ABSTRACT
Purpose
To evaluate the efficacy and safety of lotilaner ophthalmic solution, 0.25% eyedrops compared to vehicle for the treatment of Demodex blepharitis.
Methods
In this randomized, controlled, double-masked clinical trial, 54 participants were randomly assigned in a 1:1 ratio to receive either lotilaner ophthalmic solution, 0.25% (study group) or the vehicle (control group) bilaterally, twice daily for 42 days. Outcome measures were collarette cure (collarette grade 0, upper eyelid), mite eradication (mite density of 0 mites/lash), and composite cure (grade 0 for collarettes and erythema).
Results
The proportion of participants achieving collarette cure (80.0% vs 15.8%; p < .001), mite eradication (73.3% vs 21.1%, p = .003) and composite cure (73.3% vs 10.5%, p < .001) at Day 42 was statistically significantly higher in the study group than the control group.
Conclusion
Twice-daily 42-day treatment with novel lotilaner ophthalmic solution, 0.25% is safe and effective for the treatment of Demodex blepharitis compared to the vehicle control. (Registry number: ACTRN12620000320954, dated 09/03/2020).
Demodex blepharitis is an often-overlooked ocular condition associated with itching, foreign body sensation, eyelid inflammation, and the development of collarettes on the eyelids due to Demodex mite infestation.Citation1 According to a recent observational study, 69% of patients diagnosed with blepharitis that presented to eye care clinics had collarettes, a pathognomonic sign of Demodex blepharitis.Citation2 Two species of the mites, Demodex folliculorum and Demodex brevis, are known to infest the human body, primarily at the base of the eyelashes, eyelash follicles and meibomian glands.Citation3,Citation4 Demodex folliculorum mites feed on sebum as well as the lid margin epithelial cells, causing epithelial hyperplasia and hyperkeratinization, leading to the formation of collarettes or cylindrical dandruff, which is considered the pathognomonic sign of Demodex blepharitis.Citation1,Citation5–7 Mites are composed of decomposing (and sometimes live) mites, undigested material, eggs, and keratinized cells.
Demodex infestation causes damage via three different mechanisms (mechanical, bacterial, and chemical. It leads to direct mechanical damage as the mites burrow into the lash follicles and lay eggs.Citation5 It also acts as a vector for bacteria and induces inflammation due to a hypersensitivity reaction to chemicals excreted by the mites and accompanying bacteria.Citation5,Citation8–10 Demodex blepharitis has been associated with myriad clinical manifestations, including tear film disruptions, meibomian gland dysfunction, lid margin erythema, eyelash misalignment or loss and, rarely, peripheral corneal vascularization, phlyctenule-like lesions and corneal opacity.Citation5,Citation11,Citation12 Demodex infestation has also been identified as a risk factor for chalazia, hordeola, eyelid basal cell carcinoma, recurrent pterygium, rosacea and refractory herpetic keratitis.Citation11,Citation13–16
Currently, there are no FDA-approved treatments for Demodex blepharitis. Researchers have studied the effects of systemic ivermectin, metronidazole, their combination, topical tea tree oil (TTO) (3% to 100%) formulations, hypochlorous acid, mechanical debridement and intense pulsed light (IPL) on Demodex blepharitis.Citation1,Citation3,Citation9,Citation17–25 However, there is a paucity of vehicle-controlled studies to ascertain the efficacy of these agents in managing ocular Demodex, and many of these agents have significant adverse event profiles. Of significance, terpinene 4-ol (T4O), the demodicidal ingredient within TTO, has been recently discovered to be toxic to meibomian gland epithelial cells.Citation26 As a result, the lack of a gold standard for an effective treatment perpetuates the clinicians’ non-identification and disheartening reaction to Demodex blepharitis.
Lotilaner is an isoxazoline that inhibits the γ-aminobutyric acid (GABA)-gated chloride channels (GABACls) of arthropods. Ectoparasites exposed to isoxazolines exhibit a spastic paralysis leading to their starvation and death.Citation27 An oral formulation of lotilaner has been approved in several countries for use as a veterinary medicine.
The safety and efficacy of topical lotilaner ophthalmic solution, 0.25%, in humans has been evaluated previously in three clinical trials.Citation28,Citation29 In a single-arm pilot study (Mars), 28 day-treatment with lotilaner ophthalmic solution, 0.25%, resulted in a significant reduction in collarettes and mite density and was well-tolerated.Citation30 A subsequent vehicle-controlled study (Jupiter), using the same treatment regimen, demonstrated statistically significantly better outcomes with lotilaner compared to the control group.Citation29 Subsequently, in the Io study, Demodex blepharitis patients were treated with lotilaner, 0.25%, eyedrops twice a day for a longer duration (42 days).Citation28 High levels of mite eradication and collarette elimination were achieved at Day 42 in this study.
