ABSTRACT
Aim
A cross-sectional descriptive study to determine the frequency of ocular manifestations associated with systemic autoimmune diseases in a third-level hospital in Mexico.
Methods
Records from 2014 to 2017 at the Inflammatory Eye Disease Clinic of the Asociación Para Evitar la Cegueraen México were examined by both an ophthalmologist and a rheumatologist on the same day. Diagnosis was achieved from initial ocular manifestations with later systemic assessment.
Results
Out of 311 medical records, 276 were included, 75% of the patients were female. Keratoconjunctivitis sicca was the most frequent ocular manifestation (33.3%), followed by anterior uveitis (29.5%), scleritis (23.2%), and peripheral ulcerative keratitis (7.2%). The leading autoimmune diseases were spondyloarthritis (29%), rheumatoid arthritis (28.6%), primary Sjögren’s syndrome (10.5%) and granulomatosis with polyangiitis (9.1%). 41.3% of systemic disease diagnoses were made after an initial ocular manifestation.
Conclusions
Inflammatory eye manifestations can imply systemic autoimmune diseases. It is crucial to suspect and confirm this association and provide timely interdisciplinary management.
Introduction
Autoimmune diseases are a broad group of pathologies that result from immune system overactivation, which produce tissue damage in absence of any microorganism or specific exposure. The generated immune response can be related to either the innate immune system and excess inflammatory mediators, or to the adaptive immune system and T and B lymphocytes response against an antigen. There are over 80 different autoimmune diseases.Citation1 Ocular manifestations within this group of patients are diverse, but may guide us towards a concrete diagnosis according to their presentation and anatomical location.Citation2,Citation3 Furthermore, ocular manifestations may constitute an initial sign of systemic activity, which can sometimes even jeopardize patients’ lives. Clinical suspicion and an adequate approach to the medical history of these patients are decisive in order to perform tests which can rule out or confirm systemic diseases. The most common initial ocular manifestations are keratoconjunctivitis sicca (KCS), uveitis, and scleritis.Citation4
The prevalence of autoimmune diseases in the United States is around 5–7%, with females being more affected than males.Citation1,Citation5 The main autoimmune diseases associated with ocular manifestations are rheumatoid arthritis (RA),Citation4 Sjögren’s syndrome,Citation6 spondyloarthritis (SpA),Citation7,Citation8 systemic sclerosis, granulomatosis with polyangiitis (GPA),Citation9,Citation10 systemic lupus erythematosus (SLE), antiphospholipid syndrome (APS), relapsing polychondritis (RP), and juvenile idiopathic arthritis.Citation3,Citation4 Ocular presentations will depend on their underlying condition, which may be KCS, scleritis,Citation11–16 peripheral ulcerative keratitis (PUK),Citation15,Citation17–19 episcleritis,Citation20 uveitis (anterior, intermediate, posterior, and panuveitis),Citation21 vasculitis, or occlusive vasculopathy.
The purpose of the present study is to determine the prevalence of ocular manifestations associated with systemic autoimmune diseases.
Methods
A descriptive analysis of the medical records of 276 patients was made. We included adult patients (18 years-old and older) with ocular manifestations and recent diagnoses of rheumatological disease who attended medical appointments at the Inflammatory Eye Disease Clinic of the Asociación Para Evitar la Ceguera en México, I.A.P., from 2014 to 2017.
All patients were evaluated simultaneously by an ophthalmologist and rheumatologist. In most patients, a presumptive diagnosis was made from initial inflammatory eye manifestations and was corroborated with complete medical history, physical examination, and laboratory studies. All patients who were diagnosed with a systemic disease fulfilled the disease’s classification criteria. For all patients, infectious causes of ocular diseases were ruled out using Venereal Disease Research Laboratory (VDRL) and Fluorescent Treponemal Antibody-Absorption (FTA-ABS) for syphilis; Purified Protein Derivative (PPD), or QuantiFERON and chest radiography or CT thorax scan for tuberculosis; and herpes family virus, Toxocaracanis, Borrelia burgdorferi and/or Bartonella henselae antibody tests when these were suspected.
