ABSTRACT
Purpose
To describe the pathogenesis and the general immune mechanisms of the most frequent causes of bacterial uveitis.
Methodology
Narrative review
Results
Both extra- and intracellular bacteria can induce uveitis, whereas intracellular bacteria are generally transported into the inner eye via cells of the innate immune system, mainly macrophages. Systemic adaptive immunity is usually induced before the bacteria are localized to the inner eye, and once T and B cells have detected the pathogens behind the blood-eye barriers they elicit an acute and/or chronic inflammatory response deteriorating visual acuity that can severely affect the non-regenerating, intraocular tissues.
Conclusions
An understanding of pathogenic mechanisms, and its correlation with clinical and imaging features, can facilitate early recognition of microbial factors and institution of appropriate therapy.
Bacteria are a leading cause of ocular infections worldwide. Typically, the spectrum of bacterial ocular infections is described in the context of overtly infectious conditions such as conjunctivitis, keratitis, endophthalmitis (exogenous or endogenous), dacryocystitis or blepharitis.Citation1These infections can occur alone, or in combination (mono- or polymicrobial, respectively), and if not treated appropriately, may lead to visual impairment, blindness or even death. Understandably, such bacterial ocular infections have been extensively investigated for their microbiological spectrum, virulence factors and antibiotic sensitivity.
Bacteria are also a common cause of infectious uveitis across the world. Many forms of bacterial uveitis, such as tuberculosis (TB) and syphilis, have been widely reported for their clinical and imaging characteristics, diagnostic criteria, and treatment strategies.Citation2,Citation3 Yet, despite their wide prevalence, the spectrum of bacterial uveitis has not been described collectively, in recent literature. This is significant, since bacteria have unique cellular and virulence characteristics, that distinguish them from other pathogens such as viruses, fungi, or parasites. Thus, a collective study of the pathogenesis of bacterial uveitis holds the prospect of providing insights that can connect missing links in our current understanding of specific uveitis entities. This is particularly relevant, since the spectrum of bacteria causing uveitis is different from those in keratitis or endophthalmitis, and likely represents a unique set of virulence characteristics. Here, we review the pathogenesis of bacterial uveitis, with insights into the current understanding of specific infections of the inner eye, and its implications on the diagnosis and treatment of these conditions.
Classification of bacterial uveitis
Bacteria have traditionally been classified as gram-positive or gram-negative, based on the presence or absence of staining of their cell wall after application of the Gram stain.Citation4 The main difference in disease pathogenesis between these two groups is that gram-negative bacteria produce endotoxin that can lead to tissue destruction, septic shock, and death. Interestingly, two of the most common bacterial infections causing uveitis – TB and syphilis, and a relatively uncommon, but well-recognized group of infections – rickettsiosis, are caused by either weakly gram-positive or gram-negative bacteria. Mycobacteria (Mycobacterium tuberculosis, as well as atypical mycobacteria) are weakly gram-positive, and require a special technique called acid-fast staining (Ziehl-Neelsen stain) for identification. Spirochetes, including Treponema pallidum (causing syphilis), Borrelia (Lyme’s disease, relapsing fever) and Leptospira (Weil’s disease) as well asRickettsia (spotted fever, epidemic typhus, scrub typhus) are gram-negative.
Bacterial infections can also be classified based on the mode of transmission, which in turn depends on the reservoir of infection (human, animal, arthropod, water, air, soil, food). However, for the purpose of pathogenesis of bacterial uveitis, a more significant determinant is the intracellular or extracellular site of replication of the bacteria, and their dependence on host cells.Citation5 Accordingly, bacteria have been classified as extracellular, facultative intracellular (that do not depend entirely on an intracellular habitat) or obligate intracellular (that fail to survive outside host cells). Here again, the spectrum of bacterial uveitis, with the notable exception of spirochetes, consists entirely of intracellular (facultative/obligate) organisms, unlike other ocular infections (keratitis/endophthalmitis) that are caused largely by extracellular organisms. provides a list of common bacterial pathogens in uveitis along with their preferred site of replication.
Table 1. Preferred host microenvironments of common pathogens in bacterial uveitis and target cells.
Pathomechanisms of intraocular inflammation in bacterial infection
The eye as an immune privileged tissue is separated from the rest of the body by the blood-eye-barriers. The blood vessels in the eyes are sealed with tight junctions, thus not allowing microbes, non-activated cells of the immune system or macromolecules like antibodies and complement factors to enter the eye.Citation6–10 Nevertheless, the inner eye can be severely affected by pathogens like bacteria and fungi, which are usually introduced via surgery or penetrating injury, inducing endophthalmitis. In contrast, endogenous endophthalmitis and infectious uveitis result from hematogenous dissemination, when the pathogens are invading the eye via the blood circulation (hematogenous infection). In that situation, the germs frequently exploit phagocytes as shuttles and are traveling intracellularly in these innate immune cells (mostly macrophages) that have been activated by the phagocytosis of the bacteria.Citation11 Once activated, these cells can pass the blood-eye barriers and thus introduce the microbes into the eyes (also called the Trojan Horse mechanism, according to Greek mythology).
The pathogens internalized by phagocytosis are generally destroyed in the lysosomal pathway and ultimately their proteins are processed and presented to T cells on HLA molecules. Before they have reached the inner eye, the pathogens would have already elicited a systemic immune response and activated a variety of cells of the adaptive immune system, like Band especially T cells. Those lymphocytes would then be swarming out in the body seeking for their respective specific antigens. When lymphocytes are activated, they are allowed to cross all blood barriers, even those of immune privileged organs and are thus also enabled to cross the blood-eye barriers. Once they have found the bacterial antigens presented on HLA molecules of the previously invaded macrophages, or on meanwhile infected ocular cells, they get reactivated and subsequently secrete chemokines and cytokines to recruit further inflammatory cells to ocular tissues, which then induce a deleterious inflammation of the inner eye, impairing or even destroying the blood-eye barriers.Citation12–17 While in some cases clinical symptoms of the systemic infection might precede the ocular manifestation, in others, the primary infection may also have a subclinical course.