The present study was designed to evaluate the efficacy and safety of treatment with lotilaner ophthalmic solution, 0.25% versus vehicle control, administered twice a day for 6 weeks, in patients with Demodex blepharitis.
Methods
This randomized, double-masked, parallel, vehicle-controlled clinical trial was conducted at the Asociación para Evitar la Ceguera en México I.A.P in Mexico City (Registry number: ACTRN12620000320954, dated 09/03/2020). The study adhered to the tenets of the Declaration of Helsinki and was approved by the APEC Ethics Committee. All enrolled participants provided written informed consent using the APEC Ethics Committee-approved informed consent form.
Participants were screened up to 14 days prior to enrollment and treatment initiation. Eligibility criteria for participating in the study included age ≥18 years, blepharitis due to Demodex infestation with more than 10 collarettes present on the upper eyelid, at least mild upper eyelid margin erythema, and average mite density of ≥1.5 mites per lash, all in at least one eye.
Participants were excluded if they had used any systemic or topical antibacterial/ antiparasitic/steroidal drug, topical TTO, hypochlorous acid, or any other lid hygiene products (lid scrubs) within the last 14 days or were unwilling to forego the use of lid hygiene products during the study. Participants were also excluded if they had used a topical prostaglandin analogue (PGA) to promote eyelash growth within the last 30 days, had initiated PGA treatment for medical reasons within the past 30 days or planned to change or discontinue PGA treatment during the study treatment phase. Participants were also excluded if they had used contact lenses, artificial eyelashes or eyelash extensions within the last 7 days or were unwilling to forego the use of these products during the study. Participants with lid structural abnormalities, previous surgery of the eyelid margin, acute ocular infection, or inflammation other than blepharitis, severe dry eye, hypersensitivity to lotilaner or any of the formulation components, and pregnancy or lactation were also excluded.
Participants who met the eligibility criteria were enrolled and were randomly assigned in a 1:1 ratio to receive either the TP-03 study medication (topical lotilaner ophthalmic solution, 0.25%, Tarsus Pharmaceuticals, Inc., Irvine, CA, USA) (study group) or the vehicle formulation without lotilaner (control group) bilaterally. On the first treatment day, the first dose of study medication, TP-03, 0.25%, or vehicle, was administered in the clinic. Subsequent doses were applied bilaterally by the participants, one drop in each eye twice a day, morning, and evening. The dosing regimen continued for 42 days, and participants were evaluated at Days 7, 14, 28, and 42.
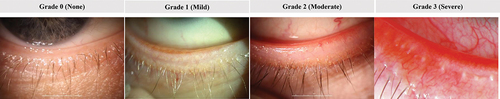
The primary efficacy outcome was elimination of collarettes or collarette cure, based on collarette grade (grade 0, upper eyelid of the analysis eye) and the secondary efficacy parameters was mite eradication (0 mites/lash for the analysis eye). Additional endpoints were composite cure, a combination of collarette and erythema grades (grade 0 for both collarettes and erythema for the upper eyelid of the analysis eye) and clinically meaningful collarette cure (grade 1 or less). Collarettes were graded for each eyelid using the grading scale shown in . Mite density (to determine mite eradication) was assessed by selecting and removing two or more lashes from each of the upper and lower eyelids, one lash from each half of each lid, using fine forceps. If present, lashes with collarettes were selected and the lashes from each lid were placed in artificial tear drops on four separate glass slides. The number of Demodex mites observed and the number of lashes epilated were recorded and mite density/lash was calculated. Mite eradication was defined as a mite density of 0 mites/lash. Erythema of the eyelid margin was graded (upper and lower eyelids separately) on a scale from 0 to 3 ().
Figure 1. Grading scale (non-linear) used for collarette grading in each eyelid. Grade 0: 0–2 lashes/eyelid with collarettes; Grade 1: 3–10 lashes/eyelid with collarettes; Grade 2: >10 to <1/3 lashes/eyelid (~50 for an upper eyelid with 150 eyelashes) with collarettes; Grade 3: ≥1/3 to <2/3 lashes/eyelid (~100 for an upper eyelid with 150 eyelashes) with collarettes; Grade 4: ≥ 2/3 lashes/eyelid with collarettes. The number of eyelashes on the upper eyelid may vary from 90 to 160.

