ABSTRACT
Purpose
To compare visual outcomes, ocular complications and therapies for patients with scleritis-associated intraocular inflammation (SAI) and patients with isolated scleritis (IS).
Results
A total of 52 patients (36 with SAI and 16 with IS) were reviewed. Mean age (standard deviation) at presentation was 48.4 years old (± 15.4) in the SAI group and 53 years old (± 17.1) in the IS group (p = .37). Visual acuity was worse at presentation and last visit for patients with SAI compared to IS (p = .04). Patients in the SAI group developed greater posterior segment complications than in the IS group (p = .002).
Conclusions
Scleritis with intraocular inflammation was associated with a higher rate of visual morbidity compared to isolated scleritis. More aggressive management strategies may be needed for patients who present with scleritis associated with inflammation.
Scleritis is an ocular inflammatory condition, categorized as anterior or posterior regarding its location in relation to the ora serrata, and mostly associated with a systemic autoimmune disease or an infectious etiology.Citation1,Citation2 Association of mild anterior chamber and/or anterior vitreous inflammation are described as complications of anterior scleritis by some authors, in addition to scleral wall thinning (scleromalacia perforans).Citation3 Posterior segment complications observed in patients with posterior scleritis, such as cystoid macular edema, optic disc edema and optic neuropathy, exudative retinal detachments, or choroidal granulomas have also been reported in prior series.Citation3
The incidence of visual loss in patients with scleritis varies between 30% and 50% in prior studies with literature suggesting a relationship to anterior or posterior segment complications (i.e. cataract, glaucoma, epiretinal membranes, cystoid macular edema, or optic atrophy).Citation3–5 Moreover, the need for corticosteroid-sparing agents including biologic therapies to reduce scleral inflammation is greater for patients with refractory or necrotizing scleritis, given the risk of vision loss and complications related to long-term corticosteroid use.Citation5,Citation6
Whether intraocular inflammation associated with scleritis increases the rate of ocular complications and the need for steroid-sparing agents is unknown. There are very few reports in the literature analyzing the role of intraocular inflammation associated with scleritis as a risk factor for poor visual outcome. Herein, we evaluated the visual outcomes, ocular complications, and therapies of patients diagnosed with scleritis associated with intraocular inflammation compared to patients with isolated scleritis over a 5-year period at a tertiary referral, university-based academic institution.
Methods
The study was approved by the Emory University Institutional Review Board. A waiver of informed consent was obtained as the study was a retrospective chart review and involved no more than minimal risks to the subjects.
Study approach, inclusion and exclusion criteria
A retrospective review of the electronic medical records of patients seen at the Uveitis clinic at Emory Eye Center with a diagnosis of scleritis on ICD-9 (ICD-9-CM 379.00) and ICD-10 (H15.009) codes between January 2014 and March 2019 was performed. One hundred and ninety-one patients were selected. We excluded patients with a diagnosis of episcleritis. Eighty-one patients were identified; among them 19 were excluded for a follow-up less than 3 months, 7 for the absence of recurrence or chronicity of scleritis in the setting of long-standing history of uveitis, 2 for lack of access to medical record, and 1 for scleral abscess secondary to endophthalmitis. A total of 52 patients (81 eyes) were included, 36 (55 eyes) with scleritis-associated intraocular inflammation (SAI) and 16 (26 eyes) with isolated scleritis (IS). For the SAI group, inclusion criteria included the presence of uveitis (including anterior, intermediate, posterior or panuveitis), either at presentation or during follow-up diagnosed by a uveitis specialist. All patients with diagnosis of scleritis in our study underwent an ultrasound. Patients with posterior scleritis were included if the diagnosis was confirmed by a positive B-mode ultrasound (increased scleral wall thickness or “T-sign” [fluid in the Tenon’s capsule]). All patients underwent an infectious and inflammatory scleritis work up upon initial diagnosis. Laboratory testing was tailored to the individual, but testing included ACE, lysozyme, RPR, FTA-ABS, HLA B-27, CBC, comprehensive metabolic panel, Quantiferon TB-Gold, rheumatoid factor, ANA, ANCA, CCP, dsDNA, HIV and HSV serology if there were concerns for suspected herpetic sclerokeratitis. Chest X-ray and/or high-resolution CT scans were ordered by the treating physician if there were concerns for sarcoidosis.
