ABSTRACT
Purpose
To investigate the role of combined systemic and local chemotherapy in improving the survival of patients with vitreoretinal lymphoma (VRL).
Methods
Patients with VRL consecutively seen from 2006 to 2020 were retrospectively reviewed; data on the presence and time of central nervous system (CNS) involvement and treatment regimen (systemic, local or combined chemotherapy) were collected. Overall survival (OS) and progression-free survival (PFS) were calculated for each group.
Results
Forty-three eyes of 22 subjects with histology-proven VRL were included. Mean time of survival was 64.8 months (SE±10.8). Twelve patients (57%) presented CNS involvement, which was significantly associated with progression (r = 0.48, P = .03) and death (r = 0.56, P = .009). The isolated primary VRL group had a 5-year OS of 80%. Combined systemic and local chemotherapy reduced the risk of death by 82% (hazard ratio 0.18[0.04– 0.85]) in the entire cohort.
Conclusion
Combined systemic and local chemotherapy significantly improved OS but not PFS of patients affected by VRL.
Vitreoretinal lymphoma (VRL), a malignant intraocular tumour that may be misdiagnosed as an inflammatory condition of the eye, is the most common uveitis masquerade syndrome.Citation1 Most cases of this rare tumour are an aggressive form of diffuse large B-cell lymphoma.Citation2 VRL is a subgroup of primary central nervous system lymphoma (PCNSL), which primarily affects the retina with or without involving the vitreous or the optic nerve.Citation3 It can present as an isolated entity or develop before, after or concurrently with central nervous system (CNS) lymphoma. In PCNSL cohorts, mean rates of concomitant VRL at diagnosis or at any time during the course of disease are 10% and 16%, respectively. Rates of CNS involvement with VRL at diagnosis or over the course of disease are 41% and 69%, respectively.Citation4 It is still not clear why some forms affect the eye first and others the CNS first.
A definitive diagnosis of VRL is not only essential to visual prognosis; it is essential, above all, to the patient’s life. Indeed, the survival of patients with VRL remains consistently poor, even with treatment, due to diagnostic delay and to the lack of a defined therapeutic strategy. CNS involvement decreases survival.Citation5 Few studies correlate the prognosis of patients with the onset of CNS involvement prior, concomitantly or subsequent to VRL diagnosis.Citation6–8 Other clinical factors predictive of a worse prognosis in patients with VRL have been investigated, and it has been shown that the presence of sub-retinal pigment epithelium (sub-RPE) infiltration can determine shorter survival.Citation9
Although the current treatment approach of local chemotherapy, frequently combined with systemic chemotherapy, distinguishes between the presence and absence of CNS involvement, this is not standardized. In cases with only one affected eye and without CNS involvement, local treatment (i.e. intravitreal methotrexate, intravitreal rituximab) or low-dose stereotactic external beam radiotherapy (30–35 Gy) to the eye is recommended; in cases of bilateral involvement without CNS involvement, there is still a preference toward local chemotherapy, but systemic treatment should not be excluded. In cases with CNS involvement, high-dose systemic chemotherapy is recommended in combination with local therapy, given the limited penetration of systemic agents into the vitreous cavity; whole brain radiotherapy in conjunction with ocular radiotherapy should be considered in those cases with CNS that have failed systemic therapy.Citation10
However, the ideal treatment approach to histology-proven VRL is controversial because of the lack of large comparative clinical trials; treatment thus depends on the preference of each clinical centre. In particular, whether to perform systemic chemotherapy even in the absence of CNS involvement is still under discussion.Citation11
The aim of our study was to evaluate survival of patients affected by VRL, with or without CNS involvement, in correlation with systemic and local chemotherapy, alone or in combination.
Material and methods
This retrospective study included consecutive patients with histologically confirmed VRL who were diagnosed between January 2006 and October 2020 at the Ocular Immunology Unit, Azienda USL-IRCCS di Reggio Emilia, Italy. The initial 7 patients have been previously reported; their outcomes after December 2014 were further examined.Citation12
Patients were referred to us for suspected uveitis. Presenting symptoms included floaters and painless loss of vision. The main signs were vitreous cellular infiltration, with cells organized into sheets or clumps, and multifocal creamy/white lesions in the outer retina. Other signs that made us suspect VRL included retinal lesions with “leopard-skin” appearance and retinal pigment epithelium atrophy. In summary, severe vitreous infiltration with characteristic retinal lesions was the most frequent presentation.
