ABSTRACT
Will optical coherence tomography angiography (OCTA) replace invasive imaging techniques like fundus fluorescein angiography (FFA) and indocyanine green (ICG) angiography entirely? While OCTA is being increasingly applied in the field of medical retina, will we see this change in the subspeciality of uveitis? In this article, five uveitis specialists with renowned imaging expertise answer to 10 specific questions to address this issue. The final verdict based on the comments of the experts suggests that FFA and ICG cannot be replaced by OCTA in uveitis, at least for now. While OCTA can offer new insights into the pathogenesis of certain inflammatory conditions and help in the diagnosis of complications like inflammatory choroidal neovascularisation, multimodal imaging is still the preferred approach in the assessment of patients with uveitis.
Optical coherence tomography (OCT) became available in clinical practice in the early 2000s and in 10 years revolutionised the world of ophthalmology.Citation1 Uveitis specialists were not among the first to appreciate its potential uses as for years they used OCT only for the evaluation of complications of inflammatory conditions like macular edema or epiretinal membranes.Citation2 Despite this, nowadays there is no uveitis clinic which could properly manage their patients without an OCT device.
Invasive imaging techniques like fundus fluorescein angiography (FFA) and indocyanine green (ICG) angiography have been traditionally more appreciated by uveitis specialists. Physical and chemical features the two dyes are responsible for their ability to highlight alterations affecting different ocular structures. In fact, approximately 30% of fluorescein is not bound to plasmatic proteins allowing the dye to leak through altered blood-retinal barriers.Citation3 On the other hand, fully bound indocyanine green cannot exit from fenestrated capillaries like those of the choriocapillaris and can be stimulated by near-infrared light,Citation4 which easily penetrates the retinal pigment epithelium, making it the perfect dye to investigate the choroid.Citation5
About 10 years ago, a new non-invasive technique called OCT angiography (OCTA) based on OCT technology but with the ability to combine flow information with structural data became available, allowing clinicians to obtain information about retinal and choroidal perfusion with no need for dye injection. OCTA was welcomed with great enthusiasm, likely due to the undisputable utility of its predecessor OCT and soon gained worldwide distribution.Citation6
The ability of OCTA to detect and characterise retinal ischemia and neovascular structures made it so useful for the evaluation of medical retinal diseases to make the use of FFA and ICG angiography redundant in many cases. However, OCTA and FFA/ICG angiography are not the same.Citation2 In fact, OCTA uses the signal coming from moving elements (i.e. blood cells) to reconstruct the vessels architecture but without injecting a dye there is no leakage, so blood–retinal barrier breakdown, and consequently inflammation, is hard to be detected with OCTA.Citation2
The thick choroid also remains a challenge to be correctly investigated due to OCT signal absorption and wide field/peripheral OCTAs have a long acquisition time and high level of collaboration from patients is necessary for images to be properly acquired and interpreted.Citation6 For these and other reasons, many uveitis specialists still believe OCTA cannot completely replace invasive imaging at least in the evaluation of uveitis patients.
The debate on whether we will still be using FFA and ICG angiography in the future or whether we will completely rely on non-invasive OCTA in the management of uveitis is more than open and in search of an answer, we tried to address 10 major questions on PROS and CONS of these imaging techniques.
Experts views
(1) Which are the advantages of FFA over OCTA in uveitis? (Answers: M. R. Munk)
Needless to say, the biggest advantage of FFA over OCTA is the ability to assess leakage and disease activity in uveitis. Leakage on FA is highly specific and sensitive for clinical disease activity in uveitis.Citation7 Vasculitis, macular edema, a hot disc and peripheral vascular leakage are important parameters for disease activity. All of them can be reliably assessed and detected using FFA, while OCTA is not directly capable of assessing these features (). Wide-field FA and ultrawide field FA have significantly impacted the way we follow our uveitis patients and have a significant impact in our treatment decisions.Citation8 The presence of peripheral vascular leakage visible on WF-FFA increases the likelihood to initiate or increase the treatment by four times.Citation9 Peripheral vascular leakage also plays a crucial role in the identification of secondary complications in active intermediate uveitis, such as peripheral retinoschisis or peripheral vasoproliferative tumours.Citation10,Citation11
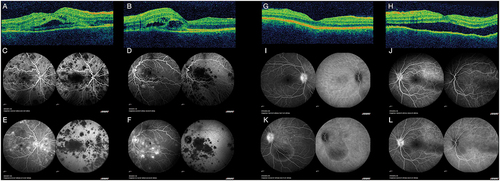
Figure 1. A-B: OCT showing bilateral (A: right eye; B: left eye) bacillary detachments with foveal involvement. C-F: Fluorescein angiography (left) and indocyanine green angiography (right) of both eyes (C&E: right eye; D&F: left eye) disclosing early (C-D) hypofluorescent lesions that become hyperfluorescent in the late frames (E-F) in fluorescein angiography, with persistent hypofluorescent lesions along the angiographic time in the indocyanine green angiography, suggestive of acute posterior multifocal placoid pigment epiteliopathy (APMPPE). G-H: OCT showing bilateral (G: right eye; H: left eye) choroidal folds with a shroud retinal detachment in the left eye. I-L: Fluorescein angiography (left) and indocyanine green angiography (right) of both eyes (I&K: right eye; J&L: left eye) disclosing bilateral optic nerve leakage and pin point pattern with filling of the serous retinal detachments in the fluorescein angiography. The indocyanine green angiography shows shadowing of the serous detachments and subtle scatter hypofluorescent lesions. The angiography findings suggest a diagnosis of Vogt-Koyanagui-Harada syndrome.