Figure 2. Lid margin erythema grading scale (non-linear). Grade 0: Normal age-related lid coloration; Grade 1: Pink capillary involvement along the lid edge, no patches of confluent capillary redness throughout the lid edge; Grade 2: Deep pink or red confluent capillary redness present locally along the lid edge; Grade 3: Deep red, diffuse confluent capillary redness present along the lid edge.
Safety parameters included assessment of adverse events as well as evaluation of any changes in corrected distance visual acuity (CDVA), IOP and slit-lamp biomicroscopy findings. CDVA was assessed using either the participant’s own spectacles or a pinhole occluder, with an ETDRS visual acuity chart at a distance of 4 meters. The number of letters read correctly was used to compute the participant’s logMAR CDVA. A change of more than two lines on the ETDRS chart (>0.2 logMAR) was considered clinically meaningful. Intraocular pressure was assessed at all visits using applanation tonometry. The IOP for each eye was measured twice and the average of the two measures was used for analysis.
Drop comfort was also assessed. Participants rated the comfort of the study medication as very comfortable, slightly comfortable, neither comfortable nor uncomfortable, slightly uncomfortable, and very uncomfortable.
Statistical analysis
One eye of each participant was chosen as the analysis eye. If both eyes met the inclusion criteria, the eye with the higher mite density at the screening visit was considered the analysis eye; if both eyes had equal mite density, the right eye was the analysis eye. All statistical analyses were performed using SAS (Version 9.4, SAS Institute Inc., Cary NC). Comparison of the efficacy measures was performed using a one-sided Fisher’s exact test and evaluated at an α of 0.025. Continuous data were described using descriptive statistics (i.e., n, mean, standard error of mean (SEM), and range) and categorical data were described using the participant count and percentage in each category. A two-sample t-test or its non-parametric counterpart Wilcoxon rank-sum test was used as appropriate to assess the statistical significance of the difference between treatment groups in the efficacy analyses. Statistical significance was set at α = 0.05. No adjustments were made for multiple comparisons.
Results
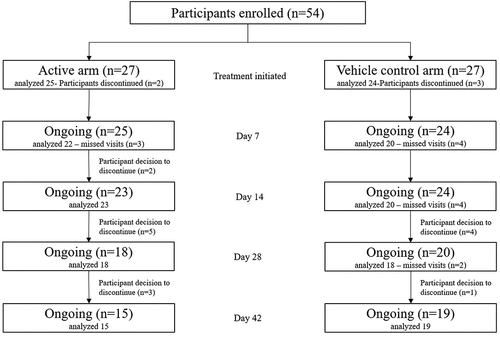
A total of 54 participants were enrolled in the study, 27 in the study group and 27 in the control group. Five participants (2 in the study group and 3 in the control group) were lost to follow-up or declined to continue in the study after receiving the first dose of medication in the clinic due to reasons unrelated to an adverse event (). These 5 participants were not included in the analysis. Of the 25 participants in the study group, the mean (± SEM) age was 58.5 ± 14.1 years and, of the 24 participants in the control group, the mean age was 62.1 ± 8.3 years. There were 64% (n = 16/25) females in the study group and 58.3% (n = 14/24) in the control group.
Collarette elimination and collarette grade
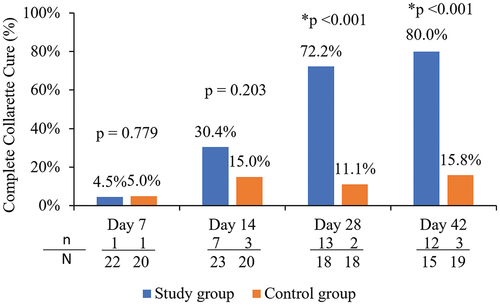
The proportion of participants in each group who achieved a “cure,” defined as elimination of lid margin collarettes (collarette grade 0 in the upper eyelid for the analysis eye) at each follow-up visit is shown in . The proportion of study group participants achieving elimination of collarettes at Days 28 and 42 was 72.2% (13/18) and 80.0% (12/15) respectively, which was statistically significantly higher than 11.1% (2/18) and 15.8% (3/19) of control group participants achieving cure at the same timepoints (p < .001). Additionally, by Day 42, 93.3% of the study eyes (14/15) had clinically meaningful collarette cure (grade 1 or less), which was statistically significantly higher than the corresponding value of 31.6% (6/19) in the control group (p = .0003).