Direct and indirect mechanisms in the pathogenesis of bacterial uveitis: tubercular uveitis as a case study
All bacterial pathogens implicated in uveitis have been isolated from ocular tissues. However, the diagnostic proof of the causative pathogen is usually rare in most clinical cases, probably due to the available samples, which are usually aqueous humor or vitreous rather than the choroidal or retinal tissue hosting the mycobacteria. Bacterial growth and replication by itself is usually only mildly pathogenic to ocular tissue unless their numbers are very high. The inflammation and ensuing tissue destruction are a consequence of the immune reaction with the release of cytokines and other inflammatory mediators such as like IL-1, TNFα, IFNγ, IL-17, MIP-1a, MCP-1, IL-8, prostaglandins, and complement factors.Citation18,Citation19 In the sections below, we describe direct (innate and adaptive immune responses to replicating bacteria) and indirect mechanisms that have been implicated in the pathogenesis of bacterial uveitis. We have interspersed this section with the example of tubercular uveitis, as it has been extensively investigated, and is also the most common cause of bacterial uveitis.
Direct mechanisms: Innate and adaptive immune responses to bacteria
Innate recognition and defense of bacteria
Bacteria are recognized by cells of the innate immune systems such as macrophages, dendritic cells, and neutrophil granulocytes via their pattern recognition receptors (PRR). These are molecules specialized for the detection of surface molecules typical for pathogens like bacteria and are called PAMPs (pathogen-associated molecular patterns). Typical bacterial PAMPs are lipopolysaccharide (LPS) recognized by Toll-like receptor 4 (TLR4), flagellin detected by TLR5 and TLR11, or lipoproteins/lipopeptides found in bacterial cell walls and recognized by heterodimeric TLRs such as TLR1/TLR2 and TLR2/TLR6.Citation20–22 In addition to the extracellular PRRs, cells are also equipped with intracellular receptors that detect pathogens internalized by phagocytosis and are located within endosomes like TLR3, TLR7, TLR8, and TLR9. The intracellular PRR TLR9 is specialized for bacteria-typical unmethylated CpG-DNA. More PRRs are found in the cytoplasm such as NLR inflammasomes (nucleotide-binding and oligomerization domain (NOD)-like receptors), RLRs (RIG-1 (retinoid acid-inducible gene I)-like receptors), AIM2 (Absent in Melanoma 2), cGAS (cyclic GMP-AMP (cGAMP) synthase), and STING (Stimulator of Interferon Genes). The latter sense cytosolic DNA.Citation23,Citation24 Inflammasomes recognize a variety of danger signals in the cytosol, which can be of exogenous and also of endogenous origin.Citation25 TLRs and inflammasomes are also expressed in healthy eyes.Citation26–28 Activation of TLRs and inflammasomes results in the production of inflammatory cytokines such as type-I interferons, IL-1β and IL-18.
Besides the professional phagocytic cells mentioned above, there are several other cell types in different organ systems that possess innate immune characteristics.Citation29 These include respiratory epithelial cells, lymphatic endothelial cells, and adipocytes. In the eye, the retinal pigment epithelium (RPE) is one such cell type that has been extensively investigated especially in the context of tubercular uveitis. RPE cells express MHC-class II antigens as well as various Toll-like receptors required for innate immune responses.Citation30,Citation31 The RPE cells are specifically important in the context of serpiginous-like choroiditis (SLC) that affects the RPE and inner choroid. The first indication of the involvement of RPE in ocular TB came from histopathological evaluation of an eye, with panuveitis showing selective distribution of acid-fast bacilli within the RPE, but not in the surrounding choroid.Citation32 In in vitro studies, human fetal RPE cells were found to have similar ability for phagocytosis of Mtb H37Ra as the human macrophage cell-line THP-1.Citation33 However, the RPE cells were able to better control bacterial growth and therefore survived longer with the infection.
Bacterial dissemination (tubercular uveitis)
The hematogenous dissemination of bacteria to the eye has been studied in animal models, in the case of tubercular uveitis. Guinea pigs infected with aerosol containing M. tuberculosisdeveloped choroidal granuloma, containing acid-fast bacilli.Citation34 This model clearly demonstrates the pathway of dissemination from the lungs to the eye. More recently, mycobacterial spreading was demonstrated by intravital microscopy in the zebrafish embryo model after caudal vein injection of M. marinum.Citation35 The bacteria localized near the inner and outer blood-retinal barriers. Chemical depletion of circulating macrophages in the zebrafish embryo showed that extracellular mycobacteria are also capable of crossing the blood-retinal barriers, even at greater frequency than their intracellular counterparts.Citation36
Elimination of bacteria
Extracellular bacteria are eliminated by phagocytosis or NETosis (NET = neutrophil extracellular trap), both usually resulting in killing of the bacteria.Citation37 While NETosis is fatal for the executing granulocyte, phagocytosis (granulocytes, macrophages, and dendritic cells) allows survival of the host cell. Phagocytosis is either mediated via the recognition of bacteria by PRRs on the cell surface, or via the Fc receptor-binding of antibodies bound to bacteria (“opsonization”). Antibody-binding of bacteria can also activate the classical complement pathway and will lead to the lysis of the bacteria when the terminal membrane attack complex is formed.