Data collected
Demographic data collected included age at diagnosis, race and sex. Ocular and medical history, presence of systemic-associated disease or infection at presentation or during follow-up, and treatment modalities of scleritis and related systemic disease at presentation, during follow-up, and at last visit were collected.
Data was collected at initial visit, 1 month, 3 months, 6 months, 12 months, and last visit. The following variables were assessed: visual acuity (Snellen visual acuity converted to log of the minimal angle of resolution [logMAR]), scleritis type and activity, anatomic location of uveitis if present and activity, and anterior and posterior segment secondary complications. Scleritis type was classified as anterior, posterior, or necrotizing according to the classification of Watson and HayrehCitation7 and graded according to Sen et al.Citation8 on a 0 to 4+ scale. Uveitis type was classified as anterior, intermediate, posterior and panuveitis, according to the International Uveitis Study Group.Citation9 Anterior segment complicationsCitation10 included scleromalacia, glaucoma, and cataract. Posterior segment complications included optic disc edema or atrophy, choroidal granulomas and scars, exudative retinal detachment, epiretinal membrane (ERM), and cystoid macular edema (CME).
Data analysis
Descriptive statistics were calculated as frequencies and proportions for categorical variables and means with standard deviations for continuous variables. Student t-tests were used to compare continuous variables, and Fisher’s exact test was used to compare categorical variables between the scleritis groups of interest.
LogMAR visual acuity was examined in a repeated measure analysis with a means model providing estimates of the means by time and scleritis group. Complication rates were also examined in a repeated measure analysis with a generalized estimating equation model by time and scleritis group. Statistical tests were done within the framework of the repeated measures models.
For all statistical tests, P ≤ .05 was considered statistically significant. Microsoft Excel (Microsoft Corporation, Redmond Washington), RStudio (version 1.3.1093; RStudio, Boston, MA), and SAS (v9.4; SAS Institute, Cary, NC) were all used for statistical analyses.
Results
Demographics, scleritis type and associated diseases at first presentation
Details of demographics, scleritis types, ocular complications and associated systemic diseases for the two groups are described in . The mean age at presentation and demographics were similar in the SAI and IS groups. The majority of patients with scleritis with and without ocular inflammation displayed anterior scleritis (50.9%, 65.4%, respectively; p = .24) and bilateral disease (52.7%, 68.8%, respectively; p = .37). A total of seven eyes in the IS group and 25 eyes in the SAI group were diagnosed with a posterior scleritis. Of the seven eyes with isolated scleritis and posterior scleritis, two eyes had positive T sign on ultrasound while the remaining five eyes had thickened sclera posteriorly on ultrasound. Of the 25 eyes with scleritis associated inflammation and posterior scleritis, 13 eyes had positive T sign on ultrasound and 12 eyes had posterior scleral thickening on ultrasound.
Table 1. Demographic and scleritis characteristics at presentation.
A larger proportion of patients of the SAI group had an idiopathic scleritis (22, 61.1%), while there was a trend towards an increased association of autoimmune disease in the IS group (9, 56.3%) (p = .07) (). A concomitant infection with herpes virus was found in one patient (2.8%) in the SAI group, and latent tuberculosis was documented in three patients (8.3%) and one patient (6.3%), respectively, in the SAI and IS groups. The median follow-up was 33.0 months (IQR 16.0–78.0) for the SAI group and 47.0 months (IQR 21.0–66.0) for the IS group.
