ABSTRACT
Objective
To highlight the safety and efficacy of Tumor Necrosis Factor inhibitors (anti-TNF) in inflammatory choroidal neovascularization (CNV) in the pediatric population.
Design
Retrospective case series.
Participants
Three patients, < 16 years old with uveitic inflammatory CNV.
Methods
Patients received systemic steroids, methotrexate (MTX), intravitreal (IVT) injections of bevacizumab, and anti-TNF (infliximab or adalimumab) in case of refractory leakage.
Results
Five eyes of three pediatric patients (mean age 6 years old) presenting with CNV and put on anti-TNF were followed up for a minimum of 32 months. Four out of five eyes had improved vision, reduced fluid on clinical exam and macular spectral-domain optical coherence tomography (SD-OCT), and cessation of leakage on fundus fluorescein angiography (FFA) after introduction of anti-TNF agents. Two patients developed minor psoriasis treated topically.
Conclusion
Anti-TNF agents showed efficacy and safety in a sustainable leakage control of inflammatory pediatric CNV along with improvement in vision.
Choroidal neovascularization (CNV) is a rare entity in the pediatric population with few case series reported in the literature. Despite its rarity, it remains a devastating disease with significant loss of visual acuity.Citation1,Citation2 Incidence is still uncertain. The British Ophthalmological Surveillance Unit (BOSU) study reported a national incidence, all etiologies combined, of 0.21 per 100,000 per year in children younger than 16 years old with no gender predominance.Citation2 Some studies found female preponderance, whereas others found males to be more affected.Citation3,Citation4 The most common cause is an underlying inflammatory disease such as multifocal choroiditis and Vogt-Kayanagi-Harada (VKH) among others, which will be the focus of this case series.Citation1,Citation2,Citation4 Unlike adult CNV, pediatric CNV has a favorable prognosis since it is usually unique, rarely associated with degeneration of the macula and myopia, and lacks Bruch’s membrane thickening.Citation4
Through history, pediatric CNV was mainly treated by observation, photodynamic therapy (PDT), laser photocoagulation, and subfoveal surgery.Citation2 More recently, anti-vascular endothelial growth factor (anti-VEGF) agents proved their efficacy and safety in this age group.Citation2,Citation4,Citation5 Furthermore, studies showed that treatment of the underlying uveitic disease is a crucial component in controlling CNV.Citation6
We hereby report a retrospective consecutive case series revealing three pediatric patients, aged between 5 and 8 years, who received IVT anti-VEGF therapy for vision threatening CNV secondary to an inflammatory etiology combined with systemic steroids, methotrexate, and anti-TNF agents. The main aim is to study the safety and efficacy of anti-TNF in controlling CNV activity and changing the course of ocular disease.
Methods
This is a retrospective case series of three pediatric patients – five eyes – younger than 16 years old, jointly followed-up in ophthalmologic and pediatric departments at the Rothschild Foundation Hospital, Paris, France. Those patients presented CNV secondary to a strictly inflammatory, non-infectious, uveitic disease. The ophthalmologic exam consisted of medical history, best-corrected visual acuity (BCVA), slit-lamp examination along with dilated fundus examination. The diagnosis was set after paraclinical tests, such as fundus fluorescein angiography (FFA) indocyanine green angiography (ICGA), macular spectral-domain optical coherence tomography (SD-OCT), and blood tests in search for etiology. Follow-up periods ranged from 32 to 71 months.