Microbial evasion and persistence
Internalization of bacteria by phagocytosis should also lead to the destruction of the bacteria, they are usually killed by cytosolic fusion of the phagosomes with lysosomes, which provide various lytic enzymes to kill and process the germs. The bacterial proteins are subsequently cleaved to the size of peptides, which are then loaded on HLA-class II molecules to serve as antigens presented for CD4 + T helper cells to initiate an adaptive immune response. However, some bacteria (especially mycobacteria) can survive the deadly environment of the phagolysosome. They either escape from the phagosome to the cytosol, or prevent the fusion of endosome and lysosome, or they can even proliferate in the phagolysosome. These mechanisms allow certain bacteria to persist in the phagocytes (mainly macrophages) and even abuse them as shuttles to travel through the body while hardly being visible for the immune system. Protected by and hidden in their shuttle cells, the bacteria can also enter the eye ().
Figure 1. Mycobacterial infection.
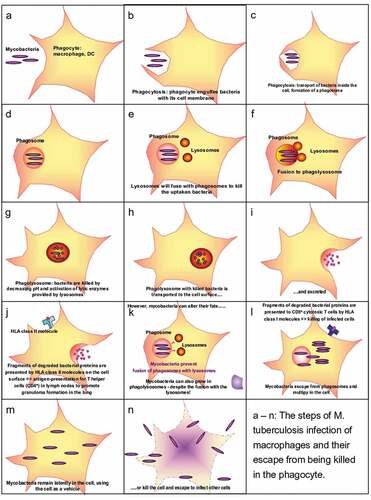
Bacteria persisting in the cytosol are noticed by their host cells via cytosolic PRR; however, the cells do not have the means to kill or eliminate the bacteria once they are in the cytoplasm. The persistence of the bacterial stimulus in the infected macrophages then leads to the formation of granulomas by aggregating and differentiating to epithelioid cells and multinucleated giant cellsCitation38 ().
Figure 2. Granuloma in M. tuberculosis infection.
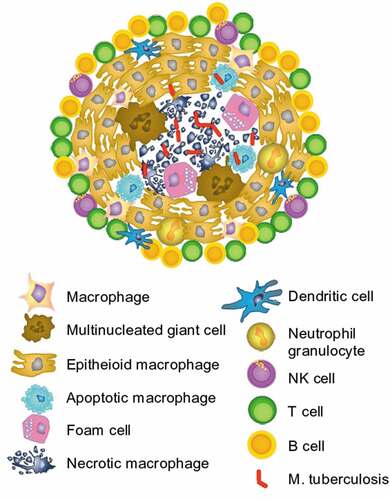
Infected macrophages are aggregating and differentiating to epithelioid cells and multinucleated giant cells and form granulomas. This is typical for Mycobacterium tuberculosis infections. Inactive tuberculosis proliferating mycobacteria can kill their host cells and leave a central necrosis within the granuloma (). The granulomas are surrounded by T, B, and NK cells. The T cells, mainly T helper cells that are expected to be specific for the trapped antigen, secrete IFN-γ and TNF-α, which support the microbicidal activity of the macrophages and promote the integrity of the granuloma.Citation38 The granulomas are a hallmark for the inflammation in ocular tuberculosis and can be detected via the slit lamp or indirectly by hypofluorescence in angiography.
Adaptive immune responses to bacteria
B-cell responses
Extracellular bacteria are easily detected by antibodies and usually elicit a B cell response. Those antibodies in the serum and sometimes in the eye can be used to monitor the infection. Intraocular antibodies can either be produced locally by B cells that have entered the eye or are serum antibodies spilling over via leaky vessels. The origin of antibodies in the eye can be determined by the Goldman-Witmer-Coefficient (GWC), which compares the levels of specific and total intraocular IgG with specific and total IgG in the serum. A GWC more than 4 is indicating local antibody production in the eye.Citation39 In addition to antibody production B cells can also serve as antigen-presenting cells for T cells augmenting T cell-mediated diseases, but some of them might as well have regulatory functions. However, B cells seem to play no pivotal role in intraocular inflammation, unless they are causing tissue destruction with the help of complement and activation of the classical complement pathway.Citation16Antibodies can as well support cell-mediated killing when antigen–antibody complexes on cells are bound by macrophages, granulocytes, or NK cells via their Fc receptors.Citation40
T-cell responses
T cells play a predominant role in intraocular inflammation; cytotoxic T cells can recognize and kill infected cells but are usually strictly controlled by T helper (Th) cells specific for the same pathogen. T helper cells play important roles in the immune response to pathogens. Th 1 cells producing IFN-γ and TNF-α promote the defense against viruses and intracellular bacteria like M. tuberculosis, while Th17 cells, secreting IL-17, TNF-α, and a variety of other cytokines are specialized in the recognition of fungi and extracellular bacteria. While Th1 cells are pivotal for fighting mycobacteria, Th17 cells are needed for the host defense against S. aureus.Citation41 Thus, the IFN-γ secretion of Th1 cells in response to M. tuberculosis antigens (IFN-γ release assay, IGRA) is used for the diagnosis of TB infection. Th1 cells are also involved in the formation of granulomas ().
The host–microbe interaction can have variable outcomes. It may lead to the elimination of the pathogen (described above), or to failure of clearance and microbial invasion of the host. Alternatively, the host and the microbe may reach a state of homeostatic existence or latency, during which the infection may persist without causing any manifestation of clinical disease. The eye, being an immune-privileged organ, provides an ideal microenvironment to support latent infections.Citation42 Such latent infections are not only prone to reactivation and chronic inflammation but may also act as a local adjuvant in apparently non-infectious uveitis.Citation43
Indirect mechanisms in bacterial uveitis
Pathogenesis of intraocular inflammation: Chronic infection and antigenic mimicry
As discussed above, several mechanisms have been proposed to explain the severity of the immunologic inflammation induced by seemingly few microbes. This apparent mismatch demonstrates the destructive power of the immune response (predominantly granulocytes and fast innate) and the development of chronic destructive and potentially autoimmune response in the eye.