Figure 4. Proportion of participants with elimination of collarettes (grade 0) in the upper eyelid of the analysis eye in the study and control groups.
shows a summary of the collarette grade for the analysis eye at each follow-up visit for both eyelids. The study group demonstrated a statistically significantly higher reduction in collarette grade compared to the control group at Day 14 onwards for the upper eyelid and Day 28 onwards for the lower eyelid of the analysis eye. At Day 14, 95.7% (22/23 lids) participants in the study group demonstrated a 1-grade improvement or better in collarette grade; at Day 42, all participants demonstrated a 2-grade improvement or better in collarette grade (). shows the change in collarettes in a representative study eye.
Figure 5. Proportion of participants with an improvement in collarettes of 1 grade or better or 2 grades or better in the analysis eye of the study group.
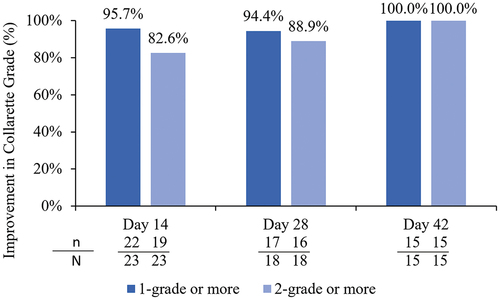
Figure 6. Representative example of the impact of treatment on collarettes. These photos show the left eye of study participant 001–312 at baseline (A) and after 42 days of treatment with lotilaner, 0.25% ophthalmic solution (B).
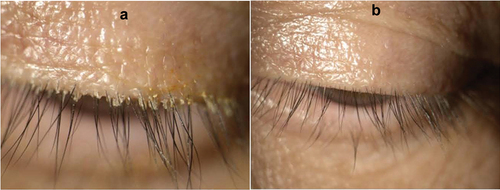
Table 1. Mean collarette grade and mean change from baseline at different time-points for the upper and lower eyelids of the analysis eye in the study and control groups.
Mite eradication and density
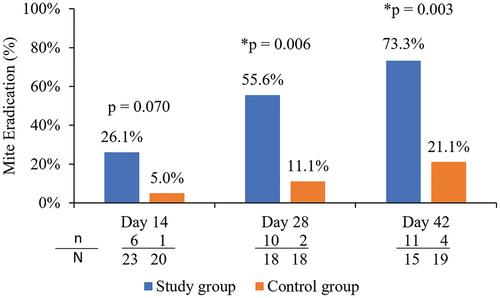
The proportion of participants in each group who achieved mite eradication (mite density of 0 mites/lash for the analysis eye) at each follow-up visit is shown in . The proportion of study group participants achieving mite eradication at Days 28 and 42 was 55.6% (10/18) and 73.3% (11/15), respectively, which was statistically significantly higher than the 11.1% (2/18) and 21.1% (4/19) of control group participants achieving eradication at the same timepoints (p = .006 at Day 28 and p = .003 at Day 42).
Figure 7. Proportion of participants with mite eradication (mite density of 0) in the analysis eye of study and control groups.
presents the mite density for the analysis eye at each follow-up visit. The study group demonstrated a statistically significantly greater reduction in mite density compared to the control group from Day 14 to Day 42 (). By Day 14, 87.0% (20/23 lids) of the analysis eyes in the study group had at least a 50% mite reduction.
Table 2. Mean mite density and mean change from baseline at different time-points for the analysis eye in the study and control groups.
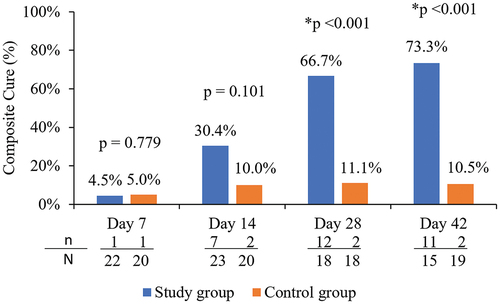
Composite cure (collarettes and lid erythema)
The proportion of participants in each group who achieved a composite cure (grade 0 for collarettes as well as grade 0 for lid erythema) of the upper eyelid of the analysis eye at each follow-up visit is shown in . The proportion of participants achieving a composite cure at Days 28 and 42 was 66.7% (12/18) and 73.3% (11/15), respectively, which was statistically significantly higher than the 11.1% (2/18) and 10.5% (2/19) of control group participants achieving the composite cure at the same timepoints (p < .001).
Drop comfort
There was no statistically significant difference in drop comfort between the study and control group. At Day 42, 93.3% (14/15) participants of the study group rated the drop as neutral to very comfortable, compared to 89.5% (17/19) of the control group (p = 1.000).