Although OCTA is capable of depicting ischemia and areas of flow void, the field of view is usually not broad enough to display peripheral capillary nonperfusion. The amount of peripheral capillary nonperfusion, however, is important to predict the risk of developing neovascularisation in retinal vasculitis. More than 3 clock hours of peripheral capillary non perfusion on UWF-FFA is associated with the development of retinal neovascularisation.Citation12
Based on FFA, the main site of retinal vasculitis can be identified, which can significantly help in the differential diagnosis. While a (peri)phlebitis can be found in patients with Birdshot, multiple sclerosis, Sarcoidosis, TB, Syphilis and pars planitis, arteritis will be seen due to herpetic causes, Behçet’s, SLE, SUSAC syndrome, Eales and Sarcoidosis. Peripheral vascular leakage may point into the direction of pars planitis, multiple sclerosis, IBD, Sarcoidosis and Lyme disease.
Finally, dye-based angiography is a dynamic exam allowing to investigate arterio-venous filling and to identify flow delays if any. These features which can be extremely helpful in the management of vasculitis cannot be seen for now by a static imaging technique like OCTA.Citation2
Despite all the advantages listed here, it must be said that OCTA is a recent imaging tool, and we are still learning about its impact, predictive value and applicability in uveitis.
(2) Which are the advantages of ICG over OCTA in uveitis? (Answers: E. Carreño)
OCTA has shown to be a very useful tool to assess the choroid and choriocapillaris in uveitis. In fact, when compared to ICG, the flow deficit in OCTA at the level of the choroid disclosed the same size in toxoplasma retinochoroiditis.Citation13 However, with the current available OCTA devices the field of imaging is very limited in comparison with wide field or ultra-wide field ICG angiography. Therefore, it could be crucial to perform ICG angiography at least at the time of diagnosis to get a better picture of the extension of the choroidal disease.
Similarly, in those diseases where subretinal fluid is the key feature, such as Vogt–Koyanagi–Harada (VKH) disease, acute posterior multifocal placoid pigment epitheliopathy (APMPPE)Citation14 or posterior scleritis, the segmentation of the OCTA may be inaccurate making the images difficult to interpret, as it is also the case in other diseases like diabetic macular edema.Citation15,Citation16 In those situations ICGA becomes fundamental for clarification of the differential diagnosis. ()
(3) Which are the advantages of OCTA compared to dye-based investigations in uveitis? (Answers: A. Invernizzi)
The major advantage of OCTA compared to dye-based investigations derives in fact from one of its greatest limitations: the lack of leakage. In fact, both neovascular and non-neovascular inflammatory lesions usually show leakage of dye on FFA making it hard to distinguish them from each other.Citation2 This is particularly true in case of uveitis forms affecting the retina, the retinal pigment epithelium and the choroid, like in multifocal choroiditis (MFC). The absence of leakage in the OCTA allows for clear visualisation of the vascular network characterising a neovascular process, in distinction from inflammatory foci where no vascular structures will be seen.Citation17 ()
Figure 2. Multimodal Imaging in a patient affected by multifocal choroiditis (MFC) with multiple active lesions.
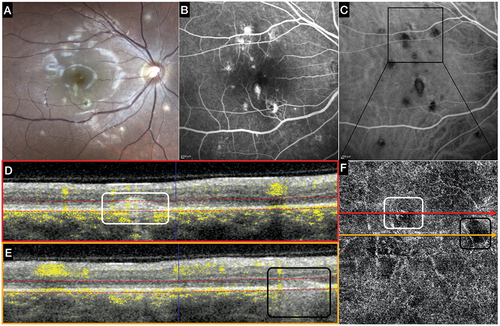
A second advantage is the high resolution of OCTA images and the possibility to combine perfusion (functional) and tomographic (structural) information. This allows an assessment and localisation of vascular alterations in far more detail than that possible with FFA/ICG angiography. A good example of this disparity is the possibility to visualise with OCTA the deep retinal plexus, which is not visible in FFA. Many studies in fact report a reduction in the vascular density at this level in inflamed eyes which was undetectable before the introduction of OCTA in clinical practice.Citation18
Finally, OCTA is completely non-invasive, allowing clinicians to repeat it many more times compared to FFA/ICG angiography, even in a busy clinic.