Autoimmune mechanisms in bacterial uveitis
“Autoimmune” uveitis is generally difficult to explain, since ocular autoantigens are usually invisible for the immune system, hidden behind the blood-eye barriers and protected by several mechanisms consolidated as the “ocular immune privilege.” Moreover, non-activated cells of the immune system cannot pass the blood-eye barriers to get activated by ocular autoantigens behind the barrier. Therefore, it has been postulated that T cells should be activated outside the eye with antigens that resemble ocular autoantigens (antigenic mimicry), and when they enter the eye and find an ocular antigen recognized by their T cell receptor these cross-reactive T cells will be re-activated. Intraocular reactivation enables these T cells to recruit inflammatory cells and induce uveitis.Citation13,Citation15 Alternatively, in autoimmune susceptible individuals, the inflammatory milieu resulting from the intraocular infection may lead to antigen-independent activation of autoreactive T and B cells. This mechanism is known as bystander activation.Citation44
Autoimmunity in tubercular uveitis
The presence of intraocular autoimmune response in infectious uveitis has been demonstrated in the context of tubercular uveitis.Citation45 It was found that the intraocular immune response in tubercular uveitis consists of both TB antigen-reactive T cells and retinal antigen-reactive (autoreactive) T cells. The autoreactive population was more pro-inflammatory and resistant to activation-induced cell death. As mentioned above, such autoimmune response could result from T cells activated in the periphery and crossing the intact blood-ocular barriers.
Peripheral activation of autoreactive T cells can be achieved by at least two mechanisms – antigenic mimicry between microbial or other environmental molecules and autoantigens, or activation by gut microbiota.Citation46–49 Ocular inflammation in the context of tuberculosis is frequent in countries with a high incidence of tuberculosis and can be explained by the phagocyte-mediated transport of mycobacteria into the eye. However, despite systemic tuberculosis, the presence of the bacteria in the eye often cannot be proven. Moreover, treatment with Bacille-Calmette-Guérin (BCG) as vaccination against tuberculosis or as therapy for bladder carcinoma has also been shown to induce uveitis, although BCG is not infectious for humans and thus usually will not enter the eye. For these cases, we have postulated antigenic mimicry of mycobacterial proteins and retinal autoantigens and provided a variety of potential mimotopes that could induce a cross-reactive T cell response resulting in uveitis, which might also be a pathomechanism in some cases of ocular tuberculosis.Citation50
Peripheral activation of autoreactive T cells by gut microbiota has also been demonstrated in experimental uveitis models, though the exact mimotopes are yet to be identified.Citation51 Several pathogens (reviewed in,Citation15) including bacteria like E. coli have been described as crossreactive with retinal antigens and causing experimental uveitis.Citation46,Citation52,Citation53 Horai et al. found a strong connection between the gut microbiome and uveitis in mice with a transgenic, retina-specific T cell receptor causing spontaneous development of uveitis that was abrogated in germ-free mice. They speculated about antigenic mimicry of gut microbiota and ocular autoantigens, but they could not identify the mimicry partners so far.Citation51,Citation54 Gut microbiota are pivotal for the differentiation of Th17 cells, which are necessary for uveitis induction in mice, therefore germ-free mice might not only lack a respective mimotope for the transgenic T cells, but are also unable to generate the respective pathologic T cell phenotype.Citation49
However, due to the extreme variety and variability of the microbiome, it is extremely difficult to identify distinct mimotopes, especially since our “microbiome” not only consists of bacteria, but also of viruses, fungi, and parasites. In addition to antigenic mimicry, there might be non-specific immunostimulatory (adjuvant) or immunoregulatory effects from microbiota, which play an important role in shaping our immune repertoire.
Another aspect of the role of gut microbiota was unraveled by Chen and colleagues defining normal-tension glaucoma (NTG) as a chronic, low-grade autoimmune disease like uveitis. They demonstrated T cell responses with cross-reactivity between heat-shock proteins from gut microbiota and the heat-shock proteins that are produced by retinal ganglion cells as a stress response to elevated intraocular pressure. Those autoreactive T cells cause a mild inflammatory response that leads to progressive destruction of retinal ganglion cells despite normal IOP, explaining the disease progression in NTG.Citation55
Non-autoimmune mechanisms in tubercular uveitis
Mycobacteria can induce intraocular inflammation by non-autoimmune indirect mechanisms as well. Nearly a century ago Finnoff demonstrated ocular inflammation in rabbits following intracarotid injection of heat-killed M. tuberculosis.Citation56 More recently, the mycobacterial-secreted protein Early Secreted Antigenic Target −6 (ESAT-6) and double-stranded RNA – both constituents of viable M. tuberculosis have been shown to induce NLRP3 inflammasome-dependent Caspase-1 activation in a human fetal RPE cell line as well as after sub-retinal injection in C57BL6/J mice.Citation57 Thus, it is possible that mycobacterial products can induce ocular inflammation by innate immune activation, or by acting as adjuvants for the adaptive immune responses, as demonstrated in animal models of experimental autoimmune uveitis that need M. tuberculosis-enhanced complete Freund’s adjuvant to obtain reliable disease induction.Citation58 Interestingly, the prevalence of TB-immunoreactivity (as measured by interferon-gamma release assays) is significantly higher among uveitis patients, than in the general population, even in non-endemic countries.Citation59,Citation60
Pathogenic mechanisms in other bacterial uveitis entities
Syphilis
Ocular manifestations occur in all stages of acquired and congenital syphilis. Uveal tract gets involved in secondary, latent, and tertiary stage of acquired syphilis and in congenital syphilis.Citation61,Citation62 The eye is embryologically derived from the brain and therefore syphilitic involvement of the optic nerve, retina, and other ocular neuroepithelial structures are regarded as a part of neurosyphilis. Pathogenesis of ocular syphilis has been inadequately reported, partly due to the fastidious nature of the T. pallidum, and partly due to the apparent decline in prevalence of syphilis in the 20th century. Historically, animal inoculation experiments to mimic systemic infection could demonstrate various forms of ocular inflammation including keratitis, anterior and posterior uveitis, and optic atrophy.Citation62–65T. pallidium has also been isolated from aqueous samples of eyes with ocular syphilis, in multiple studies.Citation66,Citation67 Thus, it is likely that the bacteria crosses the blood-retinal barriers to reach ocular tissues. Though not demonstrated in ocular syphilis, transendothelial migration by T. pallidium has been noted in in vitro experiments with different endothelial cell lines.Citation68 Theorganism can also survive in the immune privileged environment of the eye during latent syphilis, as the paucity of antigens on its cell membrane allows it to evade the immune system. The pathogenesis underlying acute syphilitic posterior placoid chorioretinitis is considered to be the immune complex deposition at the level of the pigment epithelium, choriocapillaris, and retinal vessels.