Diagnostic pars plana vitrectomy (PPV) under air was performed in patients with presumed VRL.Citation13 Before PPV we had excluded other causes of uveitis, with a workup including full blood count, protein electrophoresis, ACE, lysozyme, syphilis, HIV, hepatitis B and C serology, QuantiFERON TB Gold, Borrelia and Bartonella screening and chest computed tomography (CT). An undiluted vitreous sample was collected and immediately centrifuged. The supernatant was collected and used for the analysis of interleukin (IL)-6 and IL-10 levels (pg/ml) by cytometric bead array assay to differentiate between inflammatory and neoplastic diseases.Citation14 Cell pellets were used for cytology. Cells were placed on the coated slides and prepared for Giemsa stain. An expert pathologist examined the slides. Polymerase chain reaction (PCR) amplification was used to detect monoclonality of the malignant B‑cells and specifically, the rearrangements of the immunoglobulin heavy chain (IgH) gene.Citation15 MYD88 L265P mutations, tested in our cohort of patients with suspected VRL since 2018, have been shown to be helpful in diagnosing this tumour.Citation16 However, VRL was diagnosed with cyto-histopathology, the current gold standard for diagnosing VRL. The analysis was conducted until January 2013 at the Laboratory of Immunology of the National Eye Institute, National Institutes of Health (Bethesda, Maryland, USA), then at the Biological Haematology Department, Medical Biology and Pathology, University Hospital Pitié-Salpétrière-Charles Foix (Paris, France).
All patients underwent a brain MRI and CSF analysis as part of the workup in agreement with the haematologists.
The study was conducted in accordance with the principles of the Declaration of Helsinki and received approval by the local ethics committee (protocol n. 112655/2018 Comitato Etico dell’Area Vasta Emilia Nord, Italy). Informed consent was obtained from all subjects included in the study.
VRL and CNS involvement
We retrospectively divided the subjects with VRL into three diagnosis groups: 1) patients without CNS involvement (isolated primary VRL), 2) patients with primary VRL who later developed CNS lymphoma (primary VRL with subsequent CNS involvement) and 3) patients concurrently diagnosed with VRL and CNS lymphoma at the initial presentation or patients who developed VRL from known primary CNS lymphoma (concurrent/secondary VRL). Given the low number of subjects in these last two subgroups, they were merged to form the third group.
Treatment regimen
The subjects underwent different chemotherapy regimens: one group of patients underwent combined systemic and local chemotherapy, while the other, smaller group underwent only ocular or systemic chemotherapy. Systemic chemotherapy consisted of 4 cycles of MATRIx (methotrexate, cytarabine, thiotepa and rituximab), followed (or not) by peripheral blood stem cell transplantation, according to the therapeutic algorithm applied by the haematologists of our centre. Systemic chemotherapy was required if CNS involvement was present. Intraocular chemotherapy involved intravitreal injections of methotrexate at a dose of 400 μg (in 0.1 ml) according to the following scheme: 2 injections per week for 1 month, then 1 per week for 2 months, then 1 per month for 9 months.Citation17 However, this schedule is not always respected due to severe temporary pancytopenia resulting from concomitant systemic chemotherapy.
All patients underwent complete ophthalmological evaluation before and after treatment, including measurement of the best-corrected visual acuity (BCVA), biomicroscopic and fundus examination and optical coherence tomography (OCT) scan. We recorded vitreous haze, retinal and sub-RPE infiltrations.
Remission was determined by ocular and CNS remission. Ocular remission was based on the following: absence of cells in the vitreous and resolution of any previously documented retinal or sub-RPE infiltrations.Citation18 CNS remission was defined as the complete disappearance of all enhancing abnormalities on gadolinium-enhanced MRI.Citation19
Prognosis
Overall survival (OS) was measured from the date of confirmation of diagnosis by first positive cytological exam to the date of last follow up or death. In cases of secondary VRL, the first diagnostic exam was brain biopsy. Progression-free survival (PFS) was measured from the date of treatment start to the date of documented lymphoma relapse in any location. Relapse was defined as the reappearance of lymphoma cells in the vitreous cavity or in the retina or as CNS lymphoma lesions in patients with a previously documented remission.Citation18,Citation20
Statistical analyses
Quantitative data are presented as mean (± standard deviation [SD]) or median (interquartile range [IQR]), as appropriate, and qualitative data as absolute numbers and percentages. Shapiro–Wilk test was used to assess the normal distribution of quantitative variables. Spearman’ correlation test was performed to assess correlation among the variables. Receiver operating characteristic (ROC) curve was used to identify a cut-off value for the number of intravitreal injections and IL-6 associated with survival.