Adverse events
No serious adverse events were observed in this study. In the study group, there were four adverse events that were characterized as related to treatment: mild burning after drop instillation (n = 2), mild burning with red eye/blurriness after drop instillation (n = 1), and a mild change in taste sensation for a few hours (n = 1). In the control group, one participant experienced mild burning after drop instillation. These adverse events were transient and did not require additional intervention or result in discontinuing treatment with the study drug.
CDVA, IOP and slit-lamp biomicroscopy findings
There was little or no change in mean CDVA (logMAR) from baseline to Day 42 in either the study group or the control group (OD: 0.03 ± 0.05 vs 0.02 ± 0.05; OS: 0.06 ± 0.13 vs 0.02 ± 0.07). In the study group, two participants showed an improvement in CDVA greater than two lines and one participant demonstrated worsening in CDVA greater than two lines (0.2 logMAR). In the control group, no participants had a change in CDVA of greater than 2 lines. There was little to no change in mean IOP from baseline to Day 42 in the study group (OD: 14.5 ± 3.3 vs 13.7 ± 3.2; OS: 14.6 ± 3.3 vs 13.9 ± 3.0) or the control group (OD: 13.5 ± 2.8 vs 13.8 ± 2.4; OS: 13.6 ± 2.7 vs 13.6 ± 2.0). Clinically significant increases in corneal staining were observed in two participants in the study group and four participants in the control group.
Discussion
Currently, there are no FDA-approved treatments for Demodex blepharitis. Conventionally, lid hygiene was practiced for management of Demodex blepharitis using sulphuric ointment, yellow mercuric ointment, or pilocarpine gel; however, these methods were largely ineffective and are now obsolete.Citation6 Although other topical and systemic medications, including TTO (in the form of lid wipes or cleansing foam), systemic ivermectin, metronidazole and their combination have been tried for managing Demodex blepharitis, variable and questionable efficacy, poor tolerability and, in the case of TTO, meibomian gland cell death have been reported and there are few controlled studies.Citation6,Citation25,Citation26,Citation31
This phase 2b clinical trial was designed to evaluate the safety and efficacy of lotilaner ophthalmic solution, 0.25% BID for the treatment of Demodex blepharitis. The present vehicle-controlled clinical trial corroborates the safety and efficacy of lotilaner ophthalmic solution, 0.25%, as documented in our previous single-arm study.Citation32 The 42-day treatment with lotilaner ophthalmic solution, 0.25%, in eyes with Demodex blepharitis resulted in an 80.0% cure rate based on collarette grade compared to 15.8% in the control group (p < .001); clinically meaningful collarette cure in 93.3% of eyes in the study group compared to 30.0% in the control group (p = .0003); mite eradication in 73.3% of eyes in the study group compared to 21.1% in the control group (p = .003), and a composite cure of collarettes and erythema in 73.3% of eyes in the study group compared to 10.5% of those in the control group (p < .001).
In the present study, collarette grade 0 was achieved in more than 72% of eyes after twice-daily application with topical lotilaner ophthalmic solution, 0.25% for 28 days and 80% of eyes after 42 days, compared with only 11.1% and 15.8% of eyes in the control group at 28 and 42 days, respectively. These findings are slightly better than the Day 28 (70.6%), and Day 42 (72.2%) collarette cure rates reported in our previous 42-day single-arm study.Citation32
While this study demonstrated a statistically significant differences in complete collarette cure (patients achieving collarette grade 0 at 42 days) between the study and control groups, it is also important to note that clinical benefits may extend beyond just those who achieve a collarette grade of 0. All patients in the study group achieved an improvement of at least 1 collarette grade. The extent of Demodex infestation correlates with the clinical severity of collarettes.Citation18 It can be hypothesized that there is clinical benefit in improving the collarette grade, even if it is not reduced to 0, particularly for patients with higher initial collarette grades. Of note, clinically meaningful collarette cure (grade 0 or 1) was achieved in 93% of patients in the study group at Day 42.
While eliminating collarettes, the pathognomonic sign of Demodex blepharitis, is important, eradicating mites, the root cause of Demodex blepharitis, is of greater significance. Studies using TTO scrubs and shampoos have reported low mite eradication rates.Citation1,Citation17,Citation21 The mite eradication rate in the present study (73.3%) was similar to that in our previous 42-day single-arm study (77.8%). Of note, the baseline mite density was higher (3.27 ± 0.35) in the present study compared with our previous single arm 42-day study (2.63 ± 0.39). This higher baseline mite density per lash may have contributed to the slightly lower eradication rate seen in the present study versus the previous 42-day study.