Brucellosis
Brucella infection is a zoonosis caused by gram-negative coccobacillus. B. melitensis, B. abortus, B. canisandB. suis are the causative species of brucellosis. B. melitensisis the most common species prevalent in Latin America, Mediterranean and developing countries, while B. abortusis common in Europe and North America.Citation69
The pathogen becomes sequestered within the reticuloendothelial cells and the mechanism by which it evades the host immune system is still not conclusive. The outer membrane contains a non-classical lipopolysaccharide as compared to the classic ones in Enterobacter species, which is considered to be the major virulence factor of Brucella.Citation70
Ocular involvement in brucellosis ranges from adnexal involvement in the form of dacryoadenitis to anterior segment manifestations like episcleritis, iridocyclitis, nummular keratitis as well as posterior segment manifestations including multifocal choroiditis, exudative retinal detachment, and optic neuritis. The pathogenesis of optic nerve involvement is considered secondary to meningeal inflammation and axonal flow change in the optic nerve due to degeneration.Citation69,Citation71
Bartonellosis
Cat scratch disease is a zoonosis caused by Bartonella henselae. Parinaud oculoglandular syndrome is characteristically described as an ipsilateral granulomatous follicular conjunctivitis with regional lymphadenopathy.Citation72 Bartonellosiscan also present as neuro-retinitis, optic nerve granuloma, pan-uveitis, retinal vasculitis, retinal white dot syndrome, focal choroiditis, serous retinal detachment, peripapillary angiomatous lesion, vascular occlusive events and rarely as retinal bacillary angiomatosis.Citation73
Although the clinical features are well described, the pathogenesis is still elusive. The pathogen affects the permeability of optic nerve head capillaries leading to exudation, which is regarded as the pathology underlying neuro-retinitis with macular star exudates, supported by the evidence of leakage in fundus fluorescence angiography.Citation74,Citation75
The pathology of vascular occlusion is considered to be due to a mechanical occlusion, vasculitis, or mechanical compression at the edematous disc.Citation75 Mechanical occlusion of vessels could be mediated by the chorioretinal inflammation at the site of obstruction. Obliterative vasculitis is caused by the organisms themselves because they invade vascular endothelium, which can stimulate thrombogenic mediators and thus leading to vascular occlusion. Mechanical compression of vessel by an edematous optic nerve could also cause vessel occlusion at the level of lamina cribrosa.
Borreliosis
Borrelia burgdorferi sensulato complex refers to all Borrelia species that have been isolated from small vertebrates and ticks. Only three of them are known to cause Lyme disease in humans. In North America, B. burgdorferi is the only causative pathogen of borreliosis. In Europe and Asia, all three species, B. garinii, B. afzelii, and B. burgdorferi are found, although the latter affects humans less frequently.Citation76
Tick bite causes a local inoculation of the bacteria, where they replicate and stimulate the body’s innate and adaptive cellular immune responses. The spirochete then spreads to several other areas including the eye. Histologic examination of the affected tissues shows infiltrating plasma cells, lymphocytes, and evidence of vasculitis. The spirochete survives the anti-bacterial immune response during its dissemination by a series of adaptive mechanisms. Persisting IgM specific for Borrelia antigens is often observed, and weeks to months after infection IgG antibodies increase, which are bactericidal via complement fixation and opsonization. Specific T cell responses stimulate B cell activity but are not cytotoxic.
Bacterial endogenous endophthalmitis
In the era of broad-spectrum antibiotics, immunomodulating agents, chronic illness with indwelling catheters, diabetes, and Covid-19 pandemic there is a rise in the incidence of endogenous endophthalmitis. Fungus being the predominant causative organism, gram negative (19.5%) and gram-positive (14.6%) bacteria are also isolated from the cultures.Citation77,Citation78 Endogenous endophthalmitis develops when the pathogen in the blood stream crosses the blood-ocular barrier and multiplies within the eye.
Correlation of clinical signs and laboratory investigations with pathogenic mechanisms
A clear understanding of pathogenic mechanisms facilitates the correct interpretation of clinical and laboratory data of patients with bacterial uveitis. However, such correlations are generally not straightforward, as multiple etiological entities (infectious and non-infectious) may have similar clinical manifestations, and a single entity may have multiple manifestations. Besides, multiple pathogenic mechanisms (described above) can be involved in a single infectious entity. Despite these challenges, we may find an application of our current knowledge of pathogenic mechanisms in clinical practice.
