (MUST) study assessed the benefits of systemic anti-inflammatory therapy versus 0.59 mg FA intraocular implant in 255 patients with intermediate uveitis, posterior uveitis, or panuveitis.Citation4 The patients randomized to corticosteroid implant showed superior control of inflammation at every time point through 54 months despite many patients not being reimplanted with the FA implant following depletion of their original implants, typically between months 30 and 36. Macular edema also improved significantly more often in the implant group within the first 6 months, but the systemic group gradually improved with comparable results by 36 months. At 54 months, there was similar improvement in visual acuity in both groups (mean improvement in BCVA at 54 months, 2.4 and 3.1 letters in the implant and systemic groups, respectively).
In clinical practice, however, treatment of uveitis and/or uveitic ME may require a combination of systemic and local therapies, but there is little literature on this topic to guide clinical practice. Consequently, this post hoc analysis assessed the efficacy and safety of suprachoroidal CLS-TA in PEACHTREE for uveitic ME patients receiving vs those not receiving other systemic corticosteroid and/or steroid-sparing therapy at baseline. With respect to safety, there were no meaningful imbalances between those receiving and not receiving baseline systemic therapy within the Active or Control arms. In all subgroups, cumulative AEs included typical procedure related AEs, such as subconjunctival hemorrhage, and the AEs of interest included events of IOP increased, cataracts and eye pain. Incidence rates for these AEs are in line with what is reported in the labels for other marketed intraocular steroids. For the Control group, the incidence rates for events of increased IOP, cystoid macular edema and cataract were consistent with rates of events reported in the literature for patients diagnosed with NIU. Importantly, with respect to efficacy, this post hoc assessment corroborates the prespecified study analyses in the PEACHTREE trial, demonstrating significant and clinically meaningful visual and anatomic benefits of CLS-TA over Control among patients with uveitic ME, regardless of baseline systemic corticosteroid and/or steroid-sparing therapy.
Patients receiving systemic corticosteroid and/or steroid-sparing therapy at baseline generally showed a higher frequency of panuveitis diagnoses and experienced more chronic uveitis, as well as more chronic macular edema, compared to those not receiving systemic corticosteroid and/or steroid-sparing therapy. These disease severity and chronicity features may account for the need for systemic therapies at baseline, as well as the observation that these patients showed less visual and anatomic improvement at 24 weeks, in both the Active and Control arms, compared to those not receiving systemic corticosteroid and/or steroid-sparing therapy. Nevertheless, the relative benefit of CLS-TA was notable. In addition, despite the trend toward more disease severity and chronicity features, the need for rescue therapy among the Active arm was similar in those receiving and not receiving systemic corticosteroid and/or steroid-sparing therapy at baseline. Specifically, 14.7% of patients in the Active arm who did not receive any systemic therapies at baseline received rescue therapy, compared to 10.7% of patients in the Active arm who were receiving systemic therapies.
Regardless of baseline systemic therapy status, the visual acuity outcomes compare favorably to those from the POINT study. This NIH-funded prospective clinical trial compared three commonly administered local corticosteroids for uveitic ME. At 6 months, 4 mg IVT TA and the 0.7 mg IVT dexamethasone implant outperformed 40 mg periocular TA, both achieving approximately 9 letters of improvement compared to approximately 4 letters, respectively.Citation15
Limitations of this study include its sample size and post hoc design. Despite these limitations, the efficacy and safety results of this post hoc study are consistent with the findings of PEACHTREE and are of interest to clinicians who treat uveitis patients. In particular, treatment of ME associated with NIU may require a combination of systemic and local therapies, and there is currently limited data on combination systemic/local therapy for uveitic ME. This analysis shows a clinically meaningful relative benefit of CLS-TA over sham treatment, regardless of concurrent systemic corticosteroid or steroid-sparing therapy among patients with uveitic ME.
Prior presentations
Data from this manuscript were presented at the Retina Society Annual Meeting 2020, the Association for Research in Vision and Ophthalmology Annual Meeting 2020, and the American Society of Retina Specialists Annual Meeting 2020.
Synopsis
This post hoc analysis demonstrated suprachoroidal injection of investigational triamcinolone acetonide (CLS-TA) in PEACHTREE provided clinically relevant benefits compared to control regardless of the presence of systemic therapies.
Acknowledgments
The trial was designed jointly by the investigators and the sponsor (Clearside Biomedical, Inc; Alpharetta, GA). The investigators collected the data, and the sponsor conducted the data analyses. All authors had full access to the data; there was an agreement between the investigators and the sponsor to not disclose any trial information that was not publicly available. All authors reviewed and provided feedback on the manuscript drafts and made the decision to submit the manuscript for publication; the sponsor also reviewed and approved the manuscript. All authors vouch for the completeness and accuracy of the data and analyses and affirm that the trial was conducted and reported with fidelity to the protocol.
