?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Purpose
To evaluate the efficacy and safety of 0.05% cyclosporine eye drops (II) for the treatment of primary Sjögren’s syndrome-associated dry eye (PSSDE).
Methods
Sixty patients with PSSDE were randomly divided into three groups, received treatment with 0.05% cyclosporine (C group), artificial tears (S group) or their combination (CS group). The evaluation indicators were evaluated at baseline and at weeks 2, 4 and 12.
Results
The symptoms of C and CS groups were reduced significantly. The signs [schirmer I test (F = 4.838, p = .011), ocular staining score (F = 7.961, p = .001) and tear break-up time (F = 9.283, p < .001)] were significantly different between S and C groups as well as S and CS groups. The tear meniscus height (F = 3.197, p = .048) was significantly different between S and CS groups. No serious adverse events occurred.
Conclusion
0.05% cyclosporine is an effective and safe treatment for patients with PSSDE.
Dry eye disease (DED) is a chronic ocular surface disease. It is the instability of tear film or imbalance of ocular surface microenvironment due to abnormal quality, quantity and dynamics of tears. It may be accompanied by ocular surface inflammatory reaction, tissue injury and nerve abnormalities, which lead to a variety of ocular symptoms and/or visual dysfunction.Citation1,Citation2
Sjogren’s syndrome (SS) is a chronic inflammatory autoimmune disease involving mainly the exocrine glands.Citation3 SS can be divided into primary SS and secondary SS. The typical clinical manifestations of primary SS are dry mouth, dry eyes, dry nasal cavity, dry skin and so on. Secondary SS occurs in conjunction with progressive rheumatoid immune diseases, such as systemic lupus erythematosus, rheumatoid arthritis and so on. Secondary SS is often complex, accompanied by other diseases, and the treatment of a variety of diseases needs to be performed simultaneously. The clinical manifestations of eye include dry eyes, foreign body sensation, less tears and other symptoms due to the decreased function of lacrimal gland, reduced mucin secretion, and other abnormalities involving the neurosecreting circuits. Some patients have recurrent suppurative infection of eyelid margin, conjunctivitis, keratitis, etc.Citation4,Citation5 Primary SS-associated dry eye (PSSDE) is generally of moderate to severe, and patients are affected both physically and psychologically. Its treatment has also become a major problem in the fields of ophthalmology and rheumatology.
Previous studies demonstrated that T cells and inflammatory factors in the ocular surface and lacrimal gland tissue of dry eye patients were significantly increased, and the expression of ocular surface T cytokine was significantly increased.Citation6,Citation7 Local use of glucocorticoid in clinic can reduce ocular surface inflammation, but there are many side effects; thus, it cannot be used for a long time.Citation8 On the one hand, cyclosporine acts as an effective anti-inflammatory agent by preventing the activation of T cells and release of inflammatory cytokines.Citation9,Citation10 It can also promote T cell apoptosis by upregulating the levels of Fas/FasL and caspase, while blocking the vicious cycle of inflammation.Citation11 On the other hand, cyclosporine inhibits acinar cell apoptosis,Citation12 directly promotes neurotransmitter release, and improves lacrimal nerve feedback.Citation13 Topical cyclosporine can also lead to an increase in sub-basal corneal nerve density, thus improving the clinical signs and symptoms of SSDE.Citation14 It is very suitable for the treatment of immune-related dry eye in SS patients.Citation9,Citation15,Citation16 However, some scholars have mentioned that cyclosporine seems to be more effective in the treatment of dry eye patients with non-Sjogren’s syndrome (NSS) than those with SS.Citation17 Although 0.05% cyclosporine ophthalmic emulsion has long been prescribed in the U.S. for the treatment of DED, it is not commercially available in China. Cyclosporine eye drops (II) is the first cyclosporine ophthalmic preparation approved for treating Chinese patients with dry eye, and it is a cyclosporine nanoemulsion. It has been reported that both nanoemulsion and conventional cyclosporine can improve ocular signs, symptoms, and conjunctivitis, but the nanoemulsion showed faster improvement in ocular surface staining scores than the conventional emulsion.Citation18 However, there is no universal consensus with regard to its efficacy and safety.