Demographic data included age and gender. Ophthalmologic diagnosis was based on standardization of uveitis nomenclature (SUN),Citation21 while Watson and Hayreh classification was used for patients with scleritis.Citation22 PUK, KCS, and retinal vasculitis diagnoses were made via ophthalmological examination according to clinical features described in literature.Citation18,Citation23,Citation24 We reviewed patients’ rheumatologic diagnoses and antibody reports for antinuclear antibodies (ANA), anti-DNA antibodies, anti-RNP antibodies, anti-Sm antibodies, Anti-Ro antibodies, anti-La antibodies, anti-centromere antibodies, anti-mitochondrial antibodies, anti-citrullinated protein antibodies (ACPAs), C and P anti-neutrophil cytoplasmic autoantibodies (C-ANCA and P-ANCA), anti-proteinase 3 antibodies (anti-PR3), anti-myeloperoxidase antibodies (anti-MPO), C-reactive protein (CRP), HLA-B27, angiotensin-converting enzyme (ACE), erythrocyte sedimentation rate (ESR), and rheumatoid factor. All types of SpA were included in one group. Disease laterality, and both topical and systemic treatments were registered. Patients with negative tests and in whom other underlying conditions were discarded, were included as idiopathic. Pediatric patients and patients who were not evaluated by the rheumatologist were excluded.
Data was captured in Microsoft Excel spreadsheets and processed with SPSS version 23. Descriptive statistics were used to formulate charts which presented absolute and relative frequencies. In terms of ethical considerations, patients’ lives and well-being were not endangered by this research, since all the information was obtained from medical records. The study was authorized by the Hospital’s Ethics and Research Committees (CO-17-02), including waiver of informed consent for this chart review study, which involved no contact with the human subjects.
Results
Out of 311 adult patient records, 276 met the inclusion criteria for non-infectious disease, 75% of whom (n = 207) were female. Average age was 50 ± 13.8 years (range from 20 to 77 years-old) and most patients were between 45 and 64 years old. Bilateral disease represented 48.7% of cases (n = 134). Further population characteristics can be observed in .
Table 1. Population characteristics according to most frequent rheumatological diseases (n = 228).
Table 2. Population characteristics according to idiopathic diseases (n = 27).
56.9% of our patients were diagnosed with a systemic disease at our clinic thanks to an initial ocular manifestation, which lead to directed medical questioning, laboratory tests, and rheumatological evaluation. By comparison, 34.1% of the patients had a systemic diagnosis prior to ocular manifestations, mainly RA. ()
Graph 1. Newly and previously systemic disease diagnoses (n = 249). A total of 94 patients (34.1%) had previously known systemic diagnosis, whereas 157 patients (56.9%) were diagnosed at APEC. Idiopathic causes (n = 27) were excluded from this Graph since no systemic diagnosis was made. *GPA: Granulomatosis with polyangiitis **EGPA: Eosinophilic granulomatosis with polyangiitis.
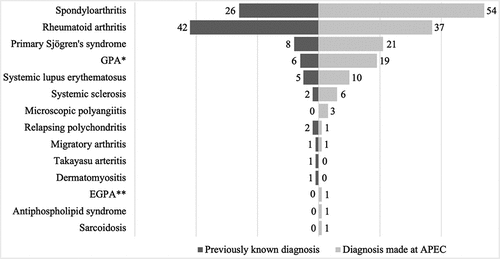
illustrates the presentation of autoimmune diseases in order of frequency. The most frequent autoimmune conditions were SpA in 29% of patients (n = 80), followed by RA in 28.6% (n = 79), primary Sjögren’s syndrome (pSS) in 10.5% (n = 29), and GPA in 9.1% (n = 25). The percentage of patients with ANCA associated small-vessel vasculitis in this study was 10.5% (n = 29), including GPA, microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis.Other conditions, such as SLE and RP, presented lower frequencies.
Table 3. Frequency of systemic diagnoses and idiopathic cases in total studied population (n = 276).
KCS was the most common ocular manifestation, occurring in 33.3% of patients (n = 92), followed by anterior uveitis in 29.5% (n = 81), scleritis in 23.6% (n = 66), and PUK in 7.2% (n = 20). Anterior uveitis was the most frequent ocular manifestation in SpA, which was the most common underlying systemic disease. .
Table 4. Ocular manifestations by order of frequency (n = 276).
PUK was presented in 7.2% (n = 20) of our patients. It was more common in patients with RA and GPA: 50% (n = 10) and 30% (n = 6), respectively. The remaining 20% (n = 4) was divided between idiopathic causes at 5% (n = 1), Sjögren’s syndrome at 5% (n = 1), MPA at 5% (n = 1), and SLE at 5% (n = 1). Thus, 95% of patients with PUK were diagnosed with a systemic disease.