Tubercular uveitis is a good example to explain this point.Citation2 The granulomatous response to mycobacteria explains most of the clinical manifestations of this condition. The ability of the host immune response to control replication of bacteria at the site of infection can explain the absence of active pulmonary TB in most cases of ocular TB. Finally, the exaggerated intraocular inflammatory response despite the paucibacillary response, and the therapeutic response to corticosteroid therapy alone in many cases can be explained by the indirect mechanisms of inflammation listed above.
Conclusion
The pathogenesis of bacterial uveitis is driven by the host immune response to the bacterial infection. The host immunity consists of innate and adaptive responses, with the latter including B and T cell responses – the nature of which is determined by the virulence characteristics of the bacteria. Apart from the direct effect of bacterial replication, several indirect mechanisms such as autoimmunity, or innate immune activation by bacterial products, may also be responsible for the intraocular inflammatory response.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Teweldemedhin M, Gebreyesus H, Atsbaha AH, Asgedom SW, Saravanan M. Bacterial profile of ocular infections: a systematic review. BMC Ophthalmol. 2017;17:212. doi:10.1186/s12886-017-0612-2.
- Basu S, Elkington P, Rao NA. Pathogenesis of ocular tuberculosis: new observations and future directions. Tuberculosis. 2020;124:101961. doi:10.1016/j.tube.2020.101961.
- Furtado JM, Simões M, Vasconcelos-Santos D, et al. Ocular syphilis. Surv Ophthalmol. 2022;67:440–462. doi:10.1016/j.survophthal.2021.06.003.
- Doron S, Gorbach SL. Bacterial infections: overview. Int Encycl Public Health. 2008;273–282. doi:10.1016/B978-012373960-5.00596-7.
- Silva M. Classical labeling of bacterial pathogens according to their lifestyle in the host: inconsistencies and alternatives. Front Microbiol. 2012;3. doi:10.3389/fmicb.2012.00071.
- Shechter R, London A, Schwartz M. Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nat Rev Immunol. 2013;13:206–218. doi:10.1038/nri3391.
- Stein-Streilein J, Lucas K. A current understanding of ocular immune privilege. Current Immunol Rev. 2011;7:336–343. doi:10.2174/157339511796196683.
- Streilein JW. Regional immunity and ocular immune privilege. Chem Immunol. 1999;73:11–38. doi:10.1159/000058741.
- Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leukoc Biol. 2003;74:179–185. doi:10.1189/jlb.1102574.
- Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–889. doi:10.1038/nri1224.
- Teng O, Ang CKE, Guan XL. Macrophage-bacteria interactions-a lipid-centric relationship. Front Immunol. 2017;8:1836. doi:10.3389/fimmu.2017.01836.
- Prendergast RA, Iliff CE, Coskuncan NM, et al. T cell traffic and the inflammatory response in experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 1998;39:754–762.
- Thurau SR, Mempel TR, Flugel A, et al. The fate of autoreactive, GFP+ T cells in rat models of uveitis analyzed by intravital fluorescence microscopy and FACS. Int Immunol. 2004;16:1573–1582. doi:10.1093/intimm/dxh158.
- Wildner G, Diedrichs-Möhring M. Resolution of uveitis. Semin Immunopathol. 2019;41:727–736. doi:10.1007/s00281-019-00758-z.
- Wildner G, Diedrichs-Möhring M. Molecular mimicry and uveitis. Front Immunol. 2020;11. doi:10.3389/fimmu.2020.580636.
- Jha P, Bora PS, Bora NS. The role of complement system in ocular diseases including uveitis and macular degeneration. Mol Immunol. 2007;44:3901–3908. doi:10.1016/j.molimm.2007.06.145.
- Busch C, Annamalai B, Abdusalamova K, et al. Anaphylatoxins activate Ca(2+), Akt/PI3-Kinase, and FOXO1/FoxP3 in the retinal pigment epithelium. Frontiers Immunol. 2017;8:703. doi:10.3389/fimmu.2017.00703.
- Wroblewski KJ, Hidayat AA, Neafie RC, Rao NA, Zapor M. Ocular tuberculosis: a clinicopathologic and molecular study. Ophthalmology. 2011;118:772–777. doi:10.1016/j.ophtha.2010.08.011.
- Basu S. Absence of evidence as the evidence of absence: the curious case of latent infection causing ocular tuberculosis. Frontiers Ophthalmol. 2022;2. doi:10.3389/fopht.2022.874400.
- Hatai H, Lepelley A, Zeng W, Hayden MS, Ghosh S. Toll-like receptor 11 (TLR11) interacts with flagellin and profilin through disparate mechanisms. PLoS One. 2016;11:e0148987. doi:10.1371/journal.pone.0148987.
- Yang J, Yan H. TLR5: beyond the recognition of flagellin. Cell Mol Immunol. 2017;14:1017–1019. doi:10.1038/cmi.2017.122.
- Takeda K, Takeuchi O, Akira S. Recognition of lipopeptides by Toll-like receptors. J Endotoxin Res. 2002;8:459–463. doi:10.1177/09680519020080060101.
- Pandey S, Kawai T, Akira S. Microbial sensing by Toll-like receptors and intracellular nucleic acid sensors. Cold Spring Harb Perspect Biol. 2014;7:a016246–a. doi:10.1101/cshperspect.a016246.
- Lugrin J, Martinon F. The AIM2 inflammasome: sensor of pathogens and cellular perturbations. Immunol Rev. 2018;281:99–114. doi:10.1111/imr.12618.
- Cassel SL, Joly S, Sutterwala FS. The NLRP3 inflammasome: a sensor of immune danger signals. Semin Immunol. 2009;21:194–198. doi:10.1016/j.smim.2009.05.002.
- Rodríguez-Martínez S, Cancino-Díaz ME, Jiménez-Zamudio L, García-Latorre E, Cancino-Díaz JC. TLRs and NODs mRNA expression pattern in healthy mouse eye. Br J Ophthalmol. 2005;89:904–910. doi:10.1136/bjo.2004.056218.