(Funded by Clearside Biomedical, Inc.; PEACHTREE ClinicalTrials.gov number NCT02595398)
Disclosure statement
Dr. Merrill reports receiving grant support from Clearside Biomedical and personal fees from Santen, Gilead, Eyepoint, and Abbvie. Dr. Henry reports receiving personal fees from Clearside Biomedical and Bausch and Lomb. Dr. Nguyen reports receiving grant support from Genentech, Regeneron, and Santen. Dr. Reddy report receiving personal fees from Clearside Biomedical. Mr. Kapik is an employee of Clearside Biomedical and holds stock in the company. Dr. Ciulla is an employee of Clearside Biomedical and holds stock in the company.
Additional information
Funding
References
- Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14(5–6):303–308. doi:10.1007/BF00163549.
- Durrani OM, Tehrani NN, Marr JE, et al. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol. 2004;88(9):1159–1162. doi:10.1136/bjo.2003.037226.
- Rothova A, Suttorp-van Schulten MS, Frits Treffers W, et al. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80(4):332–336. doi:10.1136/bjo.80.4.332.
- Multicenter Uveitis Steroid Treatment Trial Research G, Kempen JH, Altaweel MM, et al. Benefits of systemic anti-inflammatory therapy versus fluocinolone acetonide intraocular implant for intermediate uveitis, posterior uveitis, and panuveitis: fifty-four-month results of the multicenter uveitis steroid treatment (MUST) trial and follow-up study. Ophthalmology. 2015;122(10):1967–1975. doi:10.1016/j.ophtha.2015.06.042.
- Multicenter Uveitis Steroid Treatment Trial Research G, Kempen JH, Altaweel MM, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology. 2011;118(10):1916–1926. doi:10.1016/j.ophtha.2011.07.027.
- Sen HN, Vitale S, Gangaputra SS, et al. Periocular corticosteroid injections in uveitis: effects and complications. Ophthalmology. 2014;121(11):2275–2286. doi:10.1016/j.ophtha.2014.05.021.
- Writing Committee For The Multicenter Uveitis Steroid Treatment T, Follow-up Study Research G, Kempen JH, et al. Association between long-lasting intravitreous fluocinolone acetonide implant vs systemic anti-inflammatory therapy and visual acuity at 7 years among patients with intermediate, posterior, or panuveitis. JAMA. 2017;317(19):1993–2005. doi:10.1001/jama.2017.5103.
- Chen M, Li X, Liu J, et al. Safety and pharmacodynamics of suprachoroidal injection of triamcinolone acetonide as a controlled ocular drug release model. J Control Release. 2015;203:109–117. doi:10.1016/j.jconrel.2015.02.021.
- Habot-Wilner Z, Noronha G, Wykoff CC. Suprachoroidally injected pharmacological agents for the treatment of chorio-retinal diseases: a targeted approach. Acta Ophthalmol. 2019;97(5):460–472. doi:10.1111/aos.14042.
- Hancock SE, Wan CR, Fisher NE, et al. Biomechanics of suprachoroidal drug delivery: from benchtop to clinical investigation in ocular therapies. Expert Opinion on Drug Delivery 2021;1–12. doi:10.1080/17425247.2021.1867532.
- Wan C-R, Muya L, Kansara V, et al. Suprachoroidal delivery of small molecules, nanoparticles, gene and cell therapies for ocular diseases. Pharmaceutics. 2021;13(2):288. doi:10.3390/pharmaceutics13020288.
- Yeh S, Khurana RN, Shah M, et al. Efficacy and Safety of Suprachoroidal CLS-TA for Macular Edema Secondary to Noninfectious Uveitis: phase 3, Randomized Trial (PEACHTREE). Ophthalmology. 2020;127(7):948–955. doi:10.1016/j.ophtha.2020.01.006.
- Lowder C, Belfort R Jr., Lightman S, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129(5):545–553. doi:10.1001/archophthalmol.2010.339.
- Jaffe GJ, Foster CS, Pavesio CE, et al. Effect of an injectable fluocinolone acetonide insert on recurrence rates in chronic noninfectious uveitis affecting the posterior segment: twelve-month results. Ophthalmology. 2019;126(4):601–610. doi:10.1016/j.ophtha.2018.10.033.
- Thorne JE, Sugar EA, Holbrook JT, et al. Periocular triamcinolone vs. intravitreal triamcinolone vs. intravitreal dexamethasone implant for the treatment of uveitic macular edema: the periocular vs. intravitreal corticosteroids for uveitic macular edema (POINT) trial. Ophthalmology. 2019;126(2):283–295. doi:10.1016/j.ophtha.2018.08.021.