(4) Are there specific diseases where OCTA has revolutionised uveitis specialists’ clinical practice? (Answers: F. Pichi)
The main point that OCTA detractors raise when arguing against its utility in uveitis, as already mentioned above, is its inability to assess vascular leakage, one of the hallmarks of retinal inflammation that can be easily detected through fluorescein angiography. While various OCTA studies have shown a significantly lower parafoveal capillary density and lower branching complexity in the superficial retinal plexus of uveitic compared to healthy eyes, the clinical application of these findings for a uveitis specialist dealing with a vasculitis patient is dubious. On the other hand, the new OCTA machines allow for composite images that encompass up to 90° of the retina and enable clinicians to assess the vascularisation of the peripheral retina, thus looking for areas of ischemia and neovascularisation that can complicate vasculitis without the need to inject intravenous dye.
Of note, many papers have focused on the usefulness of OCTA in investigating patients with Behcet disease (BD) as this uveitis is often characterised by capillary impairment, an alteration very precisely assessed by OCTA. According to recent meta-analysis summarising the available results on this topic patients with BD show reduced perfusion affecting both the superficial and deep capillary plexa in the macular region and a larger foveal avascular zone compared to controls. The authors conclude stating that OCTA could hence assist clinicians in the diagnosis and management of these patients.Citation19
As uveitis specialists we often deal with diseases affecting the choroid, in terms of inflammatory masses (such as granulomas) or inflammatory decreased blood flow. The clinical standard for the detection of choroidal inflammatory pathology has been for years indocyanine green angiography (ICGA). However, ICGA is time and labour intensive, requires the skilled administration of intravenous dye that may be associated with rare but serious side effects and necessitates the capture of flow at appropriate time frames. Swept source OCTA, with its longer wavelengths (near 1000 nm), improves RPE penetration and allows visualisation of the choroidal vasculatureCitation20 and has been recently employed with great success to detect and follow choroidal uveitis entities without the use of intravenous dye. Our groups have shown that SS-OCTA has a detection rate of 94.2% of choroidal granulomas identified in ICGA, with good agreement between observers in identifying the space-occupying lesions.Citation21 However it has to be underlined that healed choroidal granulomas can leave a flow deficit in the choroid which can be indistinguishable from the active lesion on OCTA. As such a combined approach including ICGA is always recommended in these patients.Citation22
Various groups have utilised OCTA technology to investigate the level of disease in placoid related disorders (APMPPE, RPC, PPM) and to assess the choroid for decreased microvascular blood flow.Citation23,Citation24 OCTA shows evidence of inner choroidal or choriocapillaris flow reduction or ischemia that correspond to the area of hypo-fluorescence on ICGA. These studies indicate that OCTA may provide an important non-invasive, fast and simple imaging modality to guide diagnosis, prognosis and therapeutic response in patients with choroidal vascular uveitic disorders.
(5) Are there uveitis entities which do not benefit from OCTA examination? (Answers: A. Agarwal)
There are several precautions one must take when assessing the retina and choroid using OCTA in uveitis. While OCTA is useful in assessing the choriocapillaris in patients with choriocapillaritis (such as tubercular serpiginous-like choroiditis, punctate inner choroidopathy, and other white dot syndromes)Citation25 and stromal choroiditis (such as VKH disease and tubercular/sarcoid choroidal granulomas),Citation26,Citation27 there is limited benefit of OCTA over conventional FA in patients with infectious necrotising retinitis. For instance, in patients with acute retinal necrosis (ARN), or retinitis due to cytomegalovirus, FA is helpful in detecting intense leakage from the lesion and studying accompanying arteriolitis.Citation28 However, OCTA may show apparent areas of flow deficit especially in the deeper retina and choroid due to high hyper-reflectivity of the necrotic inner retina.Citation29 In addition, retinal necrosis may severely compromise the segmentation leading to false interpretation of abnormal retinal vasculature.
While there are studies that show that there may be retinal vascular compromise in patients with anterior uveitis such as HLA B27-associated uveitis,Citation30 or tubulointerstitial nephritis-associated uveitis,Citation31 this may not be clinically helpful in either changing the therapeutic regimen or tapering/increasing ongoing immunosuppression. These features may be relevant from a clinical research point of view but may not change management decisions in the clinics.
Entities such as birdshot retinochoroiditis frequently present with severe inflammation associated with media haze due to dense vitritis.Citation32–34 Thus, these eyes may not be amenable to good quality OCTA imaging due to these factors, as well as poor patient fixation (due to low vision). Therefore, though OCTA may be able to provide useful information related to the choroidal inflammation in birdshot retinochoroiditis, it may not be possible to perform OCTA. Due to similar reasons, OCTA is also of limited utility in endophthalmitis and other severe inflammations with media haze.
OCTA has no value in establishing a diagnosis in challenging situations, where there may be uncertainties in the aetiology. There are no publications so far that have shown the utility of OCTA in distinguishing between any two uveitic entities (unlike OCT which can help in distinguishing lesions and therefore, suggesting a particular aetiology). Thus, OCTA should not be employed as a corroborative test in establishing a diagnosis in the clinic.