Therapy decisions were taken in coordination among parents, uveitis specialists, and pediatric rheumatologists. The initial treatment regimen offered included a 3-day of intravenous methylprednisolone (IVMP) pulse (15 mg/kg/d), followed by oral steroids tapered gradually overtime, methotrexate (MTX), and intravitreal injections of bevacizumab, an anti-VEGF agent. Systemic anti-TNF (infliximab or adalimumab) were introduced when the disease course was judged refractory to steroids and MTX. The risks, benefits, and alternatives of each type of treatment were explained to the parents, along with the off-label use of bevacizumab, and informed consent was obtained. IVT injections were given under general anesthesia. All patients were monitored closely by clinical exam, SD-OCT, FFA, and ICGA. In the follow-up, FFA was used to look for active uveitis, vasculitis, and hypofluorescent nodules on ICGA. Retreatment with IVT bevacizumab was a case-by-case decision depending on the decline in BCVA and on the neovascular activity evaluated mainly by macular SD-OCT. Systemic treatment was tapered and adjusted in accordance with the pediatric rheumatologists, depending on the inflammatory ocular process activity.
All patients were tested for the NOD2 sequencing in the hypothesis of Blau syndrome.
Declaration of interest
The study was approved by Rothschild Foundation Hospital, Paris, France Review Board and was performed in accordance with the tenets of the Declaration of Helsinki. No financial disclosures to report.
Results
Patients’ characteristics are listed in . This includes descriptive eye disease, initial and final Snellen best-corrected visual acuity (BCVA), the type, and number of intravitreal anti-VEGF injections received, the systemic treatment introduced, the ocular and systemic complications, and the duration of follow-up.
Table 1. Baseline characteristics, management, and visual outcomes of 3 pediatric patients with CNV
BCVA improved significantly in 4 out of 5 eyes and 1 eye with low vision initially regressed by 1 Snellen chart line due to fibroglial atrophic macular scarring. All three patients had granulomatous panuveitis. One patient developed an elevated intraocular pressure (IOP) in both eyes at the beginning of treatment, which responded well to medical treatment. Two patients developed psoriasis consistent with a paradoxical effect of anti-TNF agents.
In all three cases, a temporary resolution of sub-retinal fluid on SD-OCT after IVT was noted, but only anti-TNF treatment halted neovessel recurrences. Patient 2 and patient 3 did not require any IVT injections after the introduction of adalimumab in addition to MTX; however, patient 1 was put on infliximab because of low compliance to treatment. The latter required 14 IVT injections of bevacizumab along with infliximab and methotrexate for a period of 21 months before stabilization of ocular inflammation.
The three cases are detailed in the following table to compare our experience to the literature.
Case Series
Patient 1
The first patient was a 5-year-old girl with a history of right retro-orbital bone destroying tumor found to be fibrotic and inflammatory with no granuloma on orbital pathology and repetitive right exophthalmia, which regresses on steroids. There was no past family medical history.
She was first seen in our clinics in 2014 and then lost to follow-up till July 2017. During the latter visit, BCVA was 20/63 right eye (OD) and 20/100 left eye (OS). Fundus examination and color photos (), FFA (), ICGA (), SD-OCT (), and OCT-A () revealed bilateral posterior uveitis, active choroidal neovascularization, macular cysts, posterior pole choroiditis lesions but without papillitis or vasculitis. Blood tests and chest X-ray (CXR) in search for etiology were all negative except for non-caseating granuloma on salivary gland biopsy. Hence, the diagnosis of bilateral posterior inflammation compatible with sarcoidosis complicated by active macular and juxtafoveal neovascularization was suspected.
Figure 1. Fundus color photography of patient 1 demonstrated parafoveal fibroglial scars on OD (A) and foveal fibroglial lesions on OS (B). Note the presence of subretinal hemorrhages (arrows) suggestive of CNV.
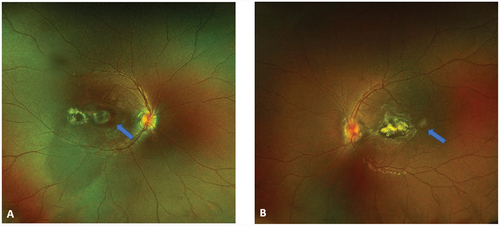
Figure 2. (A) (B) FA OD demonstrated hyperfluorescence in the early frames and late frames of CNV (yellow arrows). Note the fibroglial scars have constant hyperfluorescence. Late frame ICGA in OS (C), revealed numerous hypofluorescent spots corresponding to choroidal nodules.