Therefore, this prospective, triple-blind, randomized, controlled trial was conducted to investigate the effectiveness of cyclosporine eye drops (II) in treating Chinese patients with PSSDE. During the study, all subjects, researchers, data collectors and analysts had no prior knowledge of the grouping and medication provided to the subjects, so as to minimize the unobjective and unfair evaluation of test results caused by human factors. What is more, observation indicators are diverse and comprehensive. The purpose of this study is to evaluate the efficacy and safety of topical 0.05% cyclosporine in the treatment of PSSDE, and to promote tear production, improve symptoms, and control or delay the progression of ocular damage and secondary infection caused by immune response. We hope that the conclusion will be helpful for the diagnosis and treatment of PSSDE.
Materials and methods
General information
From January 2021 to September 2021, 60 patients with PSSDE were recruited at the Ophthalmology Clinic of The Second Hospital of Dalian Medical University. All the patients were binocular. The eyes with severe symptoms (n = 60) were selected as subjects. The right eye was chosen if both eyes were of the same severity. Inclusion criteria were as follows: (1) age ≥16 years; (2) patients who underwent anti-SSA/Ro antibody test and labial gland biopsy, and were confirmed by rheumatologist to meet the diagnosis of primary SS (according to 2016 American College of Rheumatology/European League Against Rheumatism classification criteria); (3) patients met the diagnostic criteria of dry eye if had ocular symptom, SIt ≤ 5 mm/5 min, BUT ≤ 10 s, and OSS ≥ 3; and (4) patients who are fully aware of this trial and have signed a written informed consent. Exclusion criteria were as follows: (1) patients with any other eye disease (e.g., glaucoma, blepharitis, infection, or inflammation not associated with DED within 3 months preceding the study) that affects the observation of this trial; (2) patients who had undergone any ocular surgery within 3 months preceding the study; (3) patients with systemic or local medications that might interfere with the study drug; (4) pregnant or lactating women; (5) patients suffering from mental illness and unable to cooperate; and (6) those who are allergic to the drug in this trial. This study was conducted in accordance with the Helsinki Declaration, and in line with relevant clinical trial research norms.
Methodology
Stochastic methods and blind testing
All patients were randomly divided into three groups (n = 20 per group): unit dose vials of unpreserved cyclosporine 0.05% group (C group), preservative-free hyaluronic acid artificial tears group (S group), and the combination group (CS group). C group was given 0.05% cyclosporine eye drops (II) (manufacturer: Sinqi Pharmaceutical, Shenyang, China; drug lot number: H20203239; specification: 0.4 ml: 0.2 m; drug concentration: 0.05%) twice daily, one drop each time, 12 hours apart. S group was given hyaluronic acid sodium eye drops (manufacturer: URSA PHARM Arzneimittel GmbH, Germany; drug lot number: 297927; specification: 10 ml/pcs; drug concentration: 0.1%) four times a day, one drop each time. CS group was given 0.05% cyclosporine eye drops (II) combined with hyaluronic acid sodium eye drops, and the usage and dosage of each drug were same as the first two groups. Hyaluronic acid sodium eye drop was given first, followed by 0.05% cyclosporine eye drops (II), with an interval of 15 minutes. All patients were evaluated for changes from baseline at weeks 2, 4 and 12 after administration. After the completion of the study, the blindness was tested by an individual who was not involved in the study process.
Efficacy evaluation indicators
Efficacy was evaluated according to the improvement of symptoms and signs before and after the treatment. Observation indicators were subjective symptoms [ocular dryness, foreign body sensation, photophobia, burning and ocular surface disease index score (OSDI)], and objective signs [conjunctival congestion score, schirmer I test (SIt), ocular staining score (OSS), tear break-up time (BUT) and tear meniscus height (TMH)]. Objective signs were performed in the following order: conjunctival congestion score, TMH, SIt, BUT and OSS.