In terms of antibody testing, 94.8% of the patients with RA presented a positive rheumatoid factor, 82.2% had positive ACPAs, and 66% had positive acute phase reactants. In the SpA group, 100% of patients had positive HLA-B27, and 31.1% had elevated CRP. Among patients with GPA, 95.5% had positive C-ANCA, 78.9% had positive anti-PR3 antibodies, and 52.6% had elevated CRP at initial stages. 82% of patients with pSS had positive anti-Ro antibodies, 42.3% had positive anti-La antibodies, and 83% had positive ANA titers. Finally, among patients with SLE, 93.3% had positive ANA titers and 88.8% had positive anti-dsDNA antibodies.
The treatment administered to each patient depended on their underlying pathology. The most frequent treatment was topical treatment with artificial tear drops, applied in cases of KCS. The main immunosuppressive drug administered for all five most common diseases was methotrexate, followed by sulfasalazine.
Discussion
Autoimmune diseases comprise a large and heterogenous group of systemic conditions. Ocular manifestations maybe the first clinical sign leading to the suspicion of an autoimmune disease. There are few studies describing epidemiological data which combine ophthalmological and rheumatological approaches.Citation25,Citation26
Of the 276 patients evaluated, 56.9% (n = 157), were diagnosed after their arrival at our clinic thanks to first-time questioning and both ophthalmologic and physical exploration(). We consider this to be a crucial finding in our study, since a single ocular manifestation can lead to a major change in patients’ diagnoses and subsequently their lives. A rheumatological association was found in 90.2% (n = 249) of our patients (). Idiopathic causes of ocular manifestations represented 9.8% of cases (n = 27)(). The most frequent initial ocular manifestation that oriented systemic diagnosis was HLA-B27 related non-granulomatous anterior uveitis in 87.5% of patients (n = 70), followed by RA-related PUK in 12.7% (n = 10), and GPA-related PUK in 24% (n = 6)().
Table 5. Association between systemic diseases and ophthalmological manifestations (n = 249).
The most frequent systemic condition found in this study was SpA; this diverges from epidemiological findings in Mexico, where a retrospective study of 25,587 patients in five states, reported that ankylosing spondylitis was the fourth most common rheumatologic condition, preceded (in descending order)by osteoarthritis, complex regional pain syndrome, and in third place, RA.Citation27 A Mexican study reports 57 cases of non-granulomatous anterior uveitis, mainly in patients with ankylosing spondylitis, which is similarto our findings.Citation28 We also observed few patients with GPA (n = 25) and with SLE (n = 15), which differs from typical findings in Mexican epidemiology.Citation27 We believe the small number of patients with SLE in this study is explained by the fact that patients in our clinic were initially assessed for ocular rather than systemic manifestations. This can also apply to our single case of sarcoidosis, since in Mexico this disease primarily affects the lungs and skin rather than the eyes,Citation28 and its management is therefore often directed by internal medicine instead of ophthalmology departments.
Sjögren’s syndrome is considered the second-most common rheumatic autoimmune disorder.Citation29 Similarly, in this study, it was the third-most common disorder. This condition predominantly occurs in women, with a female-to-male ratio of 9:1.Citation30,Citation31 Its prevalence depends on whether European or American diagnostic criteria are used.Citation6 Kabasakal et al. reported a prevalence of 1.56% according to European criteria and 0.72% according to U.S criteria in a Turkish population.Citation32 Our patients were predominantly women, and the prevalence of this syndrome was 10.5%. However, it should be noted that our hospital is a reference center and is therefore more likely to see a significant number of patients with this diagnosis.
ANCA-associated small-vessel vasculitis is another condition associated with ocular manifestations. Previous reports demonstrate that ocular involvement is present in approximately 29–57% of patients with this condition.Citation33 In this study, 10.5% of patients had ANCA-associated small-vessel vasculitis, all of them having ocular manifestations. The main ocular manifestation in ANCA- associated small-vessel vasculitis is scleritis,Citation9,Citation34 which is reflected by findings in our study. Scleritis can be associated with keratitis or iritis or even begin with PUK, which leads to high risk of corneal thinning and perforation. Complications are reported in up to 90% of these patients,Citation10 which makes timely diagnosis and treatment vital.