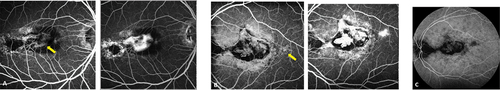
Figure 3. SD OCT (A) (B) demonstrated subretinal fibrosis, serous detachment and cystoid formations corresponding pre-epithelial choroidal neovascularization. OS (B) presence of additional macular atrophy.
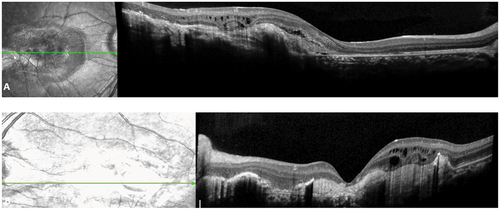
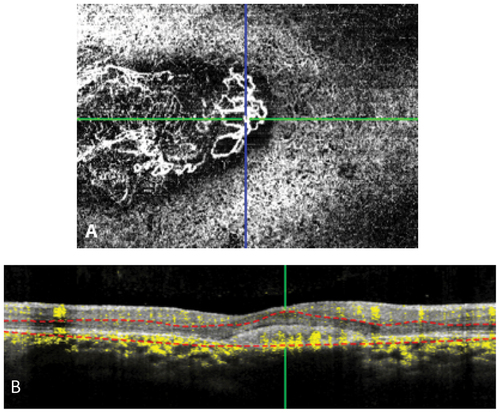
Figure 4. OCT A (A) shows CNV in the choriocapillaris layer on OS. (B) B Scan demonstrated persistent flow in CNV.
Treatment was initiated in July 2017 with a three-day loading dose of IVMP followed by 1 mg/kg/day oral prednisone with progressive tapering. Sub-cutaneous MTX 0,4 mg/kg/week and infliximab infusion (REMICADE®, 5 to 7.5 mg/kg, because of poor compliance) every 4 weeks were started enabling the withdrawal of steroids after 18 months. Bilateral IVT bevacizumab injections were given under general anesthesia as well.
The patient was then followed up for 48 months. Depending on the neovascular activity, macular exudation, and BCVA deterioration; she received 14 IVT of bevacizumab OD and 3 IVT OS. March 2019, after 21-month series of bevacizumab IVT injections, infliximab, MTX, and steroids, she received her last IVT injection.
Since then, no inflammatory activity was registered for 27 months, and no CNV exudation activity was noticed. The patient regained 20/20 of BCVA OD; however, OS had fibroglial atrophic macular and ended up with 20/200 vision.
Noteworthy, in January 2021, she developed psoriasis as a paradoxical effect of anti-TNF agents, and she was treated with steroid creams. We changed Infliximab to Adalimumab given as 1 injection every 15 days.
Patient 2
The second patient is a 5-year-old boy who presented with decrease of vision noticed at school along with intermittent strabismus OD. He had repetitive painful red eye treated with topical steroids, 2 years before presentation. Family history included Crohn’s disease.
During his first visit, in October 2015, BCVA was 20/200 OD and 20/25 OS. Slit-lamp examination showed bilateral anterior uveitis with posterior synechiae. Fundus examination showed vitritis, papillary hyperemia, and a suspicious area of neovascularization. FFA, ICGA, and SD-OCT indicated two neovascular lesions OD with hyper-reflective juxtafoveal and temporo-macular domes. Neovessels with pre-epithelial juxta papillary hyper reflectivity was also noted in OS. No subretinal fluid or mid-periphery nodular choroiditis was noted in either eye. Blood tests and CXR in search for etiology were all negative. The genetic sequencing of NOD2 was wild type. We diagnosed an idiopathic bilateral panuveitis complicated with peripapillary neovascularization and subretinal fluid OD.