The severity scores for each symptom (ocular dryness, foreign body sensation, photophobia and burning) ranged from 0 (normal) to 4 (extremely severe), and the total ocular symptom scores ranged from 0 to 32. Ocular surface disease index score was based on patient self-evaluation;
Conjunctival congestion score: Ocular surface injury was assessed by conjunctival congestion score. Conjunctival congestion score was 0–3 according to the degree of severity;
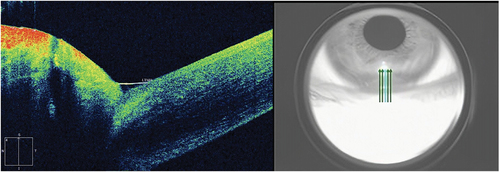
TMH: Anterior segment module of Zeiss Cirrus HD Optical Coherence Tomography 5000 was used to measure the lower tear meniscus height. The patient was asked to sit upright and look forward. The high-resolution mode of anterior segment 5-line raster was selected, and the fixed angle was adjusted to make the line of sight and optical axis consistent. The scan line was adjusted to the TMH perpendicular to the center of the cornea. When high reflection light appeared on the screen, the patient was asked to blink. After image stabilization, the images were quickly captured. Scanned three times in a row. TMH was analyzed by the built-in measurement tool. The following middle position of the lower eyelid was used for the standard scanning ().
SIt: In the absence of topical anesthesia, SIt was performed to detect tear production.
BUT: BUT without blinking was used to reflect tear film instability in clinic.
OSS: Corneal fluorescein staining combined with conjunctival lissamine green staining proposed by Sjögren’s International Collaborative Clinical Alliance (SICCA) was performed in this study. This method is more safe and convenient than Van Bijsterveld method.
All these inspections were conducted by the same operator in strict compliance with the standardized operating procedures. Each inspection was carried out in the same room using the same slit lamp, and there was no significant air convection in the room.
Safety evaluation indicators
Changes in the visual acuity and intraocular pressure before and after the treatments, as well as all adverse events were recorded.
Statistical analysis
SPSS 23.0 software was used to analyze the data. All values were expressed as mean ± standard deviation (). The comparison of measurement data among multiple groups was performed by single-factor analysis of variance, while the comparison between two groups was carried out by LSD. Paired-samples t-test was used for the comparison within the group. The data were also analyzed by Fisher’s exact probability method. P < .05 was considered statistically significant.
Results
All of the 60 patients completed the study, with 20 patients in the hyaluronic acid sodium group, 20 in the 0.05% cyclosporine group and 20 in the combination group. There were no significant differences (p > .05) in gender, age and other baseline information among the three groups ().
Table 1. Three groups of gender, age and other general information compared with the baseline evaluation index.
Comparisons of the subjective symptoms and OSDI scores before and after treatment in the three groups
After 4 weeks of treatment, ocular dryness, foreign body sensation and photophobia in S group were significantly higher than those in C and CS groups. After 12 weeks of treatment, ocular dynesia, foreign body sensation, photophobia and burning in S group were significantly higher than those in C and CS groups. There were no significant differences in these symptoms between C and CS groups ().
Table 2. Comparisons of subjective symptoms before and after treatment in the three groups.
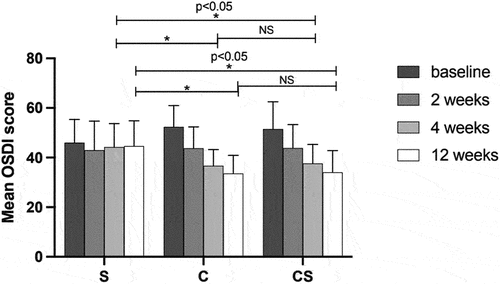
After 2, 4 and 12 weeks of treatment, OSDI scores were significantly decreased in C and CS groups, but significant change in OSDI scores was observed for S group only at 4 weeks (p < .05). After 4 (F = 5.234, p = .008) and 12 (F = 9.908, p < .001) weeks of treatment, there were significant differences in OSDI scores between S and C groups as well as S and CS groups. However, there was no significant difference between C and CS groups () ().