Visual acuity outcomes, ocular complications and immunosuppression requirement
Visual acuity outcomes
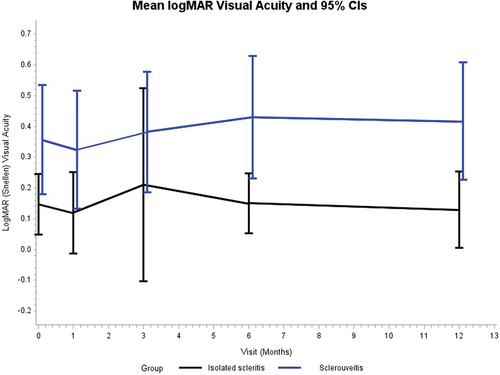
Mean LogMar visual acuity (95% CI) was significantly worse in the SAI group (0.36 [0.18–0.53]) compared to the IS group (0.15 [0.05–0.25]) at the initial visit (p = .04), at 6 months (0.43 [0.13–0.52] versus 0.15 [0.05–0.25], p = .01), and at 12 months (0.42 [0.23–0.61] versus 0.13 [0.01–0.25]; p = .01) (). This difference in vision stayed consistent over all time points. There was no progression of visual loss observed in either the SAI or IS group, defined as worsening of >1 line on Snellen visual acuity between the first and last visits (p > .05). The cause of visual acuity loss in cases with severe visual impairment (defined as LogMar of 1.0 or greater) was determined. One eye in the IS group showed severe visual impairment at final visit; specifically, the final logMAR visual acuity was 2.0 due to progression to scleromalacia perforans that eventually required a scleral patch graft and systemic immunosuppression. In the SAI group there were eight eyes in the six patients that had LogMar of 1.0 or greater. Three eyes had visual impairment secondary to complications from chronic exudative detachment, one eye had vision loss secondary to a cataract as well as an exudative detachment, two eyes had chronic cystoid macular edema, and two eyes had end-stage glaucoma.
Ocular complications
Affected eyes in the SAI group showed a significantly greater posterior segment complication rate compared to the IS group at presentation (28 of 55 eyes [50.9%] versus 3 of 26 eyes in IS group [11.5%], p = .0006) and at the last visit (30 of 55 eyes in SAI group [54.5%] and 2 of 26 eyes in IS group [7.7%], respectively, at last visit, p = .0001). For the SAI group at presentation, 8 eyes had ERM, 12 eyes had abnormal choroid (i.e. choroidal thickening, choroidal effusion, or choroiditis documented by clinical exam or imaging), 1 eye had an exudative detachment, and 7 eyes had CME; at last visit, 8 eyes had an ERM, 14 eyes had abnormal choroid, 3 eyes had exudative detachments, and 5 eyes had CME. For the IS group at presentation, 2 eyes had exudative detachment and 1 eye had an abnormal choroid; at last visit, 2 patients had ERM. The rate of anterior segment complications was lower in the SAI group compared to the IS group at presentation (16 eyes in SAI group [29.1%] versus 14 eyes in IS group [53.8%]; p = .05); however, there was no difference in the rate of anterior segment complication in the 2 groups at last visit (39 [71.0%] and 16 [62.0%]; p = .45). For the SAI group at presentation, 16 eyes had cataracts; at last visit, 29 eyes had cataracts, 5 eyes had glaucoma, and 5 eyes had scleromalacia. For the IS group at presentation, 14 eyes had cataracts; at last visit, 15 eyes had cataracts and 1 eye had glaucoma. Rate of anterior and posterior segment complications at 1, 3, 6, and 12 months in the two groups is listed in Supplemental Table S1.
Immunosuppressive treatment
The need for a corticosteroid-sparing immunosuppressive treatment did not differ in the two groups during follow-up (p = .16) or at last visit (p = .49). During the course of the disease, 22 (61.1%) patients with SAI and 10 (62.5%) with IS were treated with at least one immunosuppressive or biologic treatment, while 13 (36.1%) with SAI and 5 (31.3%) with IS were only treated with oral steroids. Methotrexate and adalimumab were the principal first-line agents in both groups for immunosuppressive and biologic treatments, respectively. The distribution of steroid-sparing agents used in both groups during the course of the disease is illustrated in Supplemental Table S2.