- Lin X, Fang D, Zhou H, Su SB. The expression of Toll-like receptors in murine Müller cells, the glial cells in retina. Neurol Sci. 2013;34:1339–1346. doi:10.1007/s10072-012-1236-1.
- Xu W-Q, Wang Y-S. The role of Toll-like receptors in retinal ischemic diseases. Int J Ophthalmol. 2016;9:1343–1351. doi:10.18240/ijo.2016.09.19.
- Mayito J, Andia I, Belay M, et al. Anatomic and cellular niches for Mycobacterium tuberculosis in latent tuberculosis infection. J Infect Dis. 2019;219:685–694. doi:10.1093/infdis/jiy579.
- Liversidge JM, Sewell HF, Forrester JV. Human retinal pigment epithelial cells differentially express MHC class II (HLA, DP, DR and DQ) antigens in response to in vitro stimulation with lymphokine or purified IFN-gamma. Clin Exp Immunol. 1988;73:489–494.
- Kumar MV, Nagineni CN, Chin MS, Hooks JJ, Detrick B. Innate immunity in the retina: toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J Neuroimmunol. 2004;153:7–15. doi:10.1016/j.jneuroim.2004.04.018.
- Rao NA, Saraswathy S, Smith RE. Tuberculous uveitis: distribution of Mycobacterium tuberculosis in the retinal pigment epithelium. Arch Ophthalmol. 2006;124:1777–1779. doi:10.1001/archopht.124.12.1777.
- Nazari H, Karakousis PC, Rao NA. Replication of Mycobacterium tuberculosis in retinal pigment epithelium. JAMA Ophthalmol. 2014;132:724–729. doi:10.1001/jamaophthalmol.2014.270.
- Rao NA, Albini TA, Kumaradas M, Pinn ML, Fraig MM, Karakousis PC. Experimental ocular tuberculosis in Guinea pigs. Arch Ophthalmol. 2009;127:1162–1166. doi:10.1001/archophthalmol.2009.220.
- Takaki K, Ramakrishnan L, Basu S. A zebrafish model for ocular tuberculosis. PLOS ONE. 2018;13:e0194982. doi:10.1371/journal.pone.0194982.
- Damera SK, Panigrahi RK, Mitra S, Basu S. Role of extracellular Mycobacteria in blood-retinal barrier invasion in a zebrafish model of ocular TB. Pathogens. 2021;10:333. doi:10.3390/pathogens10030333.
- Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi:10.1126/science.1092385.
- Pagán AJ, Ramakrishnan L. The formation and function of granulomas. Annu Rev Immunol. 2018;36:639–665. doi:10.1146/annurev-immunol-032712-100022.
- De Groot-Mijnes JD, Rothova A, Van Loon AM, et al. Polymerase chain reaction and Goldmann-Witmer coefficient analysis are complimentary for the diagnosis of infectious uveitis. Am J Ophthalmol. 2006;141:313–318. doi:10.1016/j.ajo.2005.09.017.
- Sawa T, Kinoshita M, Inoue K, Ohara J, Moriyama K. Immunoglobulin for treating bacterial infections: one more mechanism of action. Antibodies (Basel). 2019;8. doi:10.3390/antib8040052.
- Lee Y, Kuchroo V. Defining the functional states of Th17 cells. F1000Res. 2015;4:132. doi:10.12688/f1000research.6116.1.
- Forrester JV, Mölzer C, Kuffova L. Immune privilege furnishes a niche for latent infection. Frontiers Ophthalmol. 2022;2. doi:10.3389/fopht.2022.869046.
- Forrester JV, Kuffova L, Dick AD. Autoimmunity, autoinflammation, and infection in uveitis. Am J Ophthalmol. 2018;189:77–85. doi:10.1016/j.ajo.2018.02.019.
- Pacheco Y, Acosta-Ampudia Y, Monsalve DM, Chang C, Gershwin ME, Anaya JM. Bystander activation and autoimmunity. J Autoimmun. 2019;103:102301.
- Tagirasa R, Parmar S, Barik MR, Devadas S, Basu S. Autoreactive T cells in immunopathogenesis of TB-associated uveitis. Invest Ophthalmol Vis Sci. 2017;58:5682–5691. doi:10.1167/iovs.17-22462.
- Wildner G, Diedrichs-Möhring M. Autoimmune uveitis induced by molecular mimicry of peptides from rotavirus, bovine casein and retinal S-antigen. Eur J Immunol. 2003;33:2577–2587. doi:10.1002/eji.200324058.
- Lee Yun K, Menezes Juscilene S, Umesaki Y, Mazmanian Sarkis K. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc National Acad Sci. 2011;108:4615–4622. doi:10.1073/pnas.1000082107.
- Lee N, Kim WU. Microbiota in T-cell homeostasis and inflammatory diseases. Exp Mol Med. 2017;49:e340. doi:10.1038/emm.2017.36.
- Ivanov II, Frutos RdL, Manel N, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349.
- Garip A, Diedrichs-Möhring M, Thurau SR, Deeg CA, Wildner G. Uveitis in a patient treated with Bacille-Calmette-Guérin: possible antigenic mimicry of mycobacterial and retinal antigens. Ophthalmology. 2009;116:2457–62.e1–2. doi:10.1016/j.ophtha.2009.05.021.
- Horai R, Zárate-Bladés CR, Dillenburg-Pilla P, et al. Microbiota-dependent activation of an autoreactive T cell receptor provokes autoimmunity in an immunologically privileged site. Immunity. 2015;43:343–353. doi:10.1016/j.immuni.2015.07.014.