Treatment was then initiated with a three-day loading dose of 15 mg/kg/d IVMP followed by 1 mg/kg/j oral prednisone and MTX 10 mg/week. Bevacizumab IVT injection under general anesthesia was done OD.
After 20 months, no inflammatory activity or exudation occurred. Therefore, there was no need for further IVT injections. During this period, we initiated amblyopia treatment with occlusion OS, after which vision improved to 20/32 OD; and steroids were tapered to 2 mg per day.
In May 2017, he consulted for a decrease in vision OD to 20/63, and active neovascularization was noted on clinical exam. At this point, steroid dose was increased 10 mg/day and an IVT injection of bevacizumab was performed OD. The exudation improved with every IVT injection later but there were recurrences of inflammatory activity. Therefore, we increased MTX to 12,5 mg/week. The patient needed 3 IVT injections of bevacizumab up until March 2018. At the latter visit, an increase in the inflammatory activity was noted with a higher number of hypofluorescent nodules appearing on ICGA. Hence, adalimumab (HUMIRA®) 40 mg/15 days was added to his current treatment.
From March 2018 until August 2021, no inflammatory activity was noted, the nodules disappeared, and minor exudation disappeared with no additional treatments or IVT injections. BCVA was maintained at 20/32 OD, steroids were stopped in November 2018, MTX was interrupted in September 2019. We were able to augment spacing of adalimumab to once a month without any relapse of inflammation.
At his last evaluation to date August 2021, we registered 42 months with no inflammatory activity and 23 months of stability on adalimumab monotherapy. The patient overall regained visual acuity to 20/32 OD.
Patient 3
The third patient is an 8-year-old girl who presented with a bilateral decrease in vision that started 3 months prior to the visit. There was no personal or family history.
BCVA at initial visit was 20/63 in both eyes (OU). Slit lamp examination revealed bilateral anterior uveitis with posterior synechiae. Fundus examination showed vitritis with mid-periphery multifocal choroiditis lesions. Macular SD-OCT indicated an inter-papillomacular active neovessels OD and subfoveal fibrotic neovessels with lower activity OS.
Treatment began with a three-day loading dose of 15 mg/kg/d IVMP followed by 1 mg/kg oral prednisone and MTX 12.5 mg per week. Bilateral IVT bevacizumab injections under general anesthesia were initiated as well. During follow-up, an elevated intraocular pressure (IOP) was noted (30 mmHg OD and 26 mmHg OS) but controlled under local treatment. After the first injection in February 2019, imaging revealed neovascular activity OD but stable fibrogliotic lesion OS. Three other IVT injections OD were performed due to relapsing neovascular activity, along with occlusion of the right eye for left-eye amblyopia treatment. The last IVT injection was in November 2019, and she was stable after on MTX and steroid tapering to 2 mg per day. BCVA improved to 20/20 OD and 20/32 OS.
In June 2020, BCVA dropped to 20/25 OD due to macular exudation. The patient also showed MTX digestive intolerance. It was tapered and adalimumab (HUMIRA®) 40 mg every 15 days was introduced. MTX and steroids were stopped in December 2020. Since the introduction of adalimumab in June 2020 up until the last visit in August 2021, no inflammatory activity was detected, and BCVA improved to 20/20 OD and 20/32 OS, respectively.
Noteworthy, in August 2021, she developed psoriasis on her hands and feet. She was treated with a steroid cream and the adalimumab injections were spaced to 3 weeks’ interval.
Discussion
We hereby present 5 eyes of three pediatric cases with CNV of uveitic origin, treated based on its location and activity. Our study shows the importance of initiating a rapid and aggressive therapy for a better recovery of VA, and control of inflammation and neovascularization. The treatment regimen consisted of a three-day loading dose of 15 mg/kg/d IVMP followed by 1 mg/kg/d oral prednisone tapered according to the inflammatory activity. Due to severe uveitis in each child, early systemic immunosuppression (MTX 10–15 mg/m2/week) was added under the supervision of a pediatrician in the attempt to stabilize the severe inflammation and limit the use of steroids and their well-known complications. The use of IVT bevacizumab for the treatment of the CNV resulted in temporary improvement of VA and a significant resolution of fluid on OCT. The focus of this case series is to highlight the benefit of anti-TNF (infliximab or adalimumab) in sustaining a quiescent CNV, controlling the inflammatory process, avoiding multiple general anesthesia in young children, and recuperating a good visual acuity.