Reductions in mite density occurred early in the course of treatment, with 87% of the study eyes having a 50% reduction in mite density by Day 14. As previously mentioned, patients were not allowed to perform any mechanical lid scrubbing or use any other lid therapies during the study. This demonstrates the rapid mite eradication effect of lotilaner, 0.25% ophthalmic solution alone. Continued treatment showed greater reduction in mite density which suggests that some Demodex patients with high mite density may possibly benefit from continued treatment even after Day 42; additional studies are needed to understand the additional benefit and impact of treatment longer than 6 weeks.
From the dermatology literature, rosacea is associated with elevated Demodex mite density.Citation33 The mites promote the development of acute inflammatory morphological elements and increase the duration and probability of rosacea recurrence, resulting in a decrease in patient quality of life.Citation34 As such, it can be hypothesized that the high level of mite reduction achieved in this study may have clinical and quality-of-life benefits, even if not all patients achieved 100% eradication.
Since it is impractical to epilate lashes and count mites under microscopy in routine clinical practice, the mite eradication endpoint in this study confirms that lotilaner ophthalmic solution, 0.25%, not only reduces the presence of collarettes but also addresses the root cause of Demodex blepharitis by killing Demodex mites. The statistically significant differences between the study and vehicle groups for both collarette elimination and mite eradication provide further support for the pathognomonic nature of collarettes as a clinical sign of Demodex blepharitis and for using slit lamp examination to look for collarettes as a method to confidently diagnose Demodex blepharitis.
In addition to collarettes, erythema of the eyelid margin is also considered a common clinical sign of blepharitis.Citation35,Citation36 The chronic inflammatory state of the eyelid margin in Demodex blepharitis leads to the development of erythema. In the present study, we also evaluated the proportion of participants who achieved both grade 0 collarettes and grade 0 lid margin erythema (composite cure). After 42 days of treatment with topical lotilaner, 0.25%, the proportion of eyes with composite cure was significantly higher (p < .001) in the study group (73.3%) than the control group (10.5%).
In general, drop comfort on instillation and ocular tolerability affect patient compliance and therefore efficacy of treatment.Citation37 Methods of managing Demodex blepharitis using lid scrubs and wipes containing TTO or one of its derivatives, T4O have also been associated with side effects such as ocular irritation, burning sensation, contact dermatitis and allergy.Citation4,Citation6,Citation9,Citation21,Citation38 Additionally, T4O has been reported to be toxic to human meibomian gland epithelial cells in vitro.Citation26 In the present study, we assessed the subjective comfort of the lotilaner eye drops and found that more than 90% of participants rated the drops as comfortable or neutral. Additionally, the formulation of TP-03 as a topical ophthalmic solution vehicle, as opposed to a thicker gel or ointment, also effectively mitigated any perceived blurred vision (n = 1), and there was no reduction of visual acuity or corneal epitheliopathy. No serious adverse events were observed.
The small sample size of the present study, particularly at the last follow-up visit, can be considered a potential limitation. Of note, some of the participants who were lost to follow-up likely decided not to participate in the study due to the COVID-19 pandemic that occurred at the time of this study. A randomized, controlled trial in a larger study population with the same treatment duration and same endpoints is underway.
To conclude, the results of this randomized, controlled trial indicate that twice daily usage of a novel preparation of lotilaner ophthalmic solution, 0.25% for 6 weeks is safe and effective for the treatment of Demodex blepharitis.
Acknowledgments
Jan Beiting (Wordsmith Consulting, Cary, North Carolina) and Raman Bedi, MD (IrisARC -Analytics, Research & Consulting, Chandigarh, India) provided editorial assistance in the preparation of this manuscript.
Disclosure statement
EY and HQM have received consulting fees from Tarsus Pharmaceuticals. MH and SNB are employees of Tarsus Pharmaceuticals. For the remaining authors, no conflicts are declared.
Additional information
Funding
References
- Karakurt Y, Zeytun E. Evaluation of the efficacy of tea tree oil on the density of demodex mites (acari: demodicidae) and ocular symptoms in patients with demodectic blepharitis. J Parasitol. 2018;104(5):473–478. doi:10.1645/18-46.
- Trattler W, Karpecki P, Rapoport Y, et al. The prevalence of Demodex blepharitis in US eye care clinic patients as determined by collarettes: A pathognomonic sign. Clin Ophthalmol. 2022;16:1153–1164.
- Holzchuh FG, Hida RY, Moscovici BK, et al. Clinical treatment of ocular Demodex folliculorum by systemic ivermectin. Am J Ophthalmol. 2011;151(6):1030–1034 e1031.