Figure 2. Comparisons of mean OSDI scores before and after treatment in the three groups.
Comparison of the objective signs before and after treatment in the three groups
The conjunctive congestion scores were significantly decreased in C group after 2, 4 and 12 weeks of treatment, and in CS group at week 4 and 12 (p < .05). No significant change in conjunctive congestion scores was observed for S group. After 4 (F = 21.67, p < .001) and 12 (F = 23.318, p < .001) weeks of treatment, there were significant differences in conjunctive congestion scores between S and C groups as well as S and CS groups. However, no significant difference was found between C and CS groups.
After 2, 4 and 12 weeks of treatment, TMH were significantly increased in C and CS groups (p < .05). No significant change in TMH was observed for S group. After 12 weeks of treatment, there was a significant difference in TMH (F = 3.197, p = .048) between S and CS groups, but no significant differences were observed between C and S groups as well as C and CS groups.
SIt was significantly increased in C and CS groups at week 2, 4 and 12 (p < .05). No significant change was observed for S group. After 4 (F = 4.838, p = .011) and 12 (F = 9.177, p < .001) weeks of treatment, SIt differed significantly between S and C groups as well as S and CS groups. However, there was no significant difference between C and CS groups.
BUT was significantly increased in C and CS groups at week 2, 4 and 12, but it was decreased in S group at week 12 (p < .05). After 2 (F = 9.283, p < .001), 4 (F = 18.705, p < .001) and 12 (F = 37.959, p < .001) weeks of treatment, BUT was significantly different between S and C groups as well as S and CS groups, but not between C and CS groups ().
Table 3. Comparisons of objective signs before and after treatment in the three groups.
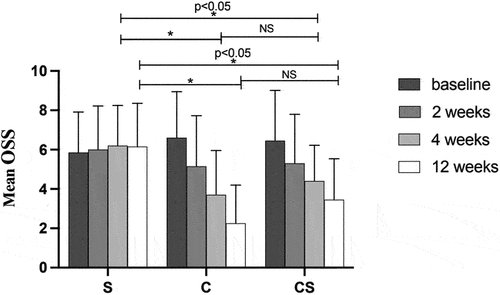
OSS was significantly decreased in both C and CS groups at week 2, 4 and 12 (p < .05), and no significant change was observed for S group. After 4 (F = 7.961, p = .001) and 12 (F = 18.397, p < .001) weeks of treatment, there were significant differences in OSS between S and C groups as well as S and CS groups, but not between C and CS groups () ().
Figure 3. Comparisons of mean OSS before and after treatment in the three groups.
Safety evaluation
After 2, 4 and 12 weeks of treatment, no patients in the three groups had visual decline, and intraocular pressure was within normal range. In the CS group, only one patient experienced eye irritation within 1–3 days and resolved after 2 days. No other adverse events were found.