(6) Is OCTA easier to interpret compared to FFA/ICGA in uveitis? What about artefacts? (Answers: A. Invernizzi)
Reading OCTA images is not easy. Being based on OCT technology, OCTA suffers from the same potential limitations and artefacts of this technique like shadowing, increased transmission and signal absorption. On top of this, flow visualisation is obtained through a software-based elaboration of two OCT scans acquired at different times. As such many artefacts can affect it and generate false positive and false negative flow signals.Citation35
Another layer of complexity is given by the fact that OCTA images have to be “explored” through different spatial plans and at different depths. A lesion can be completely missed if the wrong slab is selected.Citation36 As such, a correct interpretation of the exam requires time and a combined evaluation of the en-face image along with the B-scan showing the segmentation depth.Citation2 On the other hand, there is no need to account for the differences between images acquired at different times as OCTA is not a dynamic technique, as for FFA/ICG angiography.
In conclusion, many young clinicians find OCTA easier to read, while more senior uveitis specialists who used to rely on FFA/ICG angiography in the pre-OCT era still find these techniques more reliable. This difference is likely related just to a matter of habit. In fact, both approaches require a high level of expertise to be correctly interpreted, especially in uveitis which can be characterised by a wide range of alterations at many levels from the vitreous to the choroid.
(7) Which imaging modality do you perform at baseline/follow-up examination while assessing a uveitis patient? (Answers M. R. Munk)
This depends on the initial presentation and main site of inflammation and varies from patient to patient. In general, considering a patient with intermediate, posterior or panuveitis, I will perform a wide field CF image, an (EDI) macular OCT volume scan and a peripapillary ring scan at baseline. In intermediate uveitis ± retinal vasculitis I will also perform a wide field FFA to assess ischemia, vasculitis, peripheral vascular leakage and the presence of a hot disc.
In case of ONH swelling and vasculitis of the larger vessels, I usually add a 12 × 12 or 15 × 9 OCT SS OCT. I compare the retinal thickness maps of these scans with the FFA images. If I appreciate perivascular thickening and peripapillary thickening on the OCT thickness maps, which corresponds to the leakage seen on FFA, then I will use these thickness maps to follow the patient and guide the treatment.Citation37 In case of ischemia present within 100 degrees of FOV on FFA, I will add a montage wide field OCTA. I will then use this image modality to monitor potential progression or regression of flow void in a non-invasive way.
In case of posterior and panuveitis I will do a wide field ICG and FFA at baseline. If I appreciate hypo-fluorescence in the mid and/or late frames on ICG a wide field SS-OCTA will be added. If ICG hypo-fluorescence corresponds to areas of flow voids visible on the CC and/or choroidal slabs,Citation38 then I will stick mainly to this non-invasive image modality to monitor treatment response or disease progression at the level of the choroid.
In case of posterior uveitis affecting the posterior pole, and any suspicion of a potential secondary CNV, I will add a high resolution 6 × 6 or 9 x 9 mm OCTA to assess the potential presence of a secondary CNV.Citation39 OCTA images will be also performed in case of a steroid induced CCS, especially if a double layer sign is appreciated on OCT in the presence of (persistent) subretinal fluid. This helps to rule out any underlying potential secondary CNV as well.
(8) We know that FFA and OCT are commonly used in clinical trials, do you think OCTA could serve as an extra parameter in randomised clinical trials? If so, which OCTA parameter do you think will be the best biomarker to monitor disease activity? (Answers: F. Pichi)
At present, OCTA is not ready to find a place as imaging modality in randomised clinical trials (RCTs) because of several obstacles that are delaying its standardisation.
First, and most importantly, there is a lack of consensus on the nomenclature of its findings.Citation40 How can we use OCTA to study, as an example, the ischemic areas of the choriocapillaris in diseases such as APMPPE if one centre calls the lack of OCTA signal “flow voids,” another centre calls them “flow deficits” and a third one “non detectable flow signals”?Citation6,Citation41 Uveitis specialists are currently working hard on standardising the nomenclature of OCTA findings to give this new imaging modality a solid ground to build upon.
A second obstacle is the lack of agreement on the best way to measure areas of decreased OCTA signal, whether in the retina or choroid. Several reportsCitation42–45 have used the inbuilt software of the device to quantify signal attenuation in uveitis, but these values are not comparable among devices, even if there is a good, reported repeatability in these measurements with the same device. To obviate this problem, some researchersCitation46 have employed external processing tools such as MATLAB (Math Works, Inc., Natick, Massachusetts) or ImageJ (https://imagej.nih.gov/ij/) which result in more accurate and comparable data with similar image processing. However, due to many reasons (e.g. varying methods to capture decorrelation signal, inability to manually adjust divergent segmentation, image quality and resolution) the comparison remains problematic and prevents these methods from being employed in RCTs.