OS and PFS were estimated by the Kaplan-Meier method. Group comparisons were carried out using the log-rank test. Cox proportional hazards regression model was used for multivariate analysis.
All statistical tests were 2-tailed, and statistical significance was defined by a P value of < 0.05. Statistical analyses were performed using SPSS v.26 for Windows (IBM Statistics).
Results
Forty-five patients underwent diagnostic PPV for presumed VRL; of these, 22 patients (49%), with a mean age at diagnosis of 64 ± 12 years (12 females, 54.5%), were diagnosed with diffuse large B-cell lymphoma. Both eyes were affected by VRL in 21 patients, whereas only the left eye was involved in 1 patient, for a total of 43 eyes included in the study. One patient was lost to follow up.
The median follow-up period was 22 months (interquartile range, IQR 9–58) and the median diagnostic delay was 8 months (IQR 2–24). The clinical and demographic characteristics are listed in .
Table 1. Descriptive table of clinical and demographic characteristics. Data are presented as mean values ± SD or median (IQR) and n (%).
Twelve of the 21 (57%) patients with VRL presented CNS lymphoma: 6 were secondary or concurrent VRL and had in a median follow up of 12.5 months (IQR 4.75–48.75); the other 6 were primary VRL, with subsequent CNS involvement after a median time of 4 months (IQR −1; 20). In contrast, 9 patients did not develop CNS lymphoma during a median follow up of 22 months (IQR 8–58) and constituted the group of isolated primary VRL (). Six of the 15 (40%) patients with primary VRL developed CNS lymphoma.
Table 2. Diagnostic delay, follow-up and 5-year overall survival according to CNS involvement.
Mean OS after the diagnosis of VRL in the entire cohort was 64.8 months (SE ±10.8; median time: 38 months). OS at 12 months was 85% for all cases, while the 5-year OS rate decreased to 49%.
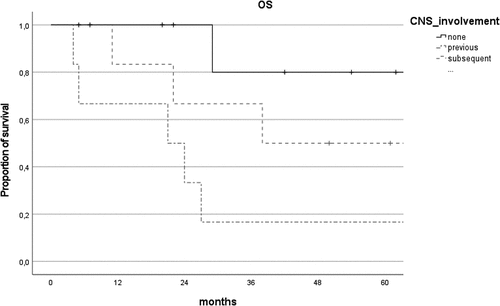
CNS involvement was significantly associated with progression (r = 0.48, P = .03) and with death (r = 0.56, P = .009). 5-year OS was 80% in the isolated primary VRL group, compared with 50% in primary VRL with subsequent CNS involvement and 16.7% in concurrent/secondary VRL (p = .03, , ). Median diagnostic delay did not differ between the three groups (P = .11).
Figure 1. Comparison of overall survival (OS) curves of patients with isolated primary VRL (none), concurrent/secondary VRL (previous) and primary VRL with subsequent CNS involvement (subsequent).
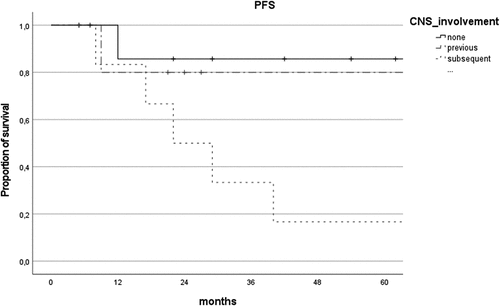
PFS was higher in patients without CNS involvement (69.0 ± 8.9 vs 41.3 ± 10.2 months), but the difference was not statistically significant (P = .07). Instead, there was a considerable reduction in PFS for the group with primary VRL with subsequent CNS involvement in comparison with primary VRL and concurrent/secondary VRL (P = .03, ).
Figure 2. Comparison of progression-free survival (PFS) curves of patients with isolated primary VRL (none), concurrent/secondary VRL (previous) and primary VRL with subsequent CNS involvement (subsequent).
Age at diagnosis was negatively correlated with CNS involvement (r = −0.48, P = .02); a lower age at diagnosis, therefore, correlates with a higher risk of progression in CNS lymphoma.