Discussion
At present, the pathogenesis of dry eye has not been fully elucidated. A large number of existing studies have proved that inflammation is an important mechanism of pathological damage in dry eye.Citation19–24 The expression of various inflammatory mediators in tears and ocular surface tissues is increased in dry eye patients, and the stability of tear film and structure of ocular surface are damaged.Citation25 Some studies have shown that a large number of lymphocytes can be seen in the lacrimal gland and conjunctival tissue of dry eye patients, pro-inflammatory factors are released from infiltrating inflammatory cells,Citation19,Citation26,Citation27 and natural anti-inflammatory factors (lactoferrin) in tears are decreased, which gradually expand the scope of inflammation-associated dry eye.Citation28–30 Anti-inflammatory treatment is a more reasonable approach for DED. Cyclosporine has been proven to exert its anti-inflammatory effects through multiple mechanisms, including the inhibition of T lymphocyte activation and subsequent cytokine production.Citation15,Citation31
Tear film plays an important role in maintaining normal eye function. SS is a complex autoimmune disorder and is a leading cause of aqueous-deficient DED worldwide.Citation24 The clinical manifestations of PSSDE are inflammatory cell infiltration, attenuated lacrimal gland function, and decreased mucin production.Citation32 In addition to its anti-inflammatory effect, previous studies have shown that 0.05% cyclosporine can increase the number of goblet cells, effectively promote mucin production and increase tear flow.Citation33,Citation34 The results of our study also indicated that 0.05% cyclosporine could improve the stability of ocular surface tear film in patients with PSSDE. After 2 weeks of treatment, BUT was better in the C group than in the S group. The main function of meibomian gland is to secrete the lipid layer of tear film in order to prevent the rapid evaporation of tears. Meibomian gland dysfunction (MGD) can also reduce tear film stability and lead to DED.Citation35,Citation36 In most cases, MGD is mild, while only more advanced forms with inflammatory processes require pharmacologic treatment.Citation37 Therefore, blepharitis patients were excluded from this study. Moreover, SS itself can affect the secretory function of meibomian gland, resulting in meibomian obstruction and dysfunction.Citation38 Thus, meibomian gland function was not evaluated separately, which was a limitation of this study. In the future, we hope to have deeper evaluation and analysis of meibomian gland function in PSSDE patients.
It has also been reported that 0.05% cyclosporine significantly improves the symptoms of SSDE, such as burning, pain, and photophobia, in both primary and secondary SS.Citation39–41 Our study also revealed significant improvement in ocular subjective symptoms and OSDI scores in the C and CS groups after 4 weeks of treatment, with no significant difference between the two groups. Several studies have shown that 0.05% cyclosporine can significantly promote tear secretion.Citation42–44 In this study, the SI t and OSS of C and CS groups were obviously improved compared with those of S group after treatment for 4 weeks. The effect of 0.05% cyclosporine on the elevation of TMH appeared later, particularly at week 12 after treatment. The TMH values of C and CS groups were higher than S group, but there was no significant difference between the two groups. This may be related to lacrimal gland tissue fibrosis and functional failure in SS patients, even if the inflammatory response is improved after drug treatment, and the tear secretion level cannot recover within a short time. Therefore, the treatment duration of 0.05% cyclosporine eye drops should be increased to obtain better ocular surface environment and therapeutic effect.Citation15
During this trial period, the clinical symptoms and laboratory indicators of PSSDE were improved in both C and CS groups, while S group did not show considerable treatment efficacy. Considering the hydrophobicity and low aqueous solubility of cyclosporine, ophthalmic emulsion was used as the vehicle in the ocular surface delivery for cyclosporine in our study. Because of the time-taking effects of cyclosporine, the benefits of cyclosporine usually begin after 4 weeks of treatment, and at least 12 weeks of treatment seem to be essential. Our study also showed that ocular symptoms in the C group improved significantly after 4 weeks of treatment, and the difference between the C group and the S group was more obvious at 12 weeks. At present, artificial tears are often used to lubricate dry eyes, because they contain the same ions, pH value and mucin composition as normal tears, and the osmotic pressure and viscosity are also very similar. The artificial tears used in this study were 0.1% hyaluronic acid eye drops, which, as the basic treatment of S group, showed a slight relief in terms of symptoms (ocular dryness, foreign body sensation, photophobia, etc.) after 1 week of treatment, but no improvement at week 4 and 12. Additionally, its use in combination with 0.05% cyclosporine did not increase the efficacy of 0.05% cyclosporine in PSSDE patients. The artificial tear can only exert a short-term relief effect, but not a long-term curative effect.
Local ocular pain and conjunctival congestion are the main adverse reactions of cyclosporine eye drops.Citation45 A clinical study showed that after treatment with 0.05% cyclosporine eye drops for 1 year, the blood cyclosporine concentrations were below 0.1 ng/ml at each time point (before treatment, 1, 6 and 9–12 months), and the blood concentration of eye medication was less than 1/500 of that of systemic medication,Citation41 suggesting a high systemic safety of this therapeutic agent. Treatment with 0.05% cyclosporine for 6 months did not affect central corneal thickness (CCT), corneal endothelial cell density (ECD), IOP, topography, and corneal biomechanics.Citation46 In our study, 0.05% cyclosporine eye drops (II) also exhibited a good ocular safety profile.