Finally, as we are just currently reaching a consensus on “biomarkers” of uveitis on OCTCitation36 (an imaging modality that has been available to us from more than 20 years) we are far from even considering them in OCTA.
(9) We know that OCTA is really good at studying retinal perfusion. Can we really assess the choroid with OCTA? (Answers A. Agarwal)
With the availability of swept-source OCTA, the ability of the device to image the choroidal vasculature has improved significantly.Citation47 However, there are no head-to-head studies comparing spectral domain OCTA with swept-source OCTA in uveitis. The OCTA can image the choriocapillaris adequately and provide useful information related to flow deficit areas in choroiditis and identify choroidal neovascular membranes (both type 1 and type 2),Citation48,Citation49 which may often not be detectable clinically, or on FA or ICGA.
There are certain caveats in assessing the choroidal vasculature using OCTA. The choriocapillaris layer may be very irregular in eyes with posterior uveitis, especially in the presence of choroidal inflammatory lesions. This may make the segmentation slabs inaccurate, giving false assessments of the choriocapillaris flow deficit areas.Citation50 Therefore, care must be taken to perform appropriate correction of the segmentation in all the scans. One may use a standardised offset of the segmentation slab beneath the Bruch’s membrane to obtain repeatable measurements. Secondly, the retinal pigment epithelium (RPE) and the outer retina may be irregular (thickened or bumpy), leading to abnormal hyper-reflectivity of this layer. This may result in back-shadowing and poor assessment of the underlying choriocapillaris. On the contrary, atrophy or thinning of the RPE and the outer retina may result in increased reflectance, giving the false impression of an underlying high flow lesion (or a CNV). These factors must be carefully considered when assessing the choroidal perfusion using OCTA in eyes with uveitis.Citation51
Stromal choroidal lesions such as tubercular or sarcoid choroidal granulomas may not be readily imaged using OCTA.Citation52,Citation53 These lesions may be deep in the choroid, and OCTA may only show the flow deficit areas corresponding to the overlying choriocapillaris which have been compressed due to the mass effect of the granuloma. OCTA cannot detect the granuloma itself. Thus, these lesions need to be assessed using OCT (either swept-source or enhanced depth imaging) and other techniques such as ICGA.
(10) Would you rely on OCTA alone to investigate a uveitis patient? (Answers E. Carreño)
Uveitis are a group of complex diseases with very different aetiologies and a wide expression of clinical features. Therefore, their diagnosis is also complex. In uveitis a complete anamnesis and clinical examination is fundamental, and in terms of imaging investigations the multimodal approach is the key. So, the quick answer, no, I wouldn’t rely on OCTA alone to investigate a uveitis patient.
Even though OCTA could be very useful, as we can have a structural b-scan, en-face reconstruction and a depth localisation of the vascular flow it is not able to completely recognise leakage and it can’t assess the health of the retinal pigment epithelium (RPE) as well as the fundus autofluorescence (FAF). So, although it can be very helpful in the follow-up, other investigations such as FAF, FFA, and ICGA are fundamental for a correct diagnosis. However, the decision regarding the best imaging test to do in each case should be based on an extensive questioning and a proper clinical examination of the patient.
Final comments and conclusions (C. Pavesio)
This set of questions gives a clear indication of the current status of the use of OCTA in uveitis. The limitations highlighted, especially the inability to assess barriers break-down, show that its use and interpretation of its findings require careful consideration. We are all a lot more familiar with the use of the traditional invasive techniques of FA and ICG as we have been using them for diagnosis and monitoring for many decades. Their repeated use in the monitoring of disease activity is time consuming, expensive and invasive, making them not ideal for this purpose. On the other hand, OCTA is not as informative for an initial assessment and is less helpful in diagnosing but represents a very good option for monitoring due to ease of being performed and the non-invasive nature, especially if a good correlation can be established with the dye tests at the start. This means that we should really consider utilising these techniques in parallel in all posterior cases, which will serve the purpose mentioned above, but also will increase the collective experience in interpreting them and building the necessary expertise for potential future expansion of their use.
The ability of OCTA to assess perfusion and identify neovascular processes is of huge importance and practical use, with the inability of detecting leakage being its major setback. Imaging of the choroid has been given more attention in recent times and limitations still exist with OCTA. As clearly stated, interpreting OCTA images is not simple and there are potentially many issues regarding the status of the retinal tissue (atrophy, thickening) or the inflammatory nature of the process (i.e., extensive sub-retinal fluid) which affect choroidal imaging.
Standardisation of nomenclature in OCTA is a very important first step in making this technique more useful in clinical practice and potentially RCTs in the future. This is not a simple piece of work since, as highlighted already, different centres are using different names for the same findings. This is work in progress and will set the stage for more widespread use of this technique.
The current status of the play indicates that the combined use of the different techniques is the best way of obtaining all the necessary information for diagnosis and monitoring. OCTA is already gaining ground regarding monitoring, and we wait for future developments which may expand its benefits.