- Savla K, Le JT, Pucker AD. Tea tree oil for Demodex blepharitis. Cochrane Database Syst Rev. 2020;6:CD013333. doi:10.1002/14651858.CD013333.pub2.
- Fromstein SR, Harthan JS, Patel J, Opitz DL. Demodex blepharitis: clinical perspectives. Clin Optom (Auckl). 2018;10:57–63. doi:10.2147/OPTO.S142708.
- Navel V, Mulliez A, Benoist d’Azy C, et al. Efficacy of treatments for Demodex blepharitis: a systematic review and meta-analysis. Ocul Surf. 2019;17(4):655–669. doi:10.1016/j.jtos.2019.06.004.
- Suresha A, Sadhwini M. Role of demodex infestation in blepharitis and coconut oil as a treatment option. Indian J Clin Exp Ophthalmol. 2020;6(2):270–275. doi:10.18231/j.ijceo.2020.058.
- Lacey N, Kavanagh K, Tseng SC. Under the lash: Demodex mites in human diseases. Biochem (Lond). 2009;31:2–6.
- Messaoud R, El Fekih L, Mahmoud A, et al. Improvement in ocular symptoms and signs in patients with Demodex anterior blepharitis using a novel terpinen-4-ol (2.5%) and hyaluronic acid (0.2%) cleansing wipe. Clin Ophthalmol. 2019;13:1043–1054. doi:10.2147/OPTH.S198585.
- Luo X, Li J, Chen C, Tseng S, Liang L. Ocular demodicosis as a potential cause of ocular surface inflammation. Cornea. 2017;36(Suppl 1):S9–S14. doi:10.1097/ICO.0000000000001361.
- Liu J, Sheha H, Tseng SC. Pathogenic role of Demodex mites in blepharitis. Curr Opin Allergy Clin Immunol. 2010;10(5):505–510. doi:10.1097/ACI.0b013e32833df9f4.
- Zhang X-B, Ding Y-H, He W. The association between demodex infestation and ocular surface manifestations in meibomian gland dysfunction. Int J Ophthalmol. 2018;11(4):589. doi:10.18240/ijo.2018.04.08.
- Erbagci Z, Erbagci I, Erkilic S. High incidence of demodicidosis in eyelid basal cell carcinomas. Int J Dermatol. 2003;42(7):567–571. doi:10.1046/j.1365-4362.2003.01928.x.
- Huang Y, He H, Sheha H, Tseng SC. Ocular demodicosis as a risk factor of pterygium recurrence. Ophthalmology. 2013;120(7):1341–1347. doi:10.1016/j.ophtha.2013.01.001.
- Liang L, Ding X, Tseng SC. High prevalence of demodex brevis infestation in chalazia. Am J Ophthalmol. 2014;157(2):342–348 e341. doi:10.1016/j.ajo.2013.09.031.
- Zhao YE, Wu L, Peng Y, Cheng H. Retrospective analysis of the association between Demodex infestation and rosacea. Arch Dermatol. 2010;146(8):896–902. doi:10.1001/archdermatol.2010.196.
- Filho PA, Hazarbassanov RM, Grisolia AB, Pazos HB, Kaiserman I, Gomes JA. The efficacy of oral ivermectin for the treatment of chronic blepharitis in patients tested positive for Demodex spp. Br J Ophthalmol. 2011;95(6):893–895. doi:10.1136/bjo.2010.201194.
- Gao YY, Di Pascuale MA, Li W, et al. High prevalence of Demodex in eyelashes with cylindrical dandruff. Invest Ophthalmol Vis Sci. 2005;46(9):3089–3094. doi:10.1167/iovs.05-0275.
- Kabat AG. Hypochlorous acid solution (Avenova((R))) is not demodicidal. Clin Optom (Auckl). 2018;10:115–117. doi:10.2147/OPTO.S182534.
- Kim JH, Chun YS, Kim JC. Clinical and immunological responses in ocular demodecosis. J Korean Med Sci. 2011;26(9):1231–1237. doi:10.3346/jkms.2011.26.9.1231.
- Koo H, Kim TH, Kim KW, Wee SW, Chun YS, Kim JC. Ocular surface discomfort and Demodex: effect of tea tree oil eyelid scrub in Demodex blepharitis. J Korean Med Sci. 2012;27(12):1574–1579. doi:10.3346/jkms.2012.27.12.1574.