Approximately half of the patients with scleritis have an associated systemic condition.Citation16 RA is the most frequent one(6.4–15.2%), followed by systemic vasculitis in 2.3–9.1%, SLE in 1.0–4.1%, SpA in 1.3–4.7%, and inflammatory bowel disease in 2.2–3.3%.Citation11–15 This contrasts with our study, where the first cause of scleritis was GPA, which was presented in 76% (n = 19) of the patients, followed by RA in 22.8% (n = 18) and SpA in 7.5% (n = 6). In previous studies, idiopathic scleritis represents up to 76.5%,Citation35 which differs from the 5.8% we observed. Prevalence of episcleritis was 0.36%, diverging from previous literature, which reports a range from 3.2–11.8%.Citation20
Prevalence of HLA-B27 associated anterior uveitis depends on the underlying condition; prevalence is 33.2–33.4% in ankylosing spondylitis, 25–36.9% in inflammatory bowel disease, 2–25% in psoriatic arthritis, 25.6% in reactive arthritis, and 13.2% in undifferentiated spondylitis.Citation7,Citation8,Citation16 As stated, we did not differentiate between different types of spondyloarthritis. This could explain the high frequency of anterior non-infectious uveitis which we found (87.5%). In this study, 80 patients were included in the SpA group, these patients met the Assessment of SpondyloArthritis international Society (ASAS) criteria for axial spondyloarthritis.Citation36
PUK is a unilateral form of stromal inflammation that involves the perilimbal cornea and consists of a progressive corneal thinning that can lead to ocular perforation. It is always associated with epithelial defects and corneal stroma loss.Citation17,Citation19 PUK is primarily related to connective tissue disorders in approximately 50% of patients.1750% of PUK cases in this study presented in patients with RA, while 30% presented in patients with GPA as a first manifestation of systemic disease.
Retinal vasculitis can be the first manifestation of inflammatory diseases. According to SUN,Citation21 it consists of various groups of clinical and angiographic changes, such as sheathing, cotton-wool spots, retinal hemorrhages, leakage or occlusion.Citation21 In endemic countries such as India, the main cause of occlusive vasculitis is tuberculosis, while in developed countries its main causes are lupus erythematosus and, in a smaller proportion, Behçet’s disease, viral uveitis, masquerade syndromes, and syphilis, among others.Citation24,Citation37 In this study, retinal vasculitis was associated with Ra in one patient, Takayasu arteritis in one patient and SpA in another patient. The remaining two patients with this diagnosis were categorized as idiopathic.
Prevalence and incidence data for ocular inflammatory diseases vary by age group and demographic area.Citation29 However, we found similarities with findings reported in Spain,Citation38,Citation39 Taiwan,Citation40 AustraliaCitation41 and the United States,Citation42 where the most frequent manifestation was anterior uveitis associated with ankylosing spondylitis. In Italy, the most frequent manifestation was anterior uveitis associated to HLA-B27 and Behçet’s disease.Citation43 In Tunisia, the most frequent manifestation was anterior uveitis associated with sarcoidosis and Behçet’s disease.Citation44 Idiopathic anterior uveitis was the most common manifestation in Egypt.Citation26 In South America, specifically in Colombia, the most frequent autoimmune disease was RA, while KCS was the most common ocular main manifestation.Citation45,Citation46
In our report, there are few cases of patients with intermediate and posterior uveitis, panuveitis, or retinal vasculitis, since these manifestations are mostly infectious, neoplasic, pharmacologic, and/or associated with autoimmune diseases which are limited to the eye, such as pars planitis and Vogt-Koyanagi-Harada disease.Citation47 Since these conditions had an accurate ophthalmological diagnosis, rheumatological evaluation was not required, and they were not included. For sarcoidosis, we found only one patient within the time frame of this research, and no patients with Behçet’s disease (BD). In Mexico, sarcoidosis and BD are rare diseases; in a rheumatological clinic, the prevalence of BD was found to be only 1.2%.Citation28 The low prevalence of these diseases is explained by of their geographic affinity and genetic heterogeneity.Citation28,Citation48
Recently, the multidisciplinary approach in autoimmune conditions has gained importance due to the long-term systemic and ocular consequences of these. Timely diagnosis, treatment, and follow-up carried out jointly by rheumatology and ophthalmology departments can reduce morbidity, mortality, and chronic complications that can affect our patients’ quality of life. Likewise, it significantly impacts the expenses of patients and governments alike, given the decrease in economic activities brought on by work incapacity derived from these diseases, expensive therapy, and long-term care.Citation49,Citation50
One of the major limitations of our study lies in its retrospective nature. Furthermore, the low frequency of retinal vasculitis among our patients may be due to our exclusion of patients among whom this condition has an infectious cause, such as tuberculosis. Another limitation lies in not including pediatric patients, which excludes juvenile idiopathic arthritis. We also did not report orbital inflammatory diseases, since no patients with these conditions arrived within the timeframe of this research. In contrast, the main advantage of this study lies in being the first Mexican study to report epidemiological data on patients with systemic autoimmune diseases and ocular manifestations who were evaluated by an ophthalmologist and a rheumatologist during the same medical appointment in an ophthalmological hospital.