Financial disclosures
Alessandro Invernizzi, Bayer (C) and Novartis (C); Allergan (R); Francesco Pichi Carl Zeiss Meditec (C), Optos (C); Marion Munk, Carl Zeiss Meditec (C); Carlos Pavesio
Aniruddha Agarwal, Ester Carreno, Manfred Zierhut no financial disclosures;
Additional information
Funding
References
- Drexler W, Fujimoto JG. State-of-the-art retinal optical coherence tomography. Prog Retin Eye Res. 2008 Jan;27(1):45–88. doi:10.1016/j.preteyeres.2007.07.005.
- Invernizzi A, Cozzi M, Staurenghi G. Optical coherence tomography and optical coherence tomography angiography in uveitis: a review. Clin Exp Ophthalmol. 2019 Apr;47(3):357–371. doi:10.1111/ceo.13470.
- Abu El-Asrar AM, Herbort CP, Tabbara KF. Retinal vasculitis. Ocul Immunol Inflamm. 2005 Dec;13(6):415–433. doi:10.1080/09273940591003828.
- Herbort CP, LeHoang P, Guex-Crosier Y. Schematic interpretation of indocyanine green angiography in posterior uveitis using a standard angiographic protocol. Ophthalmology. 1998 Mar;105(3):432–440. doi:10.1016/s0161-6420(98)93024-x.
- Herbort CP, Mantovani A, Papadia M. Use of indocyanine green angiography in uveitis. Int Ophthalmol Clin 2012 Fall;52(4);13–31. doi:10.1097/IIO.0b013e318265d48b.
- Pichi F, Sarraf D, Arepalli S, et al. The application of optical coherence tomography angiography in uveitis and inflammatory eye diseases. Prog Retin Eye Res. 2017;Jul 59:178–201. doi:10.1016/j.preteyeres.2017.04.005.
- Pecen PE, Petro KF, Baynes K, Ehlers JP, Lowder CY, Srivastava SK. Peripheral findings and retinal vascular leakage on ultra-widefield fluorescein angiography in patients with uveitis. Ophthalmol Retina. 2017 Sep-Oct;1(5):428–434. doi:10.1016/j.oret.2017.01.016.
- Campbell JP, Leder HA, Sepah YJ, et al. Wide-field retinal imaging in the management of noninfectious posterior uveitis. Am J Ophthalmol. 2012 Nov;154(5):908–911.e2. doi:10.1016/j.ajo.2012.05.019.
- Thomas AS, Redd T, Campbell JP, et al. The impact and implication of peripheral vascular leakage on ultra-widefield fluorescein angiography in uveitis. Ocul Immunol Inflamm. 2019;27(3):349–355. doi:10.1080/09273948.2017.1367406.
- Pichi F, Srivastava SK, Nucci P, Baynes K, Neri P, Lowder CY. Peripheral retinoschisis in intermediate uveitis. Retina (Philadelphia, Pa). 2017 Nov;37(11):2167–2174. doi:10.1097/iae.0000000000001463.
- Pichi F, Neri P, Agarwal A, et al. Vasoproliferative tumors in intermediate uveitis. Retina (Philadelphia, Pa). 2020 Sep;40(9):1765–1773. doi:10.1097/iae.0000000000002656.
- Sheemar A, Temkar S, Takkar B, et al. Ultra-wide field imaging characteristics of primary retinal vasculitis: risk factors for retinal neovascularization. Ocul Immunol Inflamm. 2019;27(3):383–388. doi:10.1080/09273948.2018.1508729.
- Zicarelli F, Pichi F, Parrulli S, et al., Acute posterior ocular toxoplasmosis: an optical coherence tomography angiography and dye angiography study. Ocul Immunol Inflamm. 2021 Oct 12: 1–5. doi:10.1080/09273948.2021.1977831.
- Kitamura Y, Oshitari T, Kitahashi M, Baba T, Yamamoto S. Acute posterior multifocal placoid pigment epitheliopathy sharing characteristic OCT findings of vogt-koyanagi-harada disease. Case Rep Ophthalmol Med. 2019;2019:9217656. doi:10.1155/2019/9217656.
- Ghasemi Falavarjani K, Habibi A, Anvari P, et al. Effect of segmentation error correction on optical coherence tomography angiography measurements in healthy subjects and diabetic macular oedema. Br J Ophthalmol. 2020 Feb;104(2):162–166. doi:10.1136/bjophthalmol-2019-314018.
- Ghasemi Falavarjani K, Mirshahi R, Ghasemizadeh S, Sardarinia M. Stepwise segmentation error correction in optical coherence tomography angiography images of patients with diabetic macular edema. Ther Adv Ophthalmol. Jan-Dec 2020;12:2515841420947931. doi:10.1177/2515841420947931.
- Agarwal A, Handa S, Marchese A, et al. Optical coherence tomography findings of underlying choroidal neovascularization in punctate inner choroidopathy. Front Med (Lausanne). 2021;8:758370. doi:10.3389/fmed.2021.758370.