Illustrative cases
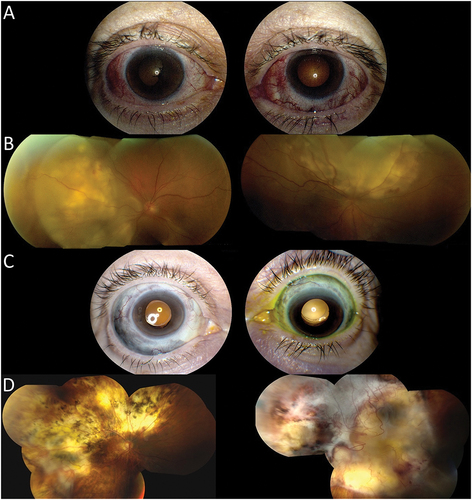
Patient 1 is a 63-year-old Caucasian female, diagnosed with rheumatoid arthritis and chronic idiopathic demyelinating polyneuropathy, who presented with bilateral necrotizing anterior scleritis and panuveitis with visual acuity of 20/30 in both eyes on her initial presentation (). Following a negative workup for inflammatory and infectious disease entities, she was started on oral corticosteroids (1 mg/kg) with methotrexate (25 mg weekly) as a steroid-sparing agent. Three months later she developed bilateral posterior scleritis with exudative retinal detachments and her vision rapidly declined to count fingers in the right eye and 20/200 in the left (). She was treated with intravenous methylprednisolone (1 gram IV infusion) and was transitioned from methotrexate to azathioprine (3 mg/kg/day, divided into twice daily dosing) for 3 months. She then developed a scleritis flare-up with worsening exudative retinal detachment and was treated with mycophenolate mofetil, followed by escalation to adalimumab (40 mg every 2 weeks). Given disease progression, she was advanced to cyclophosphamide (2 mg/kg, divided into twice daily dosing) and eventually rituximab (1000 mg divided by 2 weeks, then every 6 months thereafter) with intravenous methylprednisolone, which ultimately led to disease control. At last visit, she was in remission at 57.0 months of follow-up, bilateral cataract surgery, while treated with prednisone 10 mg and rituximab. Her visual acuity was 20/150 in the right eye and had progressed to no light perception in the left eye due to a chronic, exudative retinal detachment with severe subretinal fibrosis. Slit-lamp examination showed bilateral scleromalacia without residual inflammation (), and fundus showed bilateral chorioretinal scarring with fibrosis ().
Figure 2. 63-year-old Caucasian female presenting with bilateral necrotizing anterior scleritis and panuveitis. A) Slit lamp photos demonstrating necrotizing anterior scleritis. B) Fundus photos demonstrating posterior scleritis with exudative retinal detachments C) Slit lamp photo showing bilateral scleromalacia post-immunosuppressive therapy. D) fundus photos showing bilateral chorioretinal scarring with fibrosis.
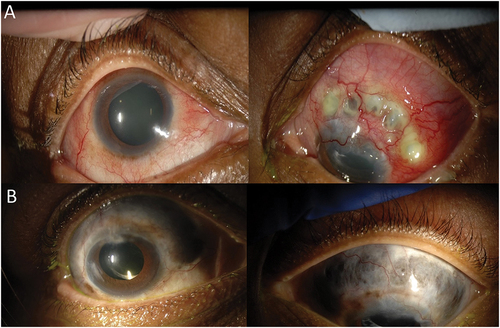
Patient 2 is a female with a history of granulomatosis with polyangiitis (GPA) who presented with a bilateral anterior diffuse and necrotizing scleritis associated with bilateral cataract and panuveitis (). Her initial visual acuity was 20/40 in the right eye and 20/25 in the left. She was initially treated with steroids, cyclophosphamide and rituximab for kidney and pulmonary complications of GPA. While her systemic findings improved, her scleritis remained refractory to numerous therapies with intermittent control from high-dose oral corticosteroids and intravenous infusions of solumedrol. Infliximab (5 mg/kg, administered every 6 weeks) and methotrexate (25 mg/week) led to partial remission, but she developed worsening scleritis after 2 years on a combination of infliximab, methotrexate and prednisone (1 mg/kg, tapering over 3 months to a maintenance dose between 10 and 15 mg/day). Other therapies attempted included cyclophosphamide, tocilizumab, mycophenolate mofetil, rituximab and repository corticotropin injection (ActharTM gel). At her last visit, after 60 months follow-up, she was affected by multiple complications of GPA and immunosuppression (pneumonia, cellulitis, hyperglycemia). She was hospitalized for suspicion of orbital cellulitis in the setting of sinusitis. Her visual acuity was 20/60 in the right eye, 20/50 in the left eye, she had extensive bilateral scleromalacia, trace cells in anterior chamber and vitreous in both eyes (). She was treated with intravenous steroids and antibiotics and cyclophosphamide was restarted after resolution of the orbital cellulitis with follow-up ongoing.
Figure 3. Photographs of patient with a history of granulomatosis with polyangiitis. A) Slit lamp photos demonstrating treatment-refractory bilateral anterior diffuse and necrotizing scleritis associated with bilateral cataract and panuveitis. B) Slit lamp photos at 43 months demonstrating extensive bilateral scleromalacia despite multiple treatments.