This emphasizes the importance of our study, in which the prevalence of idiopathic disease was only 9.8%. In other reports, variable data is associated with an idiopathic cause in 10–63% of patients.Citation29 Our having a low percentage of idiopathic diseases may be explained by the fact that the presence of a rheumatologist in the clinic has improved our accuracy in questioning patients, requesting specific laboratory and cabinet studies, and considering different diseases, thereby increasing specific diagnoses.
Conclusion
Through ocular manifestations, we achieved systemic disease diagnosis in 56.9% of our patients. Ophthalmological manifestations may be the first experienced symptom in patients with underlying autoimmune conditions, and must accordingly be considered to be indicators of systemic activity. The autoimmune conditions that showed the most correlation between ocular manifestations and later systemic diagnosis were SpA, RA, pSS, and GPA. The most frequent ocular manifestations, in descending order, were KCS, anterior uveitis, scleritis, PUK, retinal vasculitis, panuveitis, and lastly intermediate and posterior uveitis.
It is vital that multidisciplinary management be expanded between specialties such as ophthalmology, rheumatology, internal medicine, otorhinolaryngology, dermatology, gastroenterology, nephrology, cardiology, and infectiology in treating these patients. Autoimmune diseases are an important cause of inflammatory eye diseases; every case of ocular inflammatory manifestation should therefore lead us to investigate an underlying cause. Timely diagnosis and treatment can drastically affect a patient’s prognosis and quality of life.
Ethical approval
All procedures in this study were in accordance with the ethical standards of the hospital and with the 1964 Helsinki declaration and its later amendments.
Disclosure statement
Pablo Baquero-Ospina no conflict of interest, Rebeca Paquentín-Jiménez no conflict of interest, Claudia Hubbe-Tena no conflict of interest, Luz Elena Concha-del-Rio has received speaker honorarium and financial support for educational programs from Abbvie and Janssen.
Additional information
Funding
References
- Davidson A, Diamond B. The Autoimmune Diseases. Fifth ed. Boston: Academic Press; 2014:19–37. General features of autoimmune disease.
- McCluskey P, Powell RJ. The eye in systemic inflammatory diseases. Lancet. 2004;364(9451):2125–2133. doi:10.1016/S0140-6736(04)17554-5.
- Generali E, Cantarini L, Selmi C. Ocular involvement in systemic autoimmune diseases. Clin Rev Allergy Immunol. 2015;49(3):263–270. doi:10.1007/s12016-015-8518-3.
- Hamideh F, Prete PE. Ophthalmologic manifestations of rheumatic diseases. Semin Arthritis Rheum. 2001;30(4):217–241. doi:10.1053/sarh.2001.16639.
- Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84(3):223–243. doi:10.1006/clin.1997.4412.
- Brito-Zerón P, Baldini C, Bootsma H, et al. Sjögren syndrome. Nat Rev Dis Primers. 2016;2:16047. doi:10.1038/nrdp.2016.47.
- Zeboulon N, Dougados M, Gossec L. Prevalence and characteristics of uveitis in the spondyloarthropathies: a systematic literature review. Ann Rheum Dis. 2008;67(7):955–959. doi:10.1136/ard.2007.075754.
- Rosenbaum JT, Rosenzweig HL. Spondyloarthritis: the eyes have it: uveitis in patients with spondyloarthritis. Nat Rev Rheumatol. 2012;8(5):249–250. doi:10.1038/nrrheum.2012.43.
- Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi:10.1002/art.37715.
- Kubal AA, Perez VL. Ocular manifestations of ANCA-associated vasculitis. Rheum Dis Clin North Am. 2010;36(3):573–586. doi:10.1016/j.rdc.2010.05.005.