The largest study conducted regarding CNV in the pediatric population by Finn et al.Citation7 found an incidence of 0.04% all causes combined. The study focused on descriptive data and the anti-VEGF treatment.Citation7 Our case series is a continuity to their results and given the rarity of this entity we aimed our findings on patients who had bilateral inflammation and neovascularization. The latter requires a systemic control during the whole treatment process along with local treatment.
In our series, 4 out of 5 eyes showed an improvement in VA with reduction of fluid on clinical exam and SD-OCT, and cessation of leakage on FFA. VA in one out of the five eyes deteriorated since a fibroglial scar developed.
Despite its rarity in the pediatric population, CNV is an added burden in the course of an inflammatory disease. An inflammatory process disrupts the homeostasis between the retinal pigment epithelium (RPE) and Bruch’s membrane that produces soluble mediators (VEGF, IL1b, IL6, TNF-a) provided by macrophages.Citation8 The latter can trigger angiogenesis whenever there is a disparity between their inhibitory and stimulatory actions.Citation8
As stated before, this entity was treated by observation, photodynamic therapy (PDT), laser photocoagulation, and sub-foveal surgery up until the emergence of anti-VEGFs. It is unpredictable which CNV will progress and lead to visual impairment and which one will resolve spontaneously.Citation9
VEGF is an essential component in healthy angiogenesis, controlled permeability of vessels, brain endothelium development, neural cells signaling, and in the blood–brain barrier integrity.Citation4,Citation9 Hence, the use of anti-VEGF agents in the pediatric population was controversial up until safety was established through several long- and short-term studies.Citation4,Citation5,Citation9 A study on anti-VEGF injections for retinopathy of prematurity done on 153 eyes followed-up for 30.9 ± 18.4 months, showed one case of retinal detachment, one macular dragging, and one cataract; all without statistical significance.Citation10 No systemic complications occurred either.Citation10 The safety of these injections was also seen in our case series. No related side effects were reported for a median of 41 months of follow-up.
According to the literature, the pediatric population may require less anti-VEGF injections to stabilize the CNV compared to adults.Citation2 Padhi et al.Citation5 studied 43 eyes of pediatric CNV cases and reported the need for 2.11 injections on average. Besides, Kozak et al.Citation11 included 45 eyes and mentioned the need for a single injection in 60% of the uveitic cases. Finn et al. mentioned that 68.4% of eyes received 3 or less injections and 38.2% of eyes required a single injection, all etiologies of CNV combined.Citation7
In our current case series one eye required many injections (14/eye) and the others required 5 or less, with 1 eye stabilizing and regaining significant amount of vision after 1 injection. The need for multiple anti-VEGF injections in patient 1 can be explained by the delayed treatment due to loss of follow-up between 2014 and 2017, and to the severity of the ocular inflammatory process. We hypothesize that the need for more injections in our case series as compared to the literature is because it is focused on an ongoing systemic inflammatory process as compared to the literature where all causes of pediatric CNV were combined. Noteworthy is that every IVT injection subjects the child to general anesthesia (GA), hence modifying the treatment strategy by reducing the number of injections is an important point to consider to limit subjecting children to GA.