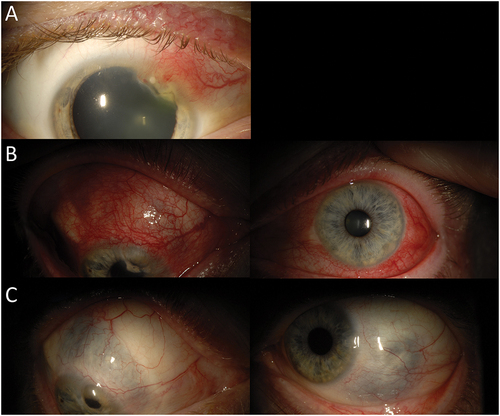
Patient 3 is a 45-year-old Caucasian man who presented with bilateral idiopathic anterior diffuse scleritis and anterior uveitis in the setting of positive rheumatoid factor and history of orofacial herpes. At presentation, his visual acuity was 20/20 in both eyes. Examination was notable for nodular anterior scleritis associated with marginal keratitis in the right eye (). He was treated with local prednisolone acetate 1% four times daily and valacyclovir 1 gram three times daily. Despite this treatment, intraocular inflammation persisted, and he was started on oral steroids and methotrexate. He then developed anterior scleritis in the left eye and bilateral anterior uveitis (). He was treated with cyclosporine (3 mg/kg, divided twice daily dosing), which had only moderate effect. He subsequently received azathioprine (2 mg/kg, divided into twice daily dosing), which led to disease control for 2 years prior to recurrent scleritis despite azathioprine therapy. Adalimumab, infliximab and rituximab were utilized serially, but were unsuccessful in controlling the patient’s scleritis. He was treated with cyclophosphamide in conjunction with repository corticotropin injection (ActharTM gel). After developing recurrent disease despite cyclophosphamide and corticotropin injection, he was started on low-dose cyclosporine (1 mg/kg, divided into twice daily dosing) and tocilizumab. Cyclosporine was eventually discontinued because of elevated creatinine. At the patient’s last visit, the scleritis remained inactive while on tocilizumab and prednisone dosed at 12.5 mg/day. His visual acuities were 20/30 in both eyes. Examination showed scleromalacia with no disease activity ().
Figure 4. 45-year-old Caucasian male with bilateral idiopathic anterior diffuse scleritis and anterior uveitis in setting of orofacial herpes. A) Slit lamp photo of the right eye demonstrating nodular anterior scleritis with marginal keratitis at presentation. B) Slit lamp photos demonstrating development of anterior scleritis in the left eye and bilateral anterior uveitis despite treatment of local steroids and antivirals. C) Post-treatment with cyclophosphamide, repository corticotropin injection, cyclosporine and tocilizumab at 29 months; slit lamp photos show bilateral scleromalacia with no residual inflammation.
Discussion
In our cohort, SAI was associated with a higher rate of visual morbidity compared to IS. Specifically, mean visual acuity was worse in the SAI group at initial presentation, 6 months, 12-month follow-up when compared to the IS group. Although both groups showed stability of vision over the time, the visual acuity difference between SAI and IS approximated 2–3 lines throughout the entire study period. A higher rate of posterior complications was also observed in the SAI group and exceeded 50% compared to 7–8% the IS group.
Our results are comparable with other cohorts that showed that posterior scleritis was associated with a higher risk of permanent visual loss, complications and disease recurrence.Citation4,Citation11,Citation12 Diaz et al. reported that posterior or necrotizing scleritis typically require more aggressive treatments.Citation6 The potential for CME, optic nerve edema, and exudative retinal detachment may contribute to visual acuity impairment, and was also illustrated by particularly aggressive and refractory cases described in this report.
The contiguous spread of scleral inflammation to intraocular structures, such as the iris, ciliary body, vitreous and retina, may be related to the presence of perivasculitis or vasculitis in patients with scleritis.Citation3,Citation12 Immune mechanisms of both scleritis and uveitis involve inflammatory cells that lead to proliferation of Muller cells in the latter stages. This causes gliotic scarring during the chronic stage of inflammation.Citation12–14 Moreover, extension of scleral inflammation to intraocular structures, inflammatory cytokine milieu, and blood-ocular barrier disruption likely contribute to CME and epiretinal membrane formation.