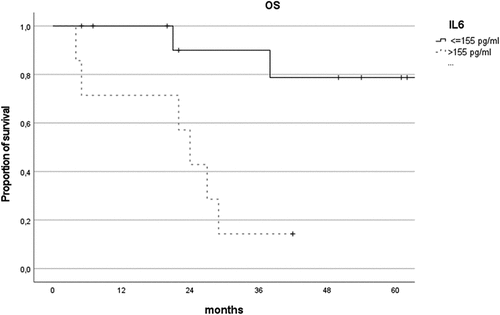
Of the 21 subjects with collected interleukin values, 18 (86%) presented an IL-10:IL-6 ratio of >1. An IL-6 value higher than 155 pg/ml was significantly associated with death (AUC = 0.77 ± 0.12). This result was confirmed in a decreased OS (), 79% vs 14% (p = .003). This effect was even more marked in the presence of CNS involvement; of the 11 subjects with CNS involvement and collected interleukin values, 5/5 subjects (100%) with IL-6 values greater than 155 pg/ml died, compared to the 2/6 subjects (33%) with IL-6 values lower than 155 pg/ml (P = .009).
Figure 3. Comparison of overall survival curves of patients depending on IL-6 cut-off value (155 pg/ml).
About half of the patients (50% right eye and 45.5% left eye) presented sub-RPE infiltrations detected on fundus and OCT examination. There were no differences in terms of OS and PFS based on the presence of this feature.
Twenty VRL patients were treated; one patient had died before starting treatment. Systemic chemotherapy was performed in 16 patients and intraocular chemotherapy in 19 patients; no significant adverse effect was recorded with either. All patients who underwent intravitreal injections of methotrexate responded to the aforementioned scheme of intraocular chemotherapy; we did not therefore have to use other intraocular chemotherapies, such as intravitreal rituximab. Fifteen patients underwent combined chemotherapy, 7 of whom presented with isolated primary VRL.
In summary, of the 22 patients considered in the study, one patient was lost to follow up and one died before starting treatment. Fifteen patients underwent combined chemotherapy, four patients had only intraocular chemotherapy and one patient had only systemic chemotherapy.
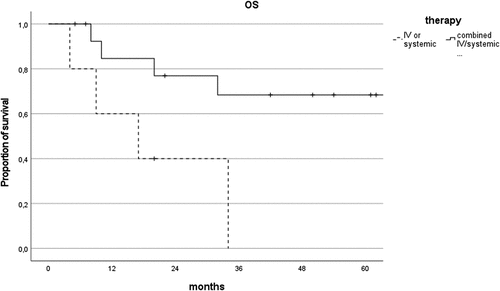
Five-year OS significantly improved in the presence of systemic chemotherapy (64% vs 0%, P = .041), and the combination of intravitreal and systemic chemotherapy increased 5-year OS even more significantly compared to local or systemic chemotherapy alone (68% vs 0%, P = .02, ). This was confirmed by the Cox regression multivariate analysis, which included age at diagnosis, CNS involvement and combined local and systemic chemotherapy. Combining local and systemic chemotherapy reduced the risk of death by 82% (HR = 0.18, 95% CI [0.03–0.96]). In particular, in the group of isolated primary VRL treated with combined chemotherapy, all patients (7 subjects) were alive at the last follow up.
Figure 4. Comparison of overall survival curves of patients treated with combined intravitreal/systemic therapy vs intravitreal or systemic alone.
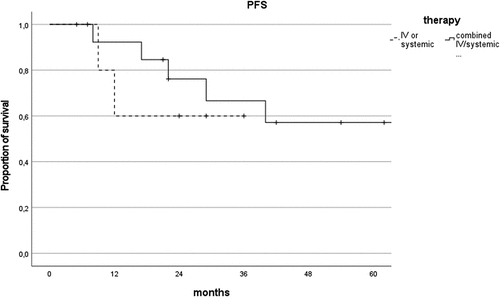
Combined chemotherapy did not extend PFS compared to systemic or local chemotherapy alone (P = .601, ).
Figure 5. Comparison of progression-free survival curves of patients treated with combined intravitreal/systemic therapy vs intravitreal or systemic alone.
Remission occurred in 12/20 (60%) subjects after treatment, with a median time of 7 months (IQR 5–10). Of these 12 patients, 8 were isolated primary VRL, 3 were primary VRL with subsequent CNS involvement and 1 was concurrent VRL.
We tried to identify a minimum number of intravitreal injections to ensure better OS and fewer relapses. More than 25 injections improved OS, but the difference was not statistically significant (p = .398).