BOSU study showed that eyes with macular CNV treated with anti-VEGF had VA improvement comparable to that of untreated eyes.Citation2 On the contrary, Rishi et al. highlighted the need for timely treatment since treated eyes showed better improvement than observation-only eyes.Citation4 In our case series, leakage stopped in 5 out of 5 eyes with bevacizumab injections and 4 out of 5 eyes showed significant improvement of vision as well. One patient only regressed into scarring. Moreover, macular SD-OCT objectified the resolution of subretinal fluid due to anti-VEGF treatment that contributed to the improved vision. However, the effect of anti-VEGF treatment was not sustainable, and recurrences were common in the 3 patients. In younger uveitic patients, higher recurrences of the initial disease and the neovascularization prompt extended follow ups.Citation1 This was seen in our case series that showed the importance of regular follow-ups to control the CNV activity and initiate prompt re-injections.
Kramer et al.Citation6 showed that bevacizumab IVT is efficient for CNV of inflammatory origin if the underlying pathology is well managed. A case series of 10 pediatric patients with inflammatory origin CNV concluded that the resolution of the latter depends on systemic managements, such as immunosuppression along with local anti-VEGF treatment.Citation1 They concluded that, in the aim of better visual outcomes an early and aggressive uveitic disease control is required.Citation1 Another study highlighted that oral corticosteroids diminished leakage from vessels thus stabilizing VA in choroiditis.Citation12 It was proven that steroids weaken the inflammatory factor contributing to neovessel formation thus preventing their development.Citation13 Moreover, they are efficient on limiting proliferating CNV by stabilizing the basement membranes, reducing permeability and leakage, and leading to the resolution of CNV.Citation12,Citation14 A study reports effective control of CNV in the pediatric population when inflammation is treated with systemic corticosteroids with or without additional immunosuppressive medication.Citation15 That being mentioned, we highlight the importance of aggressive initial corticosteroid treatment and tapering over time; however, in severe disease a broader treatment will be required.
The latest publication on the updates in the treatment of chronic non-infectious uveitisCitation16 in children mentioned the necessity of prompt treatment with disease-modifying anti-rheumatic drugs (DMARDs) and biological agents, especially for severe presentations. Higher doses, early in the course of the disease contribute to a well-controlled uveitic disease and better VA.Citation16 Methotrexate being the first in line to be prescribed spares children from devastating complications of the long-term treatment with steroids.Citation16 In our case series, all were started early on MTX along with steroids and bevacizumab IVT injections. Despite this optimal treatment, leakage from CNV kept recurring after different time intervals. One patient had digestive intolerance to MTX after 16 months of treatment.
Also, in all patients, we realized that an additional agent was needed to control the CNV leakage and maintain a dry retina. Among immunomodulatory biologics, anti-TNF as well as anti-IL6 were discussed with pediatricians. Monoclonal anti TNFs, particularly Adalimumab, were chosen due to the combination of their high efficacy in pediatric uveitis and their tolerance in children shown in recent recommendations.Citation16 According to the APTITUDE study, sub-cutaneous anti IL6 did not reach the primary end point in phase 2 trial in pediatric refractory uveitis and consequently not selected for the treatment of our study population.Citation17 Adalimumab and infliximab are anti-TNF-alpha agents used primarily if the uveitic disease is considered severe and complicated.Citation18 Both may control inflammatory recurrences, maintaining a good VA, reducing uveitic ophthalmic complications, and reducing retinal vasculitis.Citation18,Citation19 For instance, Ho et al. showed the efficacy of Adalimumba in reducing relapse episodes in five pediatric patients with uveitis related to Behcet’s disease from a mean of 5 relapses to a mean of 0.2 after initiation of this treatment in 2 years time.Citation20
In our case series, judging by the severity of the uveitic cases with neovascular complications and recurrences, despite systemic steroid and MTX, reduced VA, and the critical age with the risk of amblyopia, we decided to timely start anti-TNF agents. Patient 1 had a history of non-compliance to treatment and follow-ups, so we decided to add monthly infliximab. Her disease activity was high and required the continuation of IVT bevacizumab. After 6 months, we were able to space out the IVT injections up to a complete stop in 11 months and CNV activity ceased thereafter. Patients 2 and 3 faced a period of refractory uveitis and CNV recurrences but showed prompt disease stability after the beginning of adalimumab with no need for IVT bevacizumab injections afterward and an improvement of VA.