- Ngo W, Jones L, Bitton E. Short-term comfort responses associated with the use of eyelid cleansing products to manage Demodex folliculorum. Eye Contact Lens. 2018;44(Suppl 2):S87–S92. doi:10.1097/ICL.0000000000000415.
- Salem DA, El-Shazly A, Nabih N, El-Bayoumy Y, Saleh S. Evaluation of the efficacy of oral ivermectin in comparison with ivermectin-metronidazole combined therapy in the treatment of ocular and skin lesions of Demodex folliculorum. Int J Infect Dis. 2013;17(5):e343–347. doi:10.1016/j.ijid.2012.11.022.
- Tighe S, Gao -Y-Y, Tseng SC. Terpinen-4-ol is the most active ingredient of tea tree oil to kill Demodex mites. Transl Vis Sci Technol. 2013;2(7):2. doi:10.1167/tvst.2.7.2.
- Zhang AC, Muntz A, Wang MTM, Craig JP, Downie LE. Ocular Demodex: a systematic review of the clinical literature. Ophthalmic Physiol Opt. 2020;40(4):389–432. doi:10.1111/opo.12691.
- Chen D, Wang J, Sullivan DA, Kam WR, Liu Y. Effects of terpinen-4-ol on meibomian gland epithelial cells in vitro. Cornea. 2020;39(12):1541–1546. doi:10.1097/ICO.0000000000002506.
- Toutain CE, Seewald W, Jung M. The intravenous and oral pharmacokinetics of lotilaner in dogs. Parasit Vectors. 2017;10(1):522. doi:10.1186/s13071-017-2475-z.
- Gonzalez-Salinas R, Yeu E, Holdbrook M, et al. Collarette elimination and demodex mite eradication with topical lotilaner ophthalmic solution, 0.25%. J Ocul Pharmacol Ther. 2021;37(8):479–484. doi:10.1089/jop.2021.0011.
- Salinas RG, Karpecki P, Yeu E, et al. Safety and efficacy of lotilaner ophthalmic solution, 0.25% for the treatment of blepharitis due to demodex infestation: a randomized, controlled, double-masked clinical trial. Cont Lens Anterior Eye. 2021:101492. doi:10.1016/j.clae.2021.101492.
- Gonzalez-Salinas R, Yeu E, Holdbrook M, et al. Safety and efficacy of topical lotilaner ophthalmic solution 0.25% for the treatment of demodex blepharitis: a pilot study. J Ophthalmol. 2021;2021:3862684. doi:10.1155/2021/3862684.
- Hirsch-Hoffmann S, Kaufmann C, Banninger PB, Thiel MA. Treatment options for demodex blepharitis: patient choice and efficacy. Klin Monbl Augenheilkd. 2015;232(4):384–387. doi:10.1055/s-0035-1545780.
- Tarsus Pharmaceuticals. Tarsus releases data from io and Europa trials for TP-03 to treat demodex blepharitis and begins enrollment and treatment in phase 2b/3 Saturn-1 trial [Press release]. 2020, October 6. https://ir.tarsusrx.com/news-releases/news-release-details/tarsus-releases-data-io-and-europa-trials-tp-03-treat-demodex. Accessed May 21, 2021.
- Casas C, Paul C, Lahfa M, et al. Quantification of Demodex folliculorum by PCR in rosacea and its relationship to skin innate immune activation. Exp Dermatol. 2012;21(12):906–910. doi:10.1111/exd.12030.
- Kubanov A, Gallyamova Y, Kravchenko A. Clinical picture, diagnosis and treatment of rosacea, complicated by Demodex mites. Dermatol Reports. 2019;11(1):7675. doi:10.4081/dr.2019.7675.
- Milner MS, Beckman KA, Luchs JI, et al. Dysfunctional tear syndrome: dry eye disease and associated tear film disorders–new strategies for diagnosis and treatment. Curr Opin Ophthalmol. 2017;28(Suppl 1):3. doi:10.1097/01.icu.0000512373.81749.b7.
- Raval Y, Gomes PJ, Abelson MB. Standardized lid margin redness scale for blepharitis. Invest Ophthalmol Vis Sci. 2019;60:6270.
- Nichols KK, Holland E, Toyos MM, et al. Ocular comfort assessment of lifitegrast ophthalmic solution 5.0% in OPUS-3, a Phase III randomized controlled trial. Clin Ophthalmol. 2018;12:263. doi:10.2147/OPTH.S152841.
- Arita R, Fukuoka S. Non‐pharmaceutical treatment options for meibomian gland dysfunction. Clin Exp Optom. 2020;103(6):742–755. doi:10.1111/cxo.13035.