Few studies have shown that 0.05% cyclosporine eye drops can effectively treat various types of dry eyes via immunosuppression, anti-apoptosis, increasing goblet cell density, mucin production and increasing tear flow.Citation17,Citation47 Our clinical findings also support these studies, in which 0.05% cyclosporine eye drops can relieve dry eye symptoms, promote tear production, increase tear film stability early, and prevent corneal damage without serious adverse reactions. Therefore, 0.05% cyclosporine eye drops are effective and safe for PSSDE patients. The length of our study might not be long enough to prove the long-term effects of 0.05% cyclosporine on PSSDE. Further prospective multi-center clinical studies with large sample size are needed to verify the long-term efficacy, so as to provide evidence-based guidance for clinical medication.
Author contributions
Conceptualization & Methodology, Mingjun Gao, Lin Zhao; Data Collection, Ran Liang; Software & Data Analysis, Qing Zhu; Writing-Review & Editing, Mingjun Gao; Conceptualization, Methodology and Support Contributions, Qi Zhao, Xiaodan Kong.
Acknowledgments
The authors would like to express their gratitude to the ophthalmological nurses of The Second Hospital of Dalian Medical University for their help and EditSprings (https://www.editsprings.cn/) for the expert linguistic services provided.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi:10.1016/j.jtos.2017.05.008.
- Lemp M, Foulks GN. The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international dry eye workshop. Ocul Surf. 2007;5(2):75–92. doi:10.1016/s1542-0124(12)70081-2.
- Sumida T, Azuma N, Moriyama M, et al. Clinical practice guideline for Sjogren’s syndrome 2017. Mod Rheumatol. 2017;28(3):383–408. doi:10.1080/14397595.2018.1438093.
- Bjordal O, Norheim KB, Rødahl E, Jonsson R, Omdal R. Primary Sjögren’s syndrome and the eye. Surv Ophthalmol. 2020;65(2):119–132. doi:10.1016/j.survophthal.2019.10.004.
- Ciurtin C, Ostas A, Cojocaru VM, Walsh SB, Isenberg DA. Advances in the treatment of ocular dryness associated with Sjögren׳s syndrome. Semin Arthritis Rheum. 2015;45(3):321–327. doi:10.1016/j.semarthrit.2015.06.007.
- Zhang C, Xi L, Zhao S, et al. Interleukin-1β and tumour necrosis factor-α levels in conjunctiva of diabetic patients with symptomatic moderate dry eye: case-control study. BMJ Open. 3. 2016;6(8):e010979. doi:10.1136/bmjopen-2015-010979.
- Youinou P, Pers JO. Disturbance of cytokine networks in Sjögren’s syndrome. Arthritis Res Ther. 6. 2011;13(4):227. doi:10.1186/ar3348.
- Zhang XB, M VJ, Qu Y, et al. Dry eye management: targeting the ocular surface microenvironment. Int J Mol Sci. 29. 2017;18(7):1398. doi:10.3390/ijms18071398.
- Donnenfeld E, Pflugfelder SC. Topical ophthalmic cyclosporine: pharmacology and clinical uses. Surv Ophthalmol. 2009;54(3):321–338. doi:10.1016/j.survophthal.2009.02.002.
- Wilson SE. Inflammation: a unifying theory for the origin of dry eye syndrome. Manag Care. 2003;12:14–19.
- Gao J, Sana R, Calder V, et al. Mitochondrial permeability transition pore in inflammatory apoptosis of human conjunctival epithelial cells and T cells: effect of cyclosporin A. Invest Ophthalmol Vis Sci. 16. 2013;54(7):4717–4733. doi:10.1167/iovs.13-11681.
- Tsubota K, Fujita H, Tadano K, et al. Improvement of lacrimal function by topical application of CyA in murine models of Sjögren’s syndrome. Invest Ophthalmol Vis Sci. 2001;42(1):101–110.
- Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA phase 3 study group. Ophthalmology. 2000;107(4):631–639. doi:10.1016/s0161-6420(99)00176-1.
- Levy O, Labbé A, Borderie V, et al. Increased corneal sub-basal nerve density in patients with Sjögren syndrome treated with topical cyclosporine A. Clin Exp Ophthalmol. 2017;45(5):455–463. doi:10.1111/ceo.12898.
- Chen D, Zhang S, Bian A, et al. Efficacy and safety of 0.05% cyclosporine ophthalmic emulsion in treatment of Chinese patients with moderate to severe dry eye disease: a 12-week, multicenter, randomized, double-masked, placebo-controlled phase III clinical study. Medicine (Baltimore). 2019;98(31):e16710. doi:10.1097/MD.0000000000016710.
- Brito-Zerón P, Sisó-Almirall A, Bové A, Kostov BA, Ramos-Casals M. Primary Sjögren syndrome: an update on current pharmacotherapy options and future directions. Expert Opin Pharmacother. 2013;14(3):279–289. doi:10.1517/14656566.2013.767333.
- Cubuk MO, Ucgul AY, Ozgur A, Ozulken K, Yuksel E. Topical cyclosporine a (0.05%) treatment in dry eye patients: a comparison study of Sjogren’s syndrome versus non-Sjogren’s syndrome. Int Ophthalmol. 2021;41(4):1479–1485. doi:10.1007/s10792-021-01708-1.
- Kang MJ, Kim YH, Chou M. Evaluation of the efficacy and safety of a novel 0.05% cyclosporin a topical nanoemulsion in primary Sjögren’s syndrome dry eye. Ocul Immunol Inflammation. 2020;28(3):370–378. doi:10.1080/09273948.2019.1587470.
- Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):71–81. doi:10.3238/arztebl.2015.0071.
- Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol. 2013;32(1):19–41. doi:10.3109/08830185.2012.748052.
- Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2013;130(1):90–100. doi:10.1001/2Farchophthalmol.2011.364.
- Stern ME, Beuerman RW, Fox RI, et al. A unified theory of the role of the ocular surface in dry eye. Adv Exp Med Biol. 1998;438:643–651. doi:10.1007/978-1-4615-5359-5_91.
- Stern ME, Beuerman RW, Fox RI, et al. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17(6):584–589. doi:10.1097/00003226-199811000-00002.
- Bron A, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438–510. doi:10.1016/j.jtos.2017.05.011.
- Pflugfelder SC, de Paiva CS. The pathophysiology of dry eye disease: what we know and future directions for research. Ophthalmology. 2017;124(11S):S4–S13. doi:10.1016/j.ophtha.2017.07.010.
- Periman LM, Perez VL, Saban DR, Lin MC, Neri P. The immunological basis of dry eye disease and current topical treatment options. J Ocul Pharmacol Ther. 2020;36(3):137–146. doi:10.1089/2Fjop.2019.0060.
- Baudouin C. The pathology of dry eye. Surv Ophthalmol. 2001;45(Suppl 2):S211–S220. doi:10.1016/s0039-6257(00)00200-9.
- Tan X, Sun S, Liu Y, et al. Analysis of Th17-associated cytokines in tears of patients with dry eye syndrome. Eye (Lond). 2014;28(5):608–613. doi:10.1038/eye.2014.38.
- Vagge A, Senni C, Bernabei F, et al. Therapeutic effects of lactoferrin in ocular diseases: from dry eye disease to infections. Int J Mol Sci. 2020;21(18):6668. doi:10.3390/ijms21186668.
- Danjo Y, Lee M, Horimoto K, Hamano T. Ocular surface damage and tear lactoferrin in dry eye syndrome. Acta Ophthalmol. 1994;72(4):433–437. doi:10.1111/j.1755-3768.1994.tb02791.
- Kunert KS, Tisdale AS, Stern ME, et al. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch Ophthalmol. 2000;118(11):1489–1496. doi:10.1001/archopht.118.11.1489.