Recurrences were managed effectively with additional intravitreal injections of methotrexate.
Discussion
The prognosis of patients with VRL remains poor, even with treatment. Due to the rarity and the different expressions of the tumour, there are no consistent reports available regarding survival in VRL: mortality rates vary from 9% to 81%, with follow up ranging from 12 to 35 months.Citation21 A timely and accurate diagnosis of VRL is difficult because the tumour mimics an inflammatory condition; patients are in fact referred to our Ocular Immunology Unit for suspected uveitis. Instead, we histologically confirmed VRL in 49% of presumed cases. In our study, we evaluated the survival of patients diagnosed with VRL in relation to CNS involvement and to the type of chemotherapy regimen.
CNS lymphoma was present in 57% of the patients in our cohort, with a median follow up of 22 months, while only 40% of subjects with initial VRL eventually developed CNS lymphoma. This low CNS involvement rate could be related to a shorter diagnostic delay compared to what has been reported in the literature.Citation4 Isolated primary VRL shows the best prognosis in terms of survival compared to VRL associated with CNS involvement; the latter is therefore confirmed to be a negative prognostic factor in patients with VRL.Citation5 In particular, OS shows a worse trend in the presence of concurrent/secondary VRL compared to primary VRL with subsequent CNS lymphoma.
Our study found that older patients at diagnosis (≥ 70 years) had less CNS involvement; they therefore usually presented an isolated primary VRL. We speculate that younger patients (< 70 years) could present more genetic aberrations, such as CD79B, increasing malignancy and CNS spread.Citation22 Studies are required to confirm this hypothesis.
Interleukin analysis represents a useful additional test to support the diagnosis of VRL.Citation14 IL-6 is a pro-inflammatory cytokine elevated in several immune-mediated diseases such as rheumatoid arthritis and uveitis.Citation23–25 IL-10 is mainly an anti-inflammatory cytokine able to promote B-cell lymphoma proliferation.Citation26 Nearly 90% of our cohort had an IL-10:IL-6 ratio of >1, in line with the literature.Citation27 In our study, we were surprised to find that levels of IL-6 greater than 155 pg/ml could represent a negative prognostic factor for the survival of patients with VRL, in particular with CNS involvement. Elevated levels of IL-6 have been associated with worse prognosis in diffuse large B-cell lymphoma.Citation28 The role of chronic, smouldering and subclinical inflammation in carcinogenesis is widely described in the literature.Citation29–31 The invasive capacity of malignant cells can increase in the presence of inflammatory cytokines such as TNF-α, IL-1β and IL-6.Citation32 Therefore, high levels of IL-6 could accelerate tumour replication and CNS spread in patients with VRL, increasing mortality. Further studies are required to confirm this association.
Dalvin et al. state that VRL with sub-RPE infiltrations could decrease mean survival time and configure a more aggressive subtype. So far, no other studies have found this correlation.Citation9 In our study we did not confirm this finding, as OS and PFS were not statistically different between the groups with and without sub-RPE infiltrations.
No VRL treatment guidelines have yet been developed. VRL mortality is strongly related to CNS extension.Citation5 Hence, reducing the diagnostic delay and immediately prescribing aggressive treatment are the mainstay in avoiding CNS involvement and in achieving better prognosis. However, part of the published literature claims that VRL without CNS involvement should only be treated with ocular chemotherapy because systemic chemotherapy does not prevent CNS involvement nor prolong the time to CNS lymphoma, nor does it increase the OS of these patients.Citation11,Citation33 Furthermore, systemic chemotherapy is associated with more severe adverse effects compared to local treatments.Citation11 In our cohort of patients, we did not find any significant adverse effect related to any chemotherapy. Other studies provide partial support for the combination of intraocular and systemic chemotherapy. Klimova et al. state that combined therapy with intravitreal methotrexate extended PFS but not OS.Citation34 Castellino et al. found that primary VRL patients should undergo combined systemic and intraocular chemotherapy to prevent CNS progression, but this approach did not significantly increase OS.Citation8 Hashida et al. concluded that while prophylactic systemic chemotherapy did not inhibit the onset of CNS involvement in most of the patients with primary VRL, it significantly prolonged the time to cerebral involvement.Citation35 