The rates of associated systemic diseases in both groups (28% for SAI group and 56% for IS group) were comparable to previous publications.Citation2–4,Citation15 Systemic diseases associated with scleritis within our cohort included rheumatoid arthritis (8% of SAI group and 44% of IS group) and GPA (11% of SAI group), diseases classically associated with scleritis in the literature.Citation2,Citation5,Citation16,Citation17 While systemic autoimmune diseases may portend a worse visual and systemic prognosis in patients with scleritis,Citation4,Citation18 we did not observe a clear association in vision when comparing scleritis patients with and without systemic autoimmunity. Notably, all four patients with anterior scleritis related to GPA developed intraocular inflammation, and three of whom developed posterior segment complications.
Although the IS group showed fewer intraocular complications and reduced visual impairment than the SAI group, the need for immunosuppressive treatment was comparable between cohorts. However, the specific number of medications, dosing, and number of medications required to achieve disease inactivity was not specifically assessed due to the varying regimens and sample size limitations. Moreover, the proportion of individuals requiring immunosuppression was subject to referral bias given the tertiary referral nature of our institution. Methotrexate was the most commonly used first-line antimetabolite, which is consistent with prior reports.Citation1,Citation6,Citation15,Citation18 Other immunosuppressive agents employed included mycophenolate mofetil, azathioprine, cyclophosphamide, or biologic agents. Adalimumab was utilized more frequently than infliximab in our cohort of patients although other series support infliximab as an appropriate biologic response modifier.Citation19–21 Several patients with refractory scleritis were treated with rituximab or tocilizumab after failure of another anti-TNF agent and patients showed response variability, which has been observed by other authors.Citation22–24
Our study was limited by the retrospective nature of data collection and the referral basis of our patient population as a tertiary referral center. Specifically, the referral basis of our patient population may have skewed our cases towards higher acuity cases including individuals with greater intraocular involvement in the SAI group and potentially, worse cases of IS with the potential for greater visual morbidity. Another potential limitation was related to the duration of disease prior to referral, which may have impacted the degree of visual acuity impairment or ocular structures affected.
Nonetheless, our study highlights the potential of a greater risk of visual loss and secondary ocular complications with SAI compared to patients with IS. Rheumatoid arthritis, GPA and other systemic autoimmune disorders can be associated with SAI and IS and may be refractory to multiple immunosuppressive medications that require multidisciplinary management with rheumatology and judicious medical management.
Future research that studies risk factors contributing to intraocular inflammation associated with scleritis, specific levels of disease control with anti-inflammatory medications over time, and immunosuppressive requirement could improve our understanding of how scleritis with or without intraocular inflammation may respond to patient and disease-specific immunosuppressive regimens.
Synopsis/precis
Scleritis with intraocular inflammation was associated with higher rates of visual morbidity compared to isolated scleritis at baseline as well as higher rates of posterior segment complications.
Supplemental Material
Download Zip (29.8 KB)Disclosure statement
No conflicting relationships or competing interests are declared for any author.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/09273948.2022.2164726.
Additional information
Funding
References
- Stem MS, Todorich B, Faia LJ. Ocular pharmacology for scleritis: review of treatment and a practical perspective. J Ocul Pharmacol Ther. 2017;33(4):240–246. doi:10.1089/jop.2016.0127.
- Akpek EK, Thorne JE, Qazi FA, et al. Evaluation of patients with scleritis for systemic disease. Ophthalmology. 2004;111:501–506. doi:10.1016/j.ophtha.2003.06.006.
- Sainz de la Maza M, Foster CS, Jabbur NS. Scleritis-associated uveitis. Ophthalmology. 1997;104(1):58–63. doi:10.1016/S0161-6420(97)30361-3.
- McCluskey PJ, Watson PG, Lightman S, et al. Posterior scleritis: clinical features, systemic associations, and outcome in a large series of patients. Ophthalmology. 1999;106(12):2380–2386. doi:10.1016/S0161-6420(99)90543-2.
- Caimmi C, Crowson CS, Smith WM, Matteson EL, Makol A. Clinical correlates, outcomes, and predictors of inflammatory ocular disease associated with Rheumatoid arthritis in the biologic era. J Rheumatol. 2018;45(5):595–603. doi:10.3899/jrheum.170437.
- Diaz JD, Sobol EK, Gritz DC. Treatment and management of scleral disorders. Surv Ophthalmol. 2016;61:702–717. doi:10.1016/j.survophthal.2016.06.002.