- Smith JR, Mackensen F, Rosenbaum JT. Therapy insight: scleritis and its relationship to systemic autoimmune disease. Nat Clin Pract Rheumatol. 2007;3:219–226.
- Akpek EK, Thorne JE, Qazi FA, Do DV, Jabs DA. Evaluation of patients with scleritis for systemic disease. Ophthalmology. 2004;111(3):501–506. doi:10.1016/j.ophtha.2003.06.006.
- Oray M, Meese H, Foster CS. Diagnosis and management of non-infectious immune-mediated scleritis: current status and future prospects. Expert Rev Clin Immunol. 2016;12(8):827–837. doi:10.1586/1744666X.2016.1171713.
- Raiji VR, Palestine AG, Parver DL. Scleritis and systemic disease association in a community-based referral practice. Am J Ophthalmol. 2009;148(6):946–950. doi:10.1016/j.ajo.2009.07.021.
- Galor A, Thorne JE. Scleritis and peripheral ulcerative keratitis. Rheum Dis Clin North Am. 2007;33(4):835–854. doi:10.1016/j.rdc.2007.08.002.
- Diaz JD, Sobol EK, Gritz DC. Treatment and management of scleral disorders. Surv Ophthalmol. 2016;61:702–717.
- Yagci A. Update on peripheral ulcerative keratitis. Clin Ophthalmol. 2012;6:747–754.
- Messmer EM, Foster CS. Vasculitic peripheral ulcerative keratitis. Surv Ophthalmol. 1999;43:379–396.
- Murray PI, Rauz S. The eye and inflammatory rheumatic diseases: the eye and rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis. Best Pract Res Clin Rheumatol. 2016;30(5):802–825. doi:10.1016/j.berh.2016.10.007.
- Akpek EK, Uy HS, Christen W, Gurdal C, Foster CS. Severity of episcleritis and systemic disease association. Ophthalmology. 1999;106(4):729–731. doi:10.1016/S0161-6420(99)90157-4.
- Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) working group. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140:509–516.
- Watson PG, Hayreh SS. Scleritis and episcleritis. Br J Ophthalmol. 1976;60(3):163–191. doi:10.1136/bjo.60.3.163.
- Lemp MA, Foulks GN. The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international dry eye workshop. Ocul Surf. 2007;5(2):75–92. doi:10.1016/S1542-0124(12)70081-2.
- Abu El-Asrar AM, Herbort CP, Tabbara KF. Retinal vasculitis. Ocul Immunol Inflamm. 2005;13(6):415–433. doi:10.1080/09273940591003828.
- Levitt AE, McManus KT, McClellan AL, et al. Ocular inflammation in the setting of concomitant systemic autoimmune conditions in an older male population. Cornea. 2015;34(7):762–767. doi:10.1097/ICO.0000000000000437.
- Hassan WA, Medhat BM, Youssef MM, et al. Characteristics, evolution, and outcome of patients with non-infectious uveitis referred for rheumatologic assessment and management: an Egyptian multicenter retrospective study. Clin Rheumatol. 2021;40(4):1599–1610.
- Peláez-Ballestas I, Sanin LH, Moreno-Montoya J, et al. Epidemiology of the rheumatic diseases in Mexico. A study of 5 regions based on the COPCORD methodology. J Rheumatol Suppl. 2011;86:3–8. doi:10.3899/jrheum.100951.
- Jiménez-Balderas FJ, Fernandez-Arrieta G, Camargo-Coronel A, et al. Uveitis in adult patients with rheumatic inflammatory autoimmune diseases at a tertiary-care hospital in Mexico city. J Rheumatol. 2011;38(2):325–330. doi:10.3899/jrheum.100015.
- Tsirouki T, Dastiridou A, Symeonidis C, et al. A focus on the epidemiology of uveitis. Ocul Immunol Inflamm. 2018;26(1):2–16. doi:10.1080/09273948.2016.1196713.
- Vivino FB. Sjogren’s syndrome: clinical aspects. Clin Immunol. 2017;182:48–54. doi:10.1016/j.clim.2017.04.005.
- Maldini C, Seror R, Fain O, et al. Epidemiology of primary Sjögren’s syndrome in a French multiracial/multiethnic area. Arthritis Care Res. 2014;66(3):454–463. doi:10.1002/acr.22115.
- Kabasakal Y, Kitapcioglu G, Turk T, et al. The prevalence of Sjögren’s syndrome in adult women. Scand J Rheumatol. 2006;35(5):379–383. doi:10.1080/03009740600759704.