Our study found very improved OS in patients who underwent combined systemic and local chemotherapy compared to those who underwent either of the chemotherapy regimens alone. In particular, we noted a very high OS (80%) in the group of isolated primary VRL compared to other studies ().Citation8,Citation11,Citation34,Citation36–38 Most of this group was treated with combined chemotherapy because we rely on the assumption that the patients have already developed subclinical lymphoma in the CNS, which cannot as yet be substantiated by magnetic resonance imaging or cerebrospinal fluid examination. The importance of systemic chemotherapy for isolated primary VRL in terms of high dose of Methotrexate is highlighted in some papers in which systemic chemotherapy is compared to radiotherapy and intraocular chemotherapy.Citation39–41 High-dose systemic MTX while avoiding the possible ocular toxicities from orbital radiation has moved the trend toward systemic chemotherapy as the initial definitive treatment, although the optimal treatment is yet to be determined.Citation42 Our results demonstrate that 4 cycles of MATRIx, followed (or not) by peripheral blood stem cell transplantation and together with intravitreal injections of methotrexate, are effective in improving OS of patients with isolated primary VRL. The reason for the effectiveness of this combined approach is to be found in the main pathogenic hypothesis of VRL: lymphoma cells originate in the bone marrow and subsequently migrate into the eye by selective and specific adhesion molecules that facilitate homing to intraocular compartments (retina, vitreous and optic nerve).Citation10,Citation43 The ultimate goal of treatment is not only to eradicate the intraocular tumour cells, which could cause recurrence or CNS extension, but also to suppress bone marrow production of pathological cells, thereby eliminating the potential reservoir of untreated disease.Citation34,Citation44 Local chemotherapy cannot eliminate systemic tumour cells. On the other hand, systemic chemotherapy does not adequately penetrate the vitreous cavity.Citation10 Therefore, only a combined approach can destroy any neoplastic clone.
Table 3. 5-year overall survival in patients with isolated primary VRL. * No difference between isolated primary VRL and primary VRL with CNS involvement.
Combined chemotherapy improved OS in our cohort, but it did not extend PFS compared to systemic or local chemotherapy alone. However, despite our patients’ having a high recurrence rate, it was mainly due to ocular relapses without documented progression to CNS; OS did not, therefore, decrease.
This study has certain limitations. First, this was a retrospective analysis, which limited the consistency of the data. Second, the small sample size decreased the power of our statistical analysis. Third, this was a single-centre study. Fourth, the treatment groups were not homogeneous. Further prospective multicentre studies considering randomized groups of treatment (systemic chemotherapy alone, intraocular chemotherapy alone, combined chemotherapy) are needed to confirm our results.
Author contributions
FG, RA, JM, LDS and LC wrote the draft of the manuscript. RA performed the statistical analyses. LC, VM, FG, LDS, DI, MC, EB, FI, AF, SL, EF, DN, IT and RV contributed to the writing of the protocol and researched data. LC, RA, SC, AZ, FM, AC, LF and CS interpreted data and critically revised the manuscript. LC is the guarantor of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Declaration of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or non- financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Acknowledgments
To Jacqueline Costa for English proofreading.To Chantal Adani for support in patients visits.
References
- Rothova A, Ooijman F, Kerkhoff F, Van der Lelij A, Lokhorst HM. Uveitis masquerade syndromes. Ophthalmology. 2001;108(2):386–399. doi:10.1016/S0161-6420(00)00499-1.
- Coupland SE, Damato B. Understanding intraocular lymphomas. Clin Exp Ophthalmol. 2008;36(6):564–578. doi:10.1111/j.1442-9071.2008.01843.x.
- Coupland SE, Chan CC, Smith J. Pathophysiology of retinal lymphoma. Ocul Immunol Inflamm. 2009;17(4):227–237. doi:10.1080/09273940903168696.
- Farrall AL, Smith JR. Eye involvement in primary central nervous system lymphoma. Surv Ophthalmol. 2020;65(5):548–561. doi:10.1016/j.survophthal.2020.02.001.
- Chan CC, Sen HN. Current concepts in diagnosing and managing primary vitreoretinal (intraocular) lymphoma. Discov Med. 2013;15:93–100.
- Cho BJ, Kim DY, Park UC, Lee JY, Yoon YH, Yu HG. Clinical Features and Treatment Outcomes of Vitreoretinal Lymphoma according to Its Association with CNS Lymphoma. Ocul Immunol Inflamm. 2018;26(3):365–371. doi:10.1080/09273948.2017.1421669.