- Wintergerst MWM, Pfau M, Müller PL, et al. Optical coherence tomography angiography in intermediate uveitis. Am J Ophthalmol. 2018;Oct 194:35–45. doi:10.1016/j.ajo.2018.06.023.
- Ji KB, Hu Z, Zhang QL, Mei HF, Xing YQ. Retinal microvasculature features in patients with Behcet’s disease: a systematic review and meta-analysis. Sci Rep. 2022 Jan 14;12(1):752. doi:10.1038/s41598-021-04730-6.
- Choi W, Moult EM, Waheed NK, et al. Ultrahigh-speed, swept-source optical coherence tomography angiography in nonexudative age-related macular degeneration with geographic atrophy. Ophthalmology. 2015 Dec;122(12):2532–2544. doi:10.1016/j.ophtha.2015.08.029.
- Pichi F, Smith SD, Neri P, et al. choroidal granulomas visualized by swept-source optical coherence tomography angiography. Retina (Philadelphia, Pa). 2021 Mar 1;41(3):602–609. doi:10.1097/iae.0000000000002864.
- Parrulli S, Invernizzi A, Monteduro D, et al. Longitudinal follow-up of choroidal granulomas with indocyanine green angiography and optical coherence tomography angiography: a lesion-based analysis. Retina (Philadelphia, Pa). 2022 May 1;42(5):906–914. doi:10.1097/iae.0000000000003405.
- Klufas MA, Phasukkijwatana N, Iafe NA, et al. Optical coherence tomography angiography reveals choriocapillaris flow reduction in placoid chorioretinitis. Ophthalmol Retina. 2017 Jan-Feb;1(1):77–91. doi:10.1016/j.oret.2016.08.008.
- Burke TR, Chu CJ, Salvatore S, et al. Application of OCT-angiography to characterise the evolution of chorioretinal lesions in acute posterior multifocal placoid pigment epitheliopathy. Eye (Lond). 2017 Oct;31(10):1399–1408. doi:10.1038/eye.2017.180.
- Agarwal A, Aggarwal K, Mandadi SKR, et al. Longitudinal follow-up of tubercular serpiginous-like choroiditis using optical coherence tomography angiography. Retina (Philadelphia, Pa). 2021 Apr 1;41(4):793–803. doi:10.1097/iae.0000000000002915.
- Pichi F, Aggarwal K, Neri P, et al. Choroidal biomarkers. Indian J Ophthalmol. 2018 Dec;66(12):1716–1726. doi:10.4103/ijo.IJO_893_18.
- Aggarwal K, Agarwal A, Mahajan S, et al. The role of optical coherence tomography angiography in the diagnosis and management of acute vogt-koyanagi-harada disease. Ocul Immunol Inflamm. 2018;26(1):142–153. doi:10.1080/09273948.2016.1195001.
- Lei B, Zhou M, Wang Z, Chang Q, Xu G, Jiang R. Ultra-wide-field fundus imaging of acute retinal necrosis: clinical characteristics and visual significance. Eye (Lond). 2020 May;34(5):864–872. doi:10.1038/s41433-019-0587-8.
- Wang JC, Lu Y, Sobrin L, Husain D. Multimodal imaging in acute retinal necrosis presenting with macular involvement. Retin Cases Brief Rep. Feb 6, 2020. doi:10.1097/icb.0000000000000978.
- Kim M, Kim RY, Park YH. Choroidal vascularity index and choroidal thickness in human leukocyte antigen-B27-associated uveitis. Ocul Immunol Inflamm. 2019;27(8):1280–1287. doi:10.1080/09273948.2018.1530364.
- Cao JL, Srivastava SK, Venkat A, Lowder CY, Sharma S. Ultra-widefield fluorescein angiography and OCT findings in tubulointerstitial nephritis and uveitis syndrome. Ophthalmol Retina. 2020 Feb;4(2):189–197. doi:10.1016/j.oret.2019.08.012.
- Bousquet E, Khandelwal N, Séminel M, et al. Choroidal structural changes in patients with birdshot chorioretinopathy. Ocul Immunol Inflamm. 2021 Feb 17;29(2):346–351. doi:10.1080/09273948.2019.1681472.
- Priem HA, Oosterhuis JA. Birdshot chorioretinopathy: clinical characteristics and evolution. Br J Ophthalmol. 1988 Sep;72(9):646–659. doi:10.1136/bjo.72.9.646.
- Lee J, Smith WM, Goldstein DA. Birdshot chorioretinopathy presenting in a teenager. Am J Ophthalmol Case Rep. Sep 2020;19:100807. doi:10.1016/j.ajoc.2020.100807.
- Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence tomography angiography. Retina (Philadelphia, Pa). 2015 Nov;35(11):2163–2180. doi:10.1097/iae.0000000000000765.
- Pichi F, Invernizzi A, Tucker WR, Munk MR. Optical coherence tomography diagnostic signs in posterior uveitis. Prog Retin Eye Res. Mar 2020;75:100797. doi:10.1016/j.preteyeres.2019.100797.