- Ogawa Y, Shimizu E, Tsubota K. Interferons and dry eye in Sjögren’s syndrome. Int J Mol Sci. 10. 2018;19(11):3548. doi:10.3390/ijms19113548.
- Moon I, Kang HG, Yeo A, et al. Comparison of ocular surface mucin expression after topical ophthalmic drug administration in dry eye-induced mouse model. J Ocul Pharmacol Ther. 2018;34(9):612–620. doi:10.1089/jop.2018.0005.
- Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002;120(3):330–337. doi:10.1001/archopht.120.3.330.
- Nichols K. The international workshop on meibomian gland dysfunction: introduction. Invest Ophthalmol Vis Sci. 2011;52(4):1917–1921. doi:10.1167/iovs.10-6997.
- Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52(4):1938–1978. doi:10.1167/iovs.10-6997c.
- Kovács B, Láng B, Takácsi-Nagy A, et al. Meibom-mirigy-diszfunkció és a száraz szem: diagnosztikai és kezelési lehetőségek [Meibomian gland dysfunction and dry eye: diagnosis and treatment]. Orv Hetil. 2021;162(2):43–51. doi:10.1556/650.2021.31958.
- Sullivan DA, Dana R, Sullivan R, et al. Meibomian gland dysfunction in primary and secondary Sjögren syndrome. Ophthalmic Res. 2018;59(4):193–205. doi:10.1159/000487487.
- Hyon JY, Lee YJ, Yun PY. Management of ocular surface inflammation in Sjögren syndrome. Cornea. 2007;26(9 Suppl 1):S13–5. doi:10.1097/ICO.0b013e31812f6782.
- Devecı H, Kobak S. The efficacy of topical 0.05 % cyclosporine A in patients with dry eye disease associated with Sjögren’s syndrome. Int Ophthalmol. 2014;34(5):1043–1048. doi:10.1007/s10792-014-9901-4.
- Small DS, Acheampong A, Reis B, et al. Blood concentrations of cyclosporin a during long-term treatment with cyclosporin a ophthalmic emulsions in patients with moderate to severe dry eye disease. J Ocul Pharmacol Ther. 2002;18(5):411–418. doi:10.1089/10807680260362696.
- Wan KH, Chen LJ, Young AL. Efficacy and safety of topical 0.05% cyclosporine eye drops in the treatment of dry eye syndrome: a systematic review and meta-analysis. Ocul Surf. 2015;13(3):213–225. doi:10.1016/j.jtos.2014.12.006.
- Chen M, Gong L, Sun X, et al. A comparison of cyclosporine 0.05% ophthalmic emulsion versus vehicle in Chinese patients with moderate to severe dry eye disease: an eight-week, multicenter, randomized, double-blind, parallel-group trial. J Ocul Pharmacol Ther. 2010;26(4):361–366. doi:10.1089/jop.2009.0145.
- Schrell C, Cursiefen C, Kruse F, Jacobi C. Topical cyclosporine A 0.05% in the treatment of keratoconjunctivitis sicca. Klin Monbl Augenheilkd. 2012;229(5):548–553. doi:10.1055/s-0031-1281862. German.
- Clearkin L. Concerns over efficacy and safety of 0.1% cyclosporine a cationic emulsion in the treatment of severe dry eye disease. Eur J Ophthalmol. 8. 2017;27(6):e193. doi:10.5301/ejo.5001044.
- Pérez-Rico C, Germain F, Castro-Rebollo M, Moreno-Salgueiro A, Teus MÁ. Effect of topical 0.05% cyclosporine A on corneal endothelium in patients with dry eye disease. Int J Ophthalmol. 18. 2013;6(4):471–474. doi:10.3980/j.2222-3959.2013.04.12.
- Othman TM, Mousa A, Gikandi PW, AbdelMabod M, Abdelrahman AM. Efficacy and safety of using topical cyclosporine A for treatment of moderate to severe dry eye disease. Saudi J Ophthalmol. 2018;32(3):217–221. doi:10.1016/j.sjopt.2018.06.001.