- Akpek EK, Ahmed I, Hochberg FH, et al. Intraocular-central nervous system lymphoma: clinical features, diagnosis, and outcomes. Ophthalmology. 1999;106(9):1805–1810.doi:10.1016/S0161-6420(99)90341-X.
- Castellino A, Pulido JS, Johnston PB, et al. Role of systemic high-dose methotrexate and combined approaches in the management of vitreoretinal lymphoma: a single center experience 1990-2018. Am J Hematol. 2019;94(3):291–298.doi:10.1002/ajh.25350.
- Dalvin LA, Lim LAS, Ancona-Lezama D, et al. Clinical features predictive of survival in patients with vitreoretinal lymphoma: analysis of 70 patients at a single ocular oncology center. Asia-Pac J Ophthalmol. 2020;9(2):110–116.doi:10.1097/APO.0000000000000274.
- Chan C, Rubenstein JL, Coupland SE, et al. Primary vitreoretinal lymphoma: a report from an international primary central nervous system lymphoma collaborative group symposium. Oncologist. 2011;16(11):1589–1599.doi:10.1634/theoncologist.2011-0210.
- Riemens A, Bromberg J, Touitou V, et al. Treatment strategies in primary vitreoretinal lymphoma: a 17-center European collaborative study. JAMA Ophthalmol. 2015;133(2):191–197.doi:10.1001/jamaophthalmol.2014.4755.
- Cimino L, Coassin M, Chan CC, et al. Vitreoretinal lymphomas misdiagnosed as uveitis: lessons learned from a case series. Indian J Ophthalmol. 2016;64(5):369–375.doi:10.4103/0301-4738.185600.
- Mura M, Buschini E, Iannetta D, De Smet MD. Vitreous biopsy under air. Retina. 2016;36(4):838–839. doi:10.1097/IAE.0000000000000939.
- Frenkel S, Pe’er J, Kaufman R, Maly B, Habot-Wilner Z. The importance of cytokines analysis in the diagnosis of vitreoretinal lymphoma. Acta Ophthalmol. 2020;98(6):e668–e673. doi:10.1111/aos.14347.
- Dalal M, Casady M, Moriarty E, et al. Diagnostic procedures in vitreoretinal lymphoma. Ocul Immunol Inflamm. 2014;22(4):270–276.doi:10.3109/09273948.2013.848905.
- Raja H, Salomão DR, Viswanatha DS, Pulido JS. Prevalence of MYD88 L265P mutation in histologically proven, diffuse large B-cell vitreoretinal lymphoma. Retina. 2016;36(3):624–628. doi:10.1097/IAE.0000000000000996.
- Smith JR, Rosenbaum JT, Wilson DJ. Role of intravitreal methotrexate in the management of primary central nervous. Ophthalmology. 2002;109(9):1709–1716. doi:10.1016/S0161-6420(02)01125-9.
- Cicinelli MV, Marchese A, Miserocchi E, et al. Retinal and choroidal changes of vitreoretinal lymphoma from active to remission phase after intravitreal rituximab. Ocul Immunol Inflamm. 2020;28(4):637–646.doi:10.1080/09273948.2019.1616769.
- Abrey LE, Batchelor TT, Ferreri AJM, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23(22):5034–5043.doi:10.1200/JCO.2005.13.524.
- Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586.doi:10.1200/JCO.2006.09.2403.
- Sagoo MS, Mehta H, Swampillai AJ, et al. Primary intraocular lymphoma. Surv Ophthalmol. 2014;59(5):503–516.doi:10.1016/j.survophthal.2013.12.001.
- Yonese I, Takase H, Yoshimori M, et al. CD79B mutations in primary vitreoretinal lymphoma: diagnostic and prognostic potential. Eur J Haematol. 2019;102(2):191–196.doi:10.1111/ejh.13191.
- Favalli EG. Understanding the role of interleukin-6 (IL-6) in the joint and beyond: a comprehensive review of il-6 inhibition for the management of rheumatoid arthritis. Rheumatol Ther. 2020;7(3):473–516. doi:10.1007/s40744-020-00219-2.
- Bonacini M, Soriano A, Cimino L, et al. Cytokine profiling in aqueous humor samples from patients with non-infectious uveitis associated with systemic inflammatory diseases. Front Immunol. 2020;11. doi:10.3389/fimmu.2020.00358.
- Abu El-Asrar AM, Berghmans N, Al-Obeidan SA, et al. The cytokine interleukin-6 and the chemokines CCL20 and CXCL13 are novel biomarkers of specific endogenous uveitic entities. Investig Ophthalmol Vis Sci. 2016;57(11):4606–4613.doi:10.1167/iovs.16-19758.