Two patients developed paradoxical psoriasis due to the anti-TNF treatment. Several authors propose the withdrawal of the Anti-TNF only in severe cases of paradoxical psoriasis where more than 5% of the body surface is affected.Citation21 The addition of immunosuppressant therapy (such as methotrexate) or switching to another agent was found to be beneficial in controlling this phenomenon in its less severe forms.Citation21
The limitation of our series is its retrospective nature as well as the limited availability of articles on pediatric non-infectious CNV and anti-TNF’s treatment. Nevertheless, our cases suggest, as elicited, that anti-TNFs by controlling the inflammatory process of choroiditis is efficient to stop or at least decrease CNV activity. More cases are required to confirm the efficacy of those agents on the long term.
Based on our case series of 5 eyes we can conclude that anti-TNF medication stops leakage from inflammatory CNV: hence, reducing the need for anti-VEGF IVT injections as well as general anesthesia and improving vision. The addition of those treatments allows us to withdraw the steroids and eventually the MTX. We also realized that minor retinal cysts regressed with adalimumab only without the need for IVT anti-VEGF or re-introduction of steroids.
The significant response of the disease to the anti-TNF treatments leads us to hypothesize that a specific molecule of choice is important for a rapid and sustainable control of the inflammation and the neovascularization. However, we cannot confirm the superiority of the adalimumab over the infliximab because of the small sample size and the severe initial presentation in patient 1. This notion requires further studies to be proven.
In the pediatric population, prompt treatment of the main disease and eye occlusion are required to avoid amblyopia.
Conclusion
Choroidal neovascularization, as a complication of pediatric non-infectious uveitis disease, is a rare but sight-threatening, quality of life affecting condition. In this case series, anti-TNF agents showed efficacy in a sustainable leakage control of the CNV and in improvement of vision. However, more evidence is required to have a more conclusive treatment regimen in such cases.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ganesh SK, Ahmed AS. Pediatric inflammatory CNV: a case series from a tertiary referral centre. Ocul Immunol Inflamm. 2020 Jan 2;28(1):26–32. doi:10.1080/09273948.2019.1576909.
- Moosajee M, Abbouda A, Foot B, Bunce C, Moore AT, Acheson J. Active surveillance of choroidal neovascularisation in children: incidence, aetiology and management findings from a national study in the UK. British J Ophthalmol. 2018 Apr 1;102(4):438–443. doi:10.1136/bjophthalmol-2017-310445.
- Barth T, Zeman F, Helbig H, Oberacher-Velten I. Etiology and treatment of choroidal neovascularization in pediatric patients. Eur J Ophthalmol. 2016 Sep;26(5):388–393. doi:10.5301/ejo.5000820.
- Rishi P, Gupta A, Rishi E, Shah BJ. Choroidal neovascularization in 36 eyes of children and adolescents. Eye. 2013 Oct;27(10):1158–1168. doi:10.1038/eye.2013.155.
- Padhi TR, Anderson BJ, Abbey AM, et al. Choroidal neovascular membrane in paediatric patients: clinical characteristics and outcomes. British J Ophthalmol. 2018 Sep 1;102(9):1232–1237. doi:10.1136/bjophthalmol-2017-310497.
- Kramer M, Axer-Siegel R, Jaouni T, et al. Bevacizumab for choroidal neovascularization related to inflammatory diseases. Retina. 2010 Jun 1;30(6):938–944. doi:10.1097/IAE.0b013e3181c96a00.
- Finn AP, Fujino D, Lum F, Rao P. Etiology, treatment patterns, and outcomes for choroidal neovascularization in the pediatric population: an intelligent research in sight (IRIS®) registry study. Ophthalmol Retina. 2022 Feb 1;6(2):130–138. doi:10.1016/j.oret.2021.05.015.