- Rothschild PR, Pagnoux C, Seror R, et al. Ophthalmologic manifestations of systemic necrotizing vasculitides at diagnosis: a retrospective study of 1286 patients and review of the literature. Semin Arthritis Rheum. 2013;42(5):507–514. doi:10.1016/j.semarthrit.2012.08.003.
- Tugal-Tutkun I. Systemic vasculitis and the eye. Curr Opin Rheumatol. 2017;29:24–32.
- Lin P, Bhullar SS, Tessler HH, Goldstein DA. Immunologic markers as potential predictors of systemic autoimmune disease in patients with idiopathic scleritis. Am J Ophthalmol. 2008;145:3. doi:10.1016/j.ajo.2007.09.024.
- Rudwaleit M, van der Heijde D, Landewe R, Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, et al. The development of assessment of SpondyloArthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68(6):777–783. doi:10.1136/ard.2009.108233.
- Agarwal A, Karkhur S, Aggarwal K, et al. Epidemiology and clinical features of inflammatory retinal vascular occlusions: pooled data from two tertiary-referral institutions. Clin Exp Ophthalmol. 2018;46(1):62–74.
- De la Mora A, Aurrecoechea E, Del Corral AD, Calvo J. Rheumatology-ophthalmology collaborative uveitis units may improve the diagnostic and therapeutic approach of this pathology: experience from a uveitis unit in a secondary spanish hospital. J Arthritis. 2016;5(5):1–3. doi:10.4172/2167-7921.1000215.
- Fanlo P, Espinosa G, Adan A, et al. Multidisciplinary care and units for uveitis in the internal medicine departments in Spain: survey of the systemic autoimmune diseases group. Rev Clín Esp. 2021;221(4):221–225. doi:10.1016/j.rce.2019.11.018.
- Tseng ST, Yao TC, Huang JL, Yeh KW, Hwang YS. Clinical manifestations in uveitis patients with and without rheumatic disease in a Chinese population in Taiwan. J Microbiol Immunol Infect. 2017;50(6):798–804. doi:10.1016/j.jmii.2015.10.007.
- Hart CT, Zhu EY, Crock C, Rogers SL, Lim LL. Epidemiology of uveitis in urban Australia. Clin Exp Ophthalmol. 2019;47:733–740.
- Bajwa A, Osmanzada D, Osmanzada S, et al. Epidemiology of uveitis in the mid-atlantic United States. Clin Ophthalmol. 2015;9:889–901.
- Cimino L, Aldigeri R, Salvarani C, et al. The causes of uveitis in a referral centre of northern Italy. Int Ophthalmol. 2010;30(5):521–529.
- Bertrand PJ, Jamilloux Y, Ecochard R, et al. Uveitis: autoimmunity … and beyond. Autoimmun Rev. 2019;18(9):102351. doi:10.1016/j.autrev.2019.102351.
- Uribe-Reina P, Muñoz-Ortiz J, Cifuentes-González C, et al. Ocular manifestations in colombian patients with systemic rheumatologic diseases. Clin Ophthalmol. 2021;15:2787–2802.
- de-la-Torre A, López-Castillo CA, Rueda JC, et al. Clinical patterns of uveitis in two ophthalmology centres in Bogota, Colombia. Clin Exp Ophthalmol. 2009;37(5):458–466. doi:10.1111/j.1442-9071.2009.02082.x.
- Talat L, Lightman S, Tomkins-Netzer O. Ischemic retinal vasculitis and its management. J Ophthalmol. 2014;2014:197675. doi:10.1155/2014/197675.
- Soto-Vega E, García-Muñoz R, Richaud-Patin Y, et al. Class I and class II MHC polymorphisms in Mexican patients with Behçet’s disease. Immunol Lett. 2004;93:211–215. doi:10.1016/j.imlet.2004.03.017.
- Leal I, Romão VC, Mano S, et al. A non-infectious uveitis multidisciplinary clinic in a tertiary referral center: clinical impact and added value. J Multidiscip Healthc. 2021;14:695–704. doi:10.2147/JMDH.S292981.
- Hysa E, Cutolo CA, Gotelli E, et al. Immunopathophysiology and clinical impact of uveitis in inflammatory rheumatic diseases: an update. Eur J Clin Invest. 2021;51(8):e13572. doi:10.1111/eci.13572.