- Shinohara T, Singh VK, Yamaki K, Abe T, Tsuda M, Suzuki S. S-antigen: molecular mimicry may play a role in autoimmune uveitis. Prog Clin Biol Res. 1991;362:163–190. Issn: 0361-7742.
- Singh VK, Yamaki K, Abe T, Shinohara T. Molecular mimicry between uveitopathogenic site of retinal S-antigen and Escherichia coli protein: induction of experimental autoimmune uveitis and lymphocyte cross-reaction. Cell Immunol. 1989;122:262–273. doi:10.1016/0008-8749(89)90166-4.
- Horai R, Sen HN, Caspi RR. Commensal microbiota as a potential trigger of autoimmune uveitis. Expert Rev Clin Immunol. 2017;13:291–293. doi:10.1080/1744666X.2017.1288098.
- Chen H, Cho K-S, Vu THK, et al. Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma. Nat Commun. 2018;9:3209. doi:10.1038/s41467-018-05681-9.
- Finnoff W. Changes in eyes of rabbits following injection of dead tubercle bacilli into common carotid artery. Am J Ophthalmol. 1924;7:365–372. doi:10.1016/S0002-9394(24)90818-X.
- Basu S, Fowler BJ, Kerur N, Arnvig KB, Rao NA. NLRP3 inflammasome activation by mycobacterial ESAT-6 and dsRNA in intraocular tuberculosis. Microb Pathog. 2018;114:219–224. doi:10.1016/j.micpath.2017.11.044.
- Caspi RR. Understanding autoimmune uveitis through animal models the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2011;52:1873–1879.
- Yakin M, Kesav N, Cheng SK, Caplash S, Gangaputra S, Sen HN. The association between QuantiFERON-TB gold test and clinical manifestations of uveitis in the United States. Am J Ophthalmol. 2021;230:181–187. doi:10.1016/j.ajo.2021.04.024.
- Groen-Hakan F, van Laar JAM, Bakker M, van Hagen PM, Hardjosantoso H, Rothova A. Prevalence of positive QuantiFERON-TB gold in-tube test in uveitis and its clinical implications in a country nonendemic for tuberculosis. Am J Ophthalmol. 2020;211:151–158. doi:10.1016/j.ajo.2019.11.009.
- Dutta Majumder P, Chen EJ, Shah J, et al. Ocular syphilis: an update. Ocul Immunol Inflamm. 2019;27:117–125. doi:10.1080/09273948.2017.1371765.
- Klauder JV, Meyer GP. Chorioretinitis of congenital syphilis. AMA Arch Ophthalmol. 1953;49:139–157. doi:10.1001/archopht.1953.00920020144002.
- Brown WH, Pearce L. Experimental syphilis in the rabbit: VII. Affections of the eyes. J Exp Med. 1921;34:167–183. doi:10.1084/jem.34.2.167.
- Smith JL, Israel CW. Treponemes in aqueous humor in late seronegative syphilis. Trans Am Acad Ophthalmol Otolaryngol. 1968;72:63–75.
- Smith JL, Singer JA, Reynolds DH, Moore MB Jr., Yobs AR, Clark JW Jr. Experimental ocular syphilis and neurosyphilis. Br J Vener Dis. 1965;41:15–23.
- Hong M-C, Sheu S-J, Wu T-T, Chuang C-T. Ocular uveitis as the initial presentation of syphilis. J Chin Med Assoc. 2007;70:274–280. doi:10.1016/S0002-9394(00)00573-0.
- Schmidt H, Goldschmidt E. Demonstration of motile treponemes in the aqueous humour in secondary syphilis. Br J Vener Dis. 1972;48:400–401. doi:10.1136/sti.48.5.400.
- Thomas DD, Fogelman AM, Miller JN, Lovett MA. Interactions of Treponema pallidum with endothelial cell monolayers. Eur J Epidemiol. 1989;5:15–21. doi:10.1007/BF00145039.
- Bazzazi N, Yavarikia A, Keramat F. Ocular involvement of brucellosis. Middle East Afr J Ophthalmol. 2013;20:95–97. doi:10.4103/0974-9233.106407.
- Christopher S, Umapathy BL, Ravikumar KL. Brucellosis: review on the recent trends in pathogenicity and laboratory diagnosis. J Lab Physicians. 2010;2:55–60. doi:10.4103/0974-2727.72149.
- Rolando I, Olarte L, Vilchez G, et al. Ocular manifestations associated with brucellosis: a 26-year experience in Peru. Clin Infect Dis. 2008;46:1338–1345. doi:10.1086/529442.
- Cunningham ET, Koehler JE. Ocular bartonellosis. Am J Ophthalmol. 2000;130:340–349.
- Ormerod LD, Dailey JP. Ocular manifestations of cat-scratch disease. Curr Opin Ophthalmol. 1999;10:209–216. doi:10.1097/00055735-199906000-00010.
- Gass JD. Diseases of the optic nerve that may simulate macular disease. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977;83:763–770.
- Solley WA, Martin DF, Newman NJ, et al. Cat scratch disease: posterior segment manifestations. Ophthalmology. 1999;106:1546–1553. doi:10.1016/S0161-6420(99)90452-9.
- Schotthoefer AM, Frost HM. Ecology and epidemiology of Lyme Borreliosis. Clin Lab Med. 2015;35:723–743.
- Connell PP, O’Neill EC, Fabinyi D, et al. Endogenous endophthalmitis: 10-year experience at a tertiary referral centre. Eye (Lond). 2011;25:66–72. doi:10.1038/eye.2010.145.
- Jackson TL, Eykyn SJ, Graham EM, Stanford MR. Endogenous bacterial endophthalmitis: a 17-year prospective series and review of 267 reported cases. Surv Ophthalmol. 2003;48:403–423. doi:10.1016/S0039-6257(03)00054-7.