- Watson PG, Hayreh SS. Scleritis and episcleritis. Br J Ophthalmol. 1976;60:163e91. doi:10.1136/bjo.60.3.163.
- Sen HN, Sangave AA, Goldstein DA, et al. A standardized grading system for scleritis. Ophthalmology. 2011;118(4):768–771. doi:10.1016/j.ophtha.2010.08.027.
- Bloch-Michel E, Nussenblatt RB. International Uveitis Study Group: recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol. 1987;103:234–235. doi:10.1016/S0002-9394(14)74235-7.
- Lavric A, Gonzalez-Lopez JJ, Majumder PD, et al. Posterior scleritis: analysis of epidemiology, clinical factors, and risk of recurrence in a cohort of 114 patients. Ocul Immunol Inflamm. 2016;24(1):6–15. doi:10.3109/09273948.2015.1005240.
- Lane J, Nyugen E, Morrison J, et al. Clinical features of scleritis across the Asia Pacific Region. Ocul Immunol Inflamm. 2019;27(6):920–926. doi:10.1080/09273948.2018.1484496.
- Fong LP, Sainz de la Marza M, Rice BA, Kupferman AE, Foster S. Immunopathology of scleritis. Ophthalmology. 1998;4:472–479.
- Chian CC, Li Q. Immunopathology of uveitis. Br J Ophthalmol. 1998;82:91–96. doi:10.1136/bjo.82.1.91.
- Sainz-de-la-Maza M, Molins B, Mesquida M, et al. Interleukin-22 serum levels are elevated in active scleritis. Acta Ophthalmol. 2016;94(6):e395–399. doi:10.1111/aos.13005.
- Sands DS, Chan SCY, Gottlieb CC. Methotrexate for the treatment of noninfectious scleritis. Can J Ophthalmol. 2018;53(4):349–353. doi:10.1016/j.jcjo.2017.11.009.
- Watkins AS, Kempen JH, Choi D, et al. Ocular disease in patients with ANCA-positive vasculitis. J Ocul Biol Dis Inform. 2010;3:12–19. doi:10.1007/s12177-009-9044-4.
- Asin MA P-J, Charles P, Rothschild PR, et al. Ocular involvement in granulomatosis with polyangiitis: a single-center cohort study on 63 patients. Autoimmun Rev. 2019;18:493–500. doi:10.1016/j.autrev.2019.03.001.
- Wieringa WG, Wieringa JE, Dam-van Loon LH, Los LI. Visual outcome, treatment results and prognostic factors in patients with scleritis. Ophthalmology. 2013;120:379–386. doi:10.1016/j.ophtha.2012.08.005.
- Ragam A, Kolomeyer AM, Fang C, Xu Y, Chu DS. Treatment of chronic, noninfectious, nonnecrotizing scleritis with tumor necrosis factor alpha inhibitors. Ocul Immunol Inflam. 2014;22(6):469–477. doi:10.3109/09273948.2013.863944.
- de Fidelix TS, Vieira LA, de Freitas D, Trevisani VF. Biologic therapy for refractory scleritis: a new treatment perspective. Int Ophthalmol. 2015;35(6):903–912. doi:10.1007/s10792-015-0124-0.
- Levy-Clarke G, Jabs DA, Read RW, Rosenbaum JT, Vitale A, Van Gelder RN. Expert panel recommendations for the use of anti-tumor necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthalmology. 2014;121(3):785–796. doi:10.1016/j.ophtha.2013.09.048.
- Silpa-Archa S, Oray M, Preble JM, Foster CS. Outcome of tocilizumab treatment in refractory ocular inflammatory diseases. Acta Ophthalmol. September, 2016;94(6):e400–6. doi:10.1111/aos.13015.
- Cao JH, Oray M, Cocho L, Foster CS. Rituximab in the treatment of refractory noninfectious scleritis. Am J Ophthalmol. April, 2016;164:22–28. doi:10.1016/j.ajo.2015.12.032.
- Yoshida A, Watanabe M, Okubo A, Kawashima H. Clinical characteristics of scleritis patients with emphasized comparison of associated systemic diseases (anti-neutrophil cytoplasmic antibody-associated vasculitis and rheumatoid arthritis). Jpn J Ophthalmol. 2019;63(5):417–424. doi:10.1007/s10384-019-00674-7.