- Tian M, Tappeiner C, Zinkernagel MS, Huf W, Wolf S, Munk MR. Evaluation of vascular changes in intermediate uveitis and retinal vasculitis using swept-source wide-field optical coherence tomography angiography. Br J Ophthalmol. 2019 Sep;103(9):1289–1295. doi:10.1136/bjophthalmol-2018-313078.
- Pepple KL, Chu Z, Weinstein J, Munk MR, Van Gelder RN, Wang RK. Use of en face swept-source optical coherence tomography angiography in identifying choroidal flow voids in 3 patients with birdshot chorioretinopathy. JAMA Ophthalmol. 2018 Nov 1;136(11):1288–1292. doi:10.1001/jamaophthalmol.2018.3474.
- Dingerkus VLS, Munk MR, Brinkmann MP, et al. Optical coherence tomography angiography (OCTA) as a new diagnostic tool in uveitis. J Ophthalmic Inflamm Infect. 2019 May 28;9(1):10. doi:10.1186/s12348-019-0176-9.
- Pichi F, Salas EC, DdS M, Gupta V, Zierhut M, Munk MR. Standardisation of optical coherence tomography angiography nomenclature in uveitis: first survey results. Br J Ophthalmol. 2021 Jul;105(7):941–947. doi:10.1136/bjophthalmol-2020-316881.
- Russell JF, Pichi F, Scott NL, et al. Masqueraders of multiple evanescent white dot syndrome (MEWDS). Int Ophthalmol. 2020 Mar;40(3):627–638. doi:10.1007/s10792-019-01223-4.
- Türkcü FM, Şahin A, Karaalp Ü, et al. Automated quantification of foveal avascular zone and vascular density in Behçet’s disease. Ir J Med Sci. 2020 Feb;189(1):349–354. doi:10.1007/s11845-019-02051-2.
- Cheng D, Shen M, Zhuang X, et al. Inner retinal microvasculature damage correlates with outer retinal disruption during remission in behçet’s posterior uveitis by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2018 Mar 1;59(3):1295–1304. doi:10.1167/iovs.17-23113.
- Goker YS, Yılmaz S, Kızıltoprak H, Tekin K, Demir G. Quantitative analysis of optical coherence tomography angiography features in patients with nonocular Behcet’s disease. Curr Eye Res. 2019 Feb;44(2):212–218. doi:10.1080/02713683.2018.1530361.
- Değirmenci MFK, Temel E, Yalçındağ FN. Quantitative evaluation of the retinal vascular parameters with OCTA in patients with Behçet disease without ocular involvement. Ophthalmic Surg Lasers Imaging Retina. 2019 Dec 1;51(1):31–34. doi:10.3928/23258160-20191211-04.
- Kim AY, Rodger DC, Shahidzadeh A, et al. Quantifying retinal microvascular changes in uveitis using spectral-domain optical coherence tomography angiography. Am J Ophthalmol. 2016;Nov 171:101–112. doi:10.1016/j.ajo.2016.08.035.
- Vira J, Marchese A, Singh RB, Agarwal A. Swept-source optical coherence tomography imaging of the retinochoroid and beyond. Expert Rev Med Devices. 2020 May;17(5):413–426. doi:10.1080/17434440.2020.1755256.
- Aggarwal K, Agarwal A, Sharma A, Sharma K, Gupta V. Detection of type 1 choroidal neovascular membranes using optical coherence tomography angiography in tubercular posterior uveitis. Retina (Philadelphia, Pa). 2019 Aug;39(8):1595–1606. doi:10.1097/iae.0000000000002176.
- Pohlmann D, Pleyer U, Joussen AM, Winterhalter S. Optical coherence tomography angiography in comparison with other multimodal imaging techniques in punctate inner choroidopathy. Br J Ophthalmol. 2019 Jan;103(1):60–66. doi:10.1136/bjophthalmol-2017-311764.
- Arora A, Agarwal A, Bansal R, et al., Subretinal Hyperreflective Material (SHRM) as biomarker of activity in Exudative and Non- exudative inflammatory choroidal neovascularization. Ocul Immunol Inflamm. 2021 Oct 14: 1–8. doi:10.1080/09273948.2021.1980813.
- Corvi F, Su L, Sadda SR. Evaluation of the inner choroid using OCT angiography. Eye (Lond). 2021 Jan;35(1):110–120. doi:10.1038/s41433-020-01217-y.
- Agarwal A, Invernizzi A, Markan A, et al. Imaging in tubercular choroiditis: current concepts. Ocul Immunol Inflamm. 2020 Nov 16;28(8):1223–1238. doi:10.1080/09273948.2020.1817500.
- Invernizzi A, Mapelli C, Viola F, et al. Choroidal granulomas visualized by enhanced depth imaging optical coherence tomography. Retina (Philadelphia, Pa). 2015 Mar;35(3):525–531. doi:10.1097/iae.0000000000000312.