- Neri P, Lettieri M, Fortuna C, Manoni M, Giovannini A. Inflammatory choroidal neovascularization. Middle East Afr J Ophthalmol. 2009 Oct;16(4):245. doi:10.4103/0974-9233.58422.
- Kohly RP, Muni RH, Kertes PJ, Lam WC. Management of pediatric choroidal neovascular membranes with intravitreal anti-VEGF agents: a retrospective consecutive case series. Can J Ophthalmol. 2011 Feb 1;46(1):46–50. doi:10.3129/i10-123.
- Kang HG, Choi EY, Byeon SH, et al. Anti-vascular endothelial growth factor treatment of retinopathy of prematurity: efficacy, safety, and anatomical outcomes. Korean J Ophthalmol. 2018 Dec 1;32(6):451–458. doi:10.3341/kjo.2018.0011.
- Kozak I, Mansour A, Diaz RI, et al. Outcomes of treatment of pediatric choroidal neovascularization with intravitreal antiangiogenic agents: the results of the KKESH international collaborative retina study group. Retina. 2014 Oct 1;34(10):2044–2052. doi:10.1097/IAE.0000000000000200.
- Flaxel CJ, Owens SL, Mulholland B, Schwartz SD, Gregor ZJ. The use of corticosteroids for choroidal neovascularisation in young patients. Eye. 1998 Mar;12(2):266–272. doi:10.1038/eye.1998.62.
- Crum R, Szabo S, Folkman J. A new class of steroids inhibits angiogenesis in the presence of heparin or a heparin fragment. Science. 1985 Dec 20;230(4732):1375–1378. doi:10.1126/science.2416056.
- Folkman JU, Ingber DE. Angiostatic steroids. Method of discovery and mechanism of action. Ann Surg. 1987 Sep;206(3):374. doi:10.1097/00000658-198709000-00016.
- Dees C, Arnold JJ, Forrester JV, Dick AD. Immunosuppressive treatment of choroidal neovascularization associated with endogenous posterior uveitis. Arch Ophthalmol. 1998 Nov 1;116(11):1456–1461. doi:10.1001/archopht.116.11.1456.
- Sood AB, Angeles-Han ST. An update on treatment of pediatric chronic non-infectious uveitis. Current Treat Options Rheumatol. 2017 Mar 1;3(1):1–6. doi:10.1007/s40674-017-0057-z.
- Ramanan AV, Dick AD, Guly C, et al. Tocilizumab in patients with anti-TNF refractory juvenile idiopathic arthritis-associated uveitis (APTITUDE): a multicentre, single-arm, phase 2 trial. Lancet Rheumatol. 2020 Mar 1;2(3):e135–41. doi:10.1016/S2665-9913(20)30008-4.
- Fabiani C, Vitale A, Rigante D, et al. Comparative efficacy between Adalimumab and infliximab in the treatment of non-infectious intermediate uveitis, posterior uveitis, and panuveitis: a retrospective observational study of 107 patients. Clin Rheumatol. 2019 Feb 14;38(2):407–415. doi:10.1007/s10067-018-4228-6.
- Aardoom MA, Veereman G, de Ridder L. A review on the use of Anti-TNF in children and adolescents with inflammatory bowel disease. Int J Mol Sci. 2019 Jan;20(10):2529. doi:10.3390/ijms20102529.
- Ho M, Chen LJ, Sin HP, et al. Experience of using Adalimumab in treating sight-threatening paediatric or adolescent Behcet’s disease-related uveitis. J Ophthalmic Inflamm Infect. 2019 Dec;9(1):1–7. doi:10.1186/s12348-019-0181-z.
- Iborra M, Beltrán B, Bastida G, Aguas M, Nos P. Infliximab and Adalimumab-induced psoriasis in Crohn’s disease: a paradoxical side effect. J Crohn’s Colitis. 2011 Apr 1;5(2):157–161. doi:10.1016/j.crohns.2010.11.001.