- Kuo DE, Wei MM, Knickelbein JE, et al. Logistic regression classification of primary vitreoretinal lymphoma versus uveitis by interleukin 6 and interleukin 10 levels. Ophthalmology:1–7. 2020;127(7):956–962. doi:10.1016/j.ophtha.2020.01.042.
- Kimura K, Usui Y, Goto H, et al. Clinical features and diagnostic significance of the intraocular fluid of 217 patients with intraocular lymphoma. Jpn J Ophthalmol. 2012;56(4):383–389.doi:10.1007/s10384-012-0150-7.
- Načinović-Duletić A, Štifter S, Š D, Ž Š, Jonjić N. Correlation of serum IL-6, IL-8 and IL-10 levels with clinicopathological features and prognosis in patients with diffuse large B-cell lymphoma. Int J Lab Hematol. 2008;30(3):230–239. doi:10.1111/j.1751-553X.2007.00951.x.
- Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–217. doi:10.1016/j.ccr.2005.02.013.
- Mantovani A, Ponzetta A, Inforzato A, Jaillon S. Innate immunity, inflammation and tumour progression: double-edged swords. J Intern Med. 2019;285(5):524–532. doi:10.1111/joim.12886.
- Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011;22(2):83–89. doi:10.1016/j.cytogfr.2011.02.003.
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205.
- Grimm SA, Pulido JS, Jahnke K, et al. Primary intraocular lymphoma: an international primary central nervous system lymphoma collaborative group report. Ann Oncol. 2007;18(11):1851–1855.doi:10.1093/annonc/mdm340.
- Klimova A, Heissigerova J, Rihova E, et al. Combined treatment of primary vitreoretinal lymphomas significantly prolongs the time to first relapse. Br J Ophthalmol. 2018;102(11):1579–1585.doi:10.1136/bjophthalmol-2017-311574.
- Hashida N, Nakai K, Saitoh N, Nishida K. Association between ocular findings and preventive therapy with onset of central nervous system involvement in patients with primary vitreoretinal lymphoma. Graefe’s Arch Clin Exp Ophthalmol. 2014;252(4):687–693. doi:10.1007/s00417-014-2584-8.
- Ahmed AH, Foster CS, Shields CL. Association of disease location and treatment with survival in diffuse large B-cell lymphoma of the eye and ocular adnexal region. JAMA Ophthalmol. 2017;135(10):1062–1068. doi:10.1001/jamaophthalmol.2017.3286.
- Ma WL, Hou HA, Hsu YJ, et al. Clinical outcomes of primary intraocular lymphoma patients treated with front-line systemic high-dose methotrexate and intravitreal methotrexate injection. Ann Hematol. 2016;95(4):593–601.doi:10.1007/s00277-015-2582-x.
- Kim MM, Dabaja BS, Medeiros J, et al. Survival outcomes of primary intraocular lymphoma a single-institution experience. Am J Clin Oncol Cancer Clin Trials. 2016;39(2):109–113. doi:10.1097/COC.0000000000000028.
- Chan CC, Wallace DJ. Intraocular lymphoma: update on diagnosis and management. Cancer Control. 2004;11(5):285–295. doi:10.1177/107327480401100502.
- Hormigo A, Abrey L, Heinemann MH, DeAngelis LM. Ocular presentation of primary central nervous system lymphoma: diagnosis and treatment. Br J Haematol. 2004;126(2):202–208. doi:10.1111/j.1365-2141.2004.05028.x.
- Vosganian GS, Boisot S, Hartmann KI, et al. Primary intraocular lymphoma: a review. J Neurooncol. 2011;105(2):127–134.doi:10.1007/s11060-011-0618-1.
- Kim SK, Chan CC, Wallace DJ. Management of primary intraocular lymphoma. Curr Oncol Rep. 2005;7(1):74–79. doi:10.1007/s11912-005-0029-6.
- McCann KJ, Ashton-Key M, Smith KA, Stevenson FK, Ottensmeier CH. Primary central nervous system lymphoma: tumor-related clones exist in the blood and bone marrow with evidence for separate development. Blood. 2009;113(19):4677–4680. doi:10.1182/blood-2008-09-179366.
- Davis JL. Intraocular lymphoma: a clinical perspective. Eye. 2013;27(2):153–162. doi:10.1038/eye.2012